Association among Abdominal Obesity Induces, Diabetic Retinopathy and Metabolic Syndrome in Community: A Cross-Sectional Study
Xin Li1, Zi-Wei Yu1, Chang-Wei Yang1, Ming Hao1, Xin-Yuan Gao1,*, Hong-Yu Kuang1,*, Yong Yu2 and Xu Peng3
1Department of Endocrinology, Harbin Medical University, Harbin, Heilongjiang, China
2Department of Endocrinology, The First People’s Hospital, Hulan, China
3Department of Endocrinology, Traditional Chinese medicine Hospital, Hulan, China
- *Corresponding Author:
- Xin-Yuan Gao
Department of Endocrinology
The First Affiliated Hospital of Harbin
Medical University, Harbin, 150001
Heilongjiang, China
Tel: +8613904517001
E-mail: m15124768260@163.com
Hong-Yu Kuang
Department of Endocrinology, The First Affiliated
Hospital of Harbin Medical University
Harbin, 150001, Heilongjiang, China
Tel: +8618247140616
E-mail: ydykuanghongyu@126.com
Received Date: July 09, 2021; Accepted Date: July 23, 2021; Published Date: July 30, 2021
Citation: Li X, Yu ZW, Yang CW, Hao M, Gao XY, et al. (2021) Association among Abdominal Obesity Induces, Diabetic Retinopathy and Metabolic Syndrome in Community: A Cross-Sectional Study. Endocrinol Metab Vol. 5 No.4: 169.
Abstract
Background and aims: Obesity often coexists with diabetes has been recognized as a risk factor for diabetic complications. Diabetic retinopathy (DR) is one of the most common microvascular complications of diabetes, and the metabolic syndrome (MetS) is one of the most common symptoms of diabetes. The purpose of this study was to explore the relationship between DR and some induces, including NC, CVAI, PWNC and so on; as well as the relationship between DR and MetS.
Methods: From 2018 to 2019, a total of 562 diabetics from the Hulan District of Harbin, Heilongjiang, were selected and completed a questionnaire survey. The questionnaire included basic patient information, anthropometric parameters, blood pressure, biochemical parameters and fundus photography results.
Results: In both men and women, a one Standard Deviation (SD) increase in NCCVAI and PWNC was not associated with the prevalence of DR (P>0.05). However, in both men and women, a one SD increase in NC、CVAI and PWNC was significantly associated with the prevalence of MetS (P<0.05). These associations were all adjusted for potential confounding factors. Moreover, DR was not associated with MetS (P>0.05).
Conclusion: NC, CVAI and PWNC are associated with the prevalence of MetS. NC in men and CVAI in women had the largest area under the ROC curve compared to the other induces, which may be convenient and valuable anthropometric measurements for early prevention of MetS. However, these induces had no association with DR and there is no relationship between DR and MetS.
Keywords
Diabetic Retinopathy (DR); Metabolic syndrome (MetS); Abdominal obesity; Neck Circumference (NC); Chinese Visceral Obesity Index (CVAI)
Abbreviations
DR: Diabetic Retinopathy; MetS: Metabolic Syndrome; BMI: Body Mass Index; WC: Waist Circumference; HC: Hip Circumference; NC: Neck Circumference; CVAI: Chinese Visceral Adiposity Index; WHR: Waist-to-Hip Ratio; PWNC: Product of WC and NC; FPG: Fasting Plasma Glucose; HbA1c: glycated hemoglobin; HDL: High-Density Lipoprotein; LDL: Low-Density Lipoprotein; TG: Triglycerides; TC: Total Cholesterol; UA: Uric Acid; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure.
Introduction
The global prevalence of diabetes is predicted to increase dramatically in the coming decades as the population grows and ages, in parallel with the rising burden of overweight and obesity, in both developed and developing countries [1]. About the epidemiological data, the worldwide prevalence of overweight and obesity has reached 33.3%, which has doubled since 1980 [2].
Moreover, DR is the most common microvascular complication in patients with diabetes and the leading cause of vision loss globally in working middle-aged adults [3] and MetS is a cluster of obesity, hypertension, dysglycemia, dyslipidemia, and insulin resistance. Because hyperglycemia, oxidative stress and inflammation are the same processes involved in DR and MetS, several population studies evaluated its association with them. However, the relationship between metabolic syndrome and diabetic microvascular complications is contradictory and needs further study.
In fact, the methods to detect abdominal adiposity include Dual- Energy X-ray Absorptiometry (DEXA), Computed Tomography (CT), Magnetic Resonance Imaging (MRI) and dual Bioelectrical Impedance Analysis (BIA). However, they are unsuitable for routine clinical practices in a general population on account of the radiation exposure, time requirements and high cost [4]. There are lots of induces to estimate obesity, such as Neck Circumference (NC), Waist Circumference (WC), Body Mass Index (BMI) and the Visceral Adiposity Index (VAI), the Lipid Accumulation Product (LAP), which are calculated using the data of WC, BMI, triglycerides (TG), and High-Density Lipoprotein (HDL) [5]. Here we must mention two new indicators-Chinese Visceral Obesity Index (CVAI), and the product of WC and NC (PWNC), which are considered to serve as a better predictor of T2DM and MetS in T2DM [6,7].
The findings of a cross-sectional study suggest that visceral adiposity is associated with DR in individuals with longstanding T2DM in Asia [8]. However, a study of 2016 found that, in Asian patients with T2DM, a higher BMI appeared to confer a protective effect on DR [9]. Therefore, the association between obesity and DR is equivocal. A new study has found that CVAI was not associated with DR in both men and women [4]. In addition, a study published in 2018 with 1986 type 2 diabetic Asian patients reported a higher prevalence of retinopathy in patients with MetS defined by NCEP-ATP III (37.9% in T2D+MetS vs. 28.6% T2D without MetS, P<0.001). And yet, in 2018, Zhou et al. published a meta-analysis compiling the results of 12 observational studies which addressed this relationship between MetS and retinopathy in diabetic patients, which reported that no association between MetS and DR in type 1 or type 2 diabetic patients and no correlation between isolated MetS components (BMI, WC, BP, HDL and triglyceride levels) and retinopathy [10].
We aimed to investigate the association between DR and some induces, including WC, NC, WHR, BMI, CVAI, PWNC; as well as the relationship between DR and Mets among people with diabetes in northeast China. Our findings may provide evidence for the early detection, prevention and treatment of MetS and diabetic complications.
Materials and Methods
Subjects
The present study was a cross-sectional study comprising 562 diabetics from the Hulan District of Harbin from 2018 to 2019 (Non-admitted patients), and completed a questionnaire survey. The diagnosis of T2DM in the subjects was consistent with the 1999 WHO diagnostic criteria [11]. Each subject was examined by a clinical ophthalmologist with the use of an ophthalmoscope. This study was approved by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University and followed the Declaration of Helsinki and STARD guidelines. Written Informed consent was obtained from all participants.
Data collection
The information on sociodemographic characteristics, medical history, family history, and lifestyle factors was accessed by doctors of the First Affiliated Hospital of Harbin Medical University through a face-to-face interview. Anthropometric measurements including weight, height, NC, WC, hip circumference (HC) and blood pressure were conducted by trained staff according to standard protocols.
Height and weight were measured with participants standing without shoes and in lightweight clothes to the nearest 0.1 cm and 0.1 kg. WC was measured on the midaxillary line between the lowest border of the rib cage and the top of the iliac crest to the nearest 0.1 cm. NC was measured below the cricoid cartilage and then at the level of the mid-cervical spine to the nearest 0.1 cm. HC was measured at the widest part of the hip at the level of the greater trochanter to the nearest 0.1 cm. BMI was calculated as weight in kilograms divided by squared height in meters. WHR was calculated as WC divided by HC. The PWNC was calculated by the product of WC and NC. And the CVAI was calculated as follows:
Males:

TG(mmol/L)-11.66 × HDL(mmol/L)
Laboratory tests of fasting blood sample were performed using standard bio-chemical analysis methods, which included Total Cholesterol (TC), Triglycerides (TG), High Density Lipoprotein (HDL), Low Density Lipoprotein (LDL), and Uric Acid (UA), and using high pressure liquid phase detection method to test glycosylated hemoglobin A1c (HbA1c), and using chemiluminescence method to test fasting C-peptide.
Definition of variables
Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, or a self- reported previous diagnosis of hypertension. Dyslipidemia was defined as TC ≥ 5.18mmol/L (200mg/dl), TC ≥ 1.70mmol/L (150mg/ dl), LDL-C ≥ 3.37mmol/L (130mg/dl), HDL-L<1.04 mmol/L (40 mg/ dl).
In accordance with the guidelines for the prevention and treatment of T2DM in China (2020), MetS was defined as the presence of 3 or more of the following features:
1. abdominal obesity (central obesity): WC ≥ 90 cm for males and 85 cm for females;
2. hyperglycemia: fasting plasma glucose level (FPG) ≥ 6.1 mmol/l or 2 hours after glucose overload blood glucose ≥ 7.8 mmol/L and/or has been diagnosed as diabetes and treated;
3. hypertension: BP ≥ 130/85 mmHg (1 mmHg=0.133 kPa) and (or) have been identified as hypertension and treated;
4. Fasting TG ≥ 1.70 mmol/L;
5. Fasting HDL-L<1.04 mmol/L.
Participants without DR were defined as having no abnormalities in fundus photographs; participants with DR included individuals with intraretinal microaneurysms, hemorrhages, venous beading, prominent microvascular abnormalities, neovascularization or vitreous/preretinal hemorrhages in accordance with the Global Diabetic Retinopathy Project Group.
Statistical analysis
Data analyses were performed with IBM SPSS Statistics, version 26. Continuous variables were expressed as the mean ± Standard Deviation (SD) or the median with an interquartile range (25%, 75%), and categorical variables were presented as percentages (%). The Student’s t test and Chi-square test were used for continuous and dichotomous variables, respectively. Logistic regression tests were used to analyze the associations between abdominal obesity indices and DR or MetS. Data were summarized as odds ratios or regression coefficients (95% CI). Chi-square test was used to analyze the relationship between DR and MetS. The receiver operating characteristics (ROC) curve was constructed to evaluate the discrimination of different induces for MetS. The optimal cut-off point was determined by the maximum Youden index. A P-value <0.05 (two-sided) was regarded as statistically significant.
Results
General characteristics of the diabetic participants
Overall, 202 men and 360 women with diabetes were involved in the basal analyses. Among men, the prevalence of DR was 29.7%, and the prevalence of MetS was 84.2%. In women, the prevalence of DR was 37.8%, and the prevalence of MetS was 83.3%.
Respective characteristics of men and women by DR or MetS
The clinical and biochemical characteristics of the subjects are shown in Tables 1 and 2. The participants were divided into two groups with or without DR, and with or without MetS. Compared with the men without DR, only CVAI was significantly higher in men with DR (P<0.05). However, no differences in BMI, WC, HC, NC, WHR and PWNC were found between the two groups (P>0.05). And BMI, WC, NC, WHR, CVAI and PWNC were all significantly higher in men with MetS (P<0.05). Compared with the women without DR, only BMI was significantly higher in women with DR (P<0.05). However, no differences in WC, HC, NC, WHR, CVAI and PWNC were found between the two groups (P>0.05). And BMI, WC, HC, NC, WHR, CVAI and PWNC were all significantly higher in women with MetS (P<0.05).
| DR-(n=142) | DR+(n=60) | P | MetS-(n=32) | MetS+(n=170) | P | |
|---|---|---|---|---|---|---|
| Age (years) | 54.97 ± 11.28 | 57.85 ± 8.26 | 0.076 | 50.84 ± 14.69 | 56.76 ± 9.32 | 0.034 |
| Duration of DM (years) | 6 (3,10) | 7 (5,10) | 0.017 | 5.5 (3,10.75) | 6 (4,10) | 0.679 |
| BMI (kg/m2) | 26.81 ± 3.08 | 27.60 ± 3.35 | 0.109 | 25.20 ± 3.40 | 27.39 ± 3.02 | <0.001 |
| WC (cm) | 99.46 ± 8.76 | 101.78 ± 7.87 | 0.077 | 92.78 ± 10.49 | 101.54 ± 7.39 | <0.001 |
| HC (cm) | 100.77 ± 8.11 | 102.78 ± 11.20 | 0.155 | 99.63 ± 7.63 | 101.70 ± 9.39 | 0.24 |
| NC (cm) | 39.14 ± 5.59 | 39.82 ± 3.15 | 0.381 | 37.53 ± 2.98 | 39.68 ± 5.22 | 0.025 |
| WHR | 0.99 ± 0.11 | 0.99 ± 0.10 | 0.697 | 0.94 ± 0.12 | 1.01 ± 0.10 | <0.001 |
| HbA1c (%) | 7.82 ± 1.62 | 8.15 ± 1.80 | 0.207 | 8.53 ± 1.72 | 7.81 ± 1.66 | 0.025 |
| TG (mmol/L) | 1.51 (1.07,1.95) | 1.49 (1.08,2.05) | 0.869 | 1.14 (0.88,1.40) | 1.59 (1.15,2.17) | <0.001 |
| TC (mmol/L) | 4.95 ± 1.13 | 4.63 ± 1.20 | 0.074 | 4.55 ± 0.83 | 4.91 ± 1.20 | 0.106 |
| LDL (mmol/L) | 3.16 ± 0.91 | 2.60 ± 0.96 | 0.157 | 2.84 ± 0.81 | 3.15 ± 0.94 | 0.087 |
| HDL (mmol/L) | 1.30 ± 0.25 | 1.38 ± 0.74 | 0.414 | 1.32 ± 0.16 | 1.33 ± 0.50 | 0.91 |
| SBP (mmHg) | 138.84 ± 17.83 | 143.42 ± 17.89 | 0.097 | 124.53 ± 16.28 | 143.15 ± 16.68 | <0.001 |
| DBP (mmHg) | 86.48 ± 11.12 | 88.67 ± 10.41 | 0.195 | 79.06 ± 9.28 | 88.65 ± 10.57 | <0.001 |
| FPG (mmol/L) | 10.91 ± 3.73 | 11.50 ± 3.87 | 0.306 | 11.04 ± 3.85 | 11.09 ± 3.77 | 0.939 |
| UA (μmol/L) | 282.03 ± 82.78 | 291.06 ± 93.66 | 0.497 | 243.61 ± 51.33 | 292.44 ± 89.08 | <0.001 |
| CVAI | 148.02 ± 50.42 | 163.12 ± 38.45 | 0.039 | 117.60 ± 41.50 | 159.10 ± 45.88 | <0.001 |
| PWNC (cm2) | 3912 ± 761 | 4068 ± 577 | 0.157 | 3507 ± 638 | 4043 ± 696 | <0.001 |
| Family history | 35 (74.5%) | 12 (25.5%) | 0.475 | 9 (19.1%) | 38 (80.9%) | 0.478 |
| Intervention time (years) | 5 (2, 9.25) | 6 (5, 10) | 0.019 | 5 (2.13, 9.75) | 6 (3, 10) | 0.531 |
Table 1: General characteristics of all male participants by DR and MetS.
| DR-(n=224) | DR+(n=136) | P | MetS-(n=60) | MetS+(n=300) | P | |
|---|---|---|---|---|---|---|
| Age (years) | 57.77 ± 10.08 | 59.71 ± 8.21 | 0.06 | 54.07 ± 11.11 | 59.39 ± 8.84 | 0.001 |
| Duration of DM (years) | 7 (4, 11) | 9 (5.25, 15) | <0.001 | 7 (3.25, 12) | 8 (4.25, 13) | 0.468 |
| BMI (kg/m2) | 26.50 ± 3.84 | 25.67 ± 2.93 | 0.026 | 23.83 ± 2.83 | 26.63 ± 3.50 | <0.001 |
| WC (cm) | 96.46 ± 9.34 | 96.68 ± 8.59 | 0.823 | 88.50 ± 9.58 | 98.15 ± 8.05 | <0.001 |
| HC (cm) | 97.75 ± 8.50 | 96.85 ± 9.33 | 0.352 | 92.58 ± 8.61 | 98.37 ± 8.55 | <0.001 |
| NC (cm) | 0.99 ± 0.11 | 1.01 ± 0.11 | 0.249 | 0.96 ± 0.14 | 1.00 ± 0.10 | 0.048 |
| WHR | 35.27 ± 2.65 | 35.11 ± 2.73 | 0.587 | 33.48 ± 2.73 | 35.56 ± 2.53 | <0.001 |
| HbA1c (%) | 7.61 ± 1.62 | 8.18 ± 1.93 | 0.004 | 7.80 ± 2.08 | 7.83 ± 1.70 | 0.865 |
| TG (mmol/L) | 1.58 (1.15, 2.00) | 1.57 (1.16, 2.35) | 0.93 | 1.14 (0.92, 1.44) | 1.71 (1.29, 2.40) | <0.001 |
| TC (mmol/L) | 4.92 ± 1.12 | 5.20 ± 1.26 | 0.029 | 4.52 ± 1.15 | 5.12 ± 1.16 | <0.001 |
| LDL(mmol/L) | 3.05 ± 0.93 | 3.22 ± 1.14 | 0.16 | 2.78 ± 0.96 | 3.18 ± 1.02 | 0.005 |
| HDL(mmol/L) | 1.29 ± 0.20 | 1.31 ± 0.27 | 0.412 | 1.34 ± 0.21 | 1.29 ± 0.23 | 0.111 |
| SBP (mmHg) | 140.49 ± 18.90 | 142.29 ± 19.27 | 0.387 | 124.50 ± 16.67 | 144.50 ± 17.70 | <0.001 |
| DBP (mmHg) | 84.83 ± 12.00 | 83.90 ± 1025 | 0.448 | 77.25 ± 8.85 | 85.93 ± 11.27 | <0.001 |
| FPG(mmol/L) | 10.90 ± 3.89 | 12.16 ± 5.11 | 0.016 | 11.27 ± 4.97 | 11.41 ± 4.32 | 0.82 |
| UA (μmol/L) | 240.63 ± 87.27 | 254.22 ± 74.16 | 0.131 | 218.90 ± 43.47 | 251.14 ± 87.58 | <0.001 |
| CVAI | 124.84 ± 36.25 | 125.27 ± 29.00 | 0.907 | 91.51 ± 34.30 | 131.70 ± 29.30 | <0.001 |
| PWNC (cm2) | 3416 ± 519 | 3406 ± 489 | 0.854 | 2976 ± 501 | 3500 ± 463 | <0.001 |
| Family history | 45 (20.1%) | 30 (22.1%) | 0.656 | 10 (16.7%) | 65 (21.7%) | 0.384 |
| Intervention time (years) | 6 (4, 11) | 8.5 (5, 13) | 0.001 | 6.5 (3.25, 10) | 7 (4, 12) | 0.175 |
Table 2: General characteristics of all female participants by DR and MetS.
In addition, the participants were also divided into four groups according to the quartiles of NC (Tables 3 and 4). Both in men and women, BMI, NC, WC, HC, CVAI, SBP, DBP, and PWNC were all significant among groups (P<0.05). However, MS was significant (P<0.001) and DR was not significant among groups (P>0.05).
| Q1 (n=46) | Q2 (n=45) | Q3 (n=62) | Q4 (n=49) | P | |
|---|---|---|---|---|---|
| Age (years) | 56.76 ± 10.49 | 55.00 ± 12.58 | 56.47 ± 10.07 | 54.90 ± 9.20 | 0.744 |
| Duration of DM (years) | 4.5 (3, 9) | 8 (4, 12.5) | 6 (3.88, 9) | 6 (3, 10.5) | 0.011 |
| BMI (kg/m2) | 24.33 ± 2.78 | 25.67 ± 2.06 | 27.79 ± 2.29 | 29.93 ± 2.52 | <0.001 |
| WC (cm) | 92.87 ± 8.78 | 96.98 ± 6.55 | 101.85 ± 5.07 | 107.73 ± 6.29 | <0.001 |
| HC (cm) | 96.87 ± 7.38 | 99.18 ± 11.28 | 101.60 ± 8.00 | 107.33 ± 6.44 | <0.001 |
| NC (cm) | 35.02 ± 1.54 | 37.38 ± 0.49 | 39.92 ± 0.75 | 44.47 ± 7.25 | <0.001 |
| WHR | 0.96 ± 0.12 | 0.99 ± 0.13 | 1.01 ± 0.09 | 1.01 ± 0.06 | 0.127 |
| HbA1c% | 7.91 ± 1.96 | 8.08 ± 1.82 | 7.75 ± 1.50 | 8.00 ± 1.50 | 0.766 |
| TG (mmol/L) | 1.24 (0.94, 1.76) | 1.41 (1.07, 1.74) | 1.43 (1.00, 2.09) | 1.78 (1.39, 2.44) | 0.919 |
| TC (mmol/L) | 4.85 ± 1.32 | 4.82 ± 1.17 | 4.93 ± 1.23 | 4.81 ± 0.90 | 0.947 |
| LDL (mmol/L) | 3.05 ± 1.12 | 3.03 ± 0.92 | 3.10 ± 0.82 | 3.20 ± 0.87 | 0.813 |
| HDL (mmol/L) | 1.39 ± 0.30 | 1.33 ± 0.30 | 1.37 ± 0.70 | 1.21 ± 0.22 | 0.199 |
| SBP (mmHg) | 138.70 ± 15.18 | 138.00 ± 14.28 | 137.98 ± 19.13 | 146.43 ± 20.62 | 0.048 |
| DBP (mmHg) | 85.43 ± 9.12 | 84.11 ± 8.07 | 86.45 ± 11.68 | 92.35 ± 12.25 | 0.001 |
| FPG (mmol/L) | 11.35 ± 4.67 | 11.14 ± 3.72 | 10.99 ± 3.69 | 10.90 ± 3.00 | 0.942 |
| UA (μmol/L) | 252.47 ± 69.17 | 262.13 ± 69.64 | 298.04 ± 93.08 | 318.84 ± 90.26 | <0.001 |
| CVAI | 122.79 ± 35.88 | 139.83 ± 29.50 | 155.84 ± 59.18 | 187.83 ± 27.32 | <0.001 |
| PWNC(cm2) | 3254 ± 345 | 3625 ± 257 | 4066 ± 216 | 4789 ± 775 | <0.001 |
| Family history | 12(26.1%) | 16(35.6%) | 12(19.4%) | 7(14.3%) | 0.08 |
| Intervention time (years) | 4 (3, 7) | 6 (4, 12.5) | 6 (3, 7.25) | 6 (3, 10) | 0.003 |
| DR | 11(23.9%) | 13(28.9%) | 18(29.0%) | 18(36.7%) | 0.588 |
| MS | 26(56.5%) | 38(84.4%) | 57(91.9%) | 49(100%) | <0.001 |
Table 3: General characteristics of male participants divided by quartiles of NC.
| Q1 (n=90) | Q2 (n=60) | Q3 (n=148) | Q4 (n=62) | P | |
|---|---|---|---|---|---|
| Age (years) | 57.2 ± 11.43 | 57.47 ± 9.50 | 59.24 ± 8.08 | 59.65 ± 9.20 | 0.238 |
| Duration of DM (years) | 8.5 (5, 12.5) | 5 (3, 9.75) | 8 (5, 13) | 7.5 (4, 13) | 0.046 |
| BMI (kg/m2) | 23.76 ± 2.91 | 25.88 ± 2.81 | 26.43 ± 3.25 | 29.30 ± 3.10 | <0.001 |
| WC (cm) | 89.68 ± 7.20 | 95.30 ± 8.81 | 97.74 ± 7.55 | 104.84 ± 7.07 | <0.001 |
| HC (cm) | 92.53 ± 9.12 | 96.95 ± 8.31 | 98.46 ± 7.82 | 102.42 ± 7.66 | <0.001 |
| NC (cm) | 32.00 ± 1.35 | 34.01 ± 0.06 | 35.95 ± 0.79 | 39.34 ± 1.49 | <0.001 |
| WHR | 0.98 ± 0.14 | 0.99 ± 0.10 | 1.00 ± 0.10 | 1.03 ± 0.07 | 0.05 |
| HbA1c% | 7.91 ± 1.88 | 7.34 ± 1.62 | 7.84 ± 1.81 | 8.11 ± 1.57 | 0.094 |
| TG (mmol/L) | 1.59 (1.03, 2.20) | 1.38 (1.03, 1.78) | 1.62 (1.26, 2.48) | 1.58 (1.35, 2.61) | 0.018 |
| TC (mmol/L) | 5.10 ± 1.10 | 4.77 ± 1.18 | 5.00 ± 1.23 | 5.23 ± 1.15 | 0.177 |
| LDL (mmol/L) | 1.31 ± 0.23 | 1.28 ± 0.21 | 1.31 ± 0.24 | 1.27 ± 0.21 | 0.522 |
| HDL (mmol/L) | 2.94 ± 0.85 | 2.86 ± 0.80 | 3.23 ± 1.12 | 3.32 ± 1.12 | 0.012 |
| SBP (mmHg) | 133.33 ± 18.41 | 140.72 ± 17.24 | 144.31 ± 19.50 | 145.48 ± 17.36 | <0.001 |
| DBP (mmHg) | 79.44 ± 9.04 | 86.00 ± 8.63 | 86.03 ± 13.12 | 86.61 ± 10.15 | <0.001 |
| FPG (mmol/L) | 12.59 ± 5.12 | 10.86 ± 4.65 | 11.13 ± 4.11 | 10.80 ± 3.56 | 0.028 |
| UA (μmol/L) | 229.03 ± 53.74 | 241.08 ± 62.71 | 251.46 ± 106.77 | 261.00 ± 63.51 | 0.082 |
| CVAI | 105.23 ± 33.10 | 116.38 ± 33.00 | 129.81 ± 28.63 | 150.57 ± 25.77 | <0.001 |
| PWNC (cm2) | 2870 ± 298 | 3241 ± 301 | 3514 ± 284 | 4124 ± 320 | <0.001 |
| Family history | 16(17.8%) | 14(23.3%) | 31(20.9%) | 14(22.6%) | 0.837 |
| Intervention time (years) | 8 (5, 12) | 5 (2.25, 9) | 8 (4, 12) | 7 (4,12) | 0.019 |
| DR | 34(37.8%) | 25(41.7%) | 56(37.8%) | 21(33.9%) | 0.852 |
| MS | 60(66.7%) | 46(76.7%) | 134(90.5%) | 59(95.2%) | <0.001 |
Table 4: General characteristics of female participants divided by quartiles of NC.
Associations between abdominal obesity indices and prevalence of DR and MetS
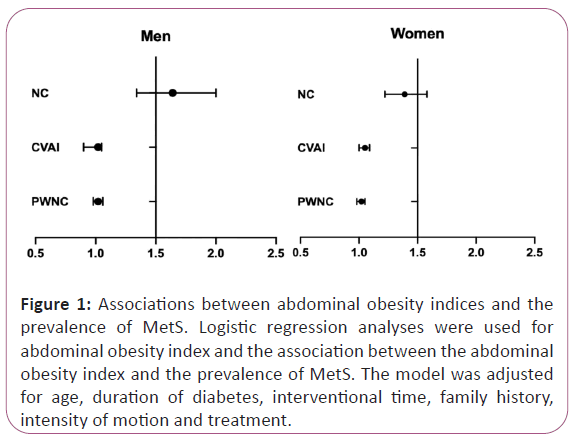
We found that in increased NC, CVAI, and PWNC were significantly associated with the prevalence of MetS both in men and women (Figure 1). In men, a one SD increase in NC (OR 1.64; 95% CI 1.34- 2.00), CVAI (OR 1.02; 95% CI 0.90-1.05), and PWNC (OR 1.02; 95% CI 0.98-1.06) was significantly associated with a greater prevalence of MetS (P<0.05). In women, a one SD increase in NC (OR 1.39; 95% CI 1.22-1.58), CVAI (OR 1.05; 95% CI 1.00- 1.09), and PWNC (OR 1.02; 95% CI 0.98-1.05) was significantly associated with a greater prevalence of MetS (P<0.05). And these associations were adjusted for age, duration of diabetes, interventional time, and family history (Figure 1).
Figure 1: Associations between abdominal obesity indices and the prevalence of MetS. Logistic regression analyses were used for abdominal obesity index and the association between the abdominal obesity index and the prevalence of MetS. The model was adjusted for age, duration of diabetes, interventional time, family history, intensity of motion and treatment.
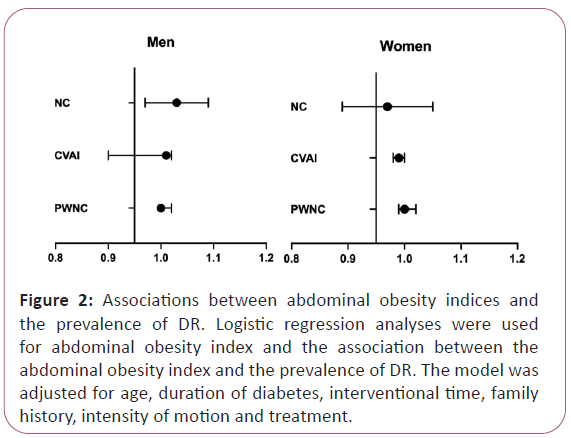
Moreover, after adjusting for age, duration of diabetes, interventional time, and family history, in both men and women, a one SD increase in NC, CVAI, and PWNC was not associated with the prevalence of DR (all P for trend >0.05) (Figure 2).
Figure 2: Associations between abdominal obesity indices and the prevalence of DR. Logistic regression analyses were used for abdominal obesity index and the association between the abdominal obesity index and the prevalence of DR. The model was adjusted for age, duration of diabetes, interventional time, family history, intensity of motion and treatment.
Receiver-Operating Characteristics (ROC) curve analysis
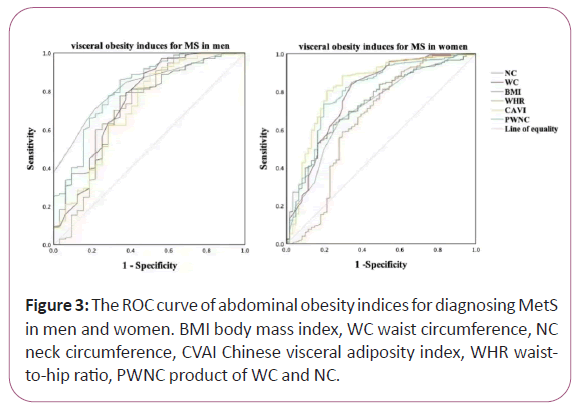
We found that the diagnostic ability of abdominal obesity indices including BMI, WC, NC, WHR, CVAI and PWNC for MetS among men and women, respectively, analyzed by ROC curve. The differences between the area under the curve of CVAI and that of BMI, WC, NC, WHR, and PWNC for CVD and DKD both in men and women were all significant (P<0.05). However, the differences between the area under the curve of these indices in DR were not significant (P>0.05).
In men, area under ROC curve of BMI, WC, NC, WHR, CVAI and PWNC for MetS was 0.730, 0.746, 0.824, 0.718, 0.717 and 0.816 respectively (all P<0.001). NC had the largest area under the ROC curve compared to the other induces, and the cut-off with the biggest Youden index of NC was 37.50 cm with a sensitivity of 71.2% and a specificity of 78.1%. In women, area under ROC curve of BMI, WC, NC, WHR, CVAI and PWNC for MetS was 0.741, 0.786, 0.719, 0.658, 0.828 and 0.800 respectively (all P<0.001). CVAI had the largest area under the ROC curve compared to the other induces, and the cut-off with the biggest Youden index of CVAI was 109.01 with a sensitivity of 80.6% and a specificity of 78.7% (Figure 3).
Discussion
Obesity due to poor diet and lifestyle habits is a time bomb for diabetes and its complications in the community population. As we all know, obesity frequently coexists with type 2 diabetes mellitus (T2DM), leading to the so-called “diabesity epidemic” [12]. However, the relationship between obesity and diabetes complications is ambiguous. A meta-analysis in 2018 (n=14,575; 13 clinical studies) reported that obesity (assessed by BMI) significantly increased the risk of DR; this effect mainly referred to non-proliferative DR and to patients with T2DM, as shown in subgroup analysis [13]. Moreover, another cross-sectional study (n=1,414 DM patients) showed that abdominal obesity (assessed by WC) also correlated with DR [14]. Also, abdominal obesity (defined by WHR) was positively related to mild-moderate and severe DR in T2DM women [9]. These results suggest that these induce may be associated with DR, however, our findings showed that BMI, WC, NC, CVAI, WHR and PWNC were not associated with the prevalence of DR, which is consistent with the latest research [4].
Evolving body of evidence suggests that the susceptibility to obesity-associated metabolic disorders is not mediated by the amount of fatness per se, but by the inability for excess energy to be stored appropriately in adipose tissue after reaching an individual’s fat threshold [8]. Adipose tissue plays a pivotal role in storing excess nutrients, sensing nutrient status, and regulating energy mobilization. In the face of long-term excessive nutrition, exhaustion of adipose tissue expandability creates stress on adipocytes and elicits a transition from an adaptive to a maladaptive inflammatory response over time, leading to increased inflammation as characterized by deranged secretion of adipokines and proinflammatory cytokines, abnormal tissue remodeling and fibrosis, and eventually insulin resistance and its manifestations [15]. Visceral fat is closely related to inflammation and increased risk for metabolic disorders, whereas subcutaneous adiposity is comparatively less harmful [16].
MetS is a cluster of obesity, hypertension, dysglycemia, dyslipidemia, and insulin resistance, which abdominal obesity and insulin resistance seem to play a central role in promoting the development of MetS [17,18]. MetS is a risk factor for cardiovascular complications of DM, but the association between MetS and microvascular complications of DM is limited. Moreover, the relationship between the components of metabolic syndrome and DR remains to be studied. NC has been considered a marker of upper body subcutaneous fat deposits and a simple and valuable screening tool for identifying individuals with obesity [5,19], which is independently associated with MetS [19,20]. CVAI is a novel visceral adiposity index developed in Chinese adults that is associated with visceral fat area and insulin resistance [6,21]. And PWNC is a novel anthropometric index, as an obesity indicator for MetS [7]. In our study, we found NC, CVAI and PWNC were significantly associated with a greater prevalence of MetS. However, they were not associated with DR.
In addition, we also studied the differences among groups grouped by the cervical quartile. We found that NC, CVAI and PWNC are all significant among groups. These induces are all significant with MetS but not significant with DR. Moreover, NC had the largest area under the ROC curve in men, however, CVAI had the largest area under the ROC curve in women. This may be due to the uneven distribution of body fat between men and women. In men, the cut-off with the biggest Youden index of NC was 37.50 cm, which has a higher specificity among these induces. And in women, the cut-off with the biggest Youden index of CVAI was 109.01, but the specificity of PWNC is higher than CVAI.
Hyperglycemia, oxidative stress, and inflammation are processes involved in MetS and DR, so several population studies evaluated its association with DR. A large multicenter clinic-based study from Italy reported an increased risk of type 2 diabetic retinopathy (T2DR) rather than type 1 diabetic retinopathy (T1DR) in patients with MetS [22]. Indeed, a study showed a 2.7 times higher risk of DR in patients with MetS which comprised of 3 components, while a 4.4 times higher risk of DR in patients with MetS which comprised of 5 components [23]. However, neither MetS nor its components are associated with an increased risk of DR based on recent published data [24], which is consistent with our findings. This may be due to the fact that most studies are cross-sectional and could not confirm a causal relationship. Moreover, it was associated with differences in race and metabolic markers.
This study has several strengths. Firstly, we investigated for the first time the relationship among abdominal obesity induces and DR and MetS. Secondly, our sample came from a community in northeast China, which the selection of non-admitted patients reduces the selection bias to a certain extent. Thirdly, we investigated for the first time the relationship between PWNC and DR. However, there are also some limitations in our study. First, being a cross-sectional study, causal inference between obesity phenotype indices and diabetic complications cannot be established. Second, the ethnic group investigated was only Han Chinese, thus generalizing the results to other ethnic groups should be done cautiously. Third, the questionnaire of our study did not address whether the patients were taking lipid-lowering drugs or had no history of smoking or alcohol consumption.
Conclusion
The present study demonstrates that NC, CVAI and PWNC are associated with the prevalence of MetS. In men, NC may be a convenient and valuable anthropometric measurement for early prevention of MetS. And in women, CVAI may be more suitable. However, these induces had no association with DR and there is no relationship between DR and MetS. Further prospective studies are necessary to examine our findings in external populations.
Acknowledgements
The authors thank the participants for participating in the study and the medical staff for their work on information collection.
Authors’ Contributions
All the authors contributed significantly to the manuscript. Xin-Li conceived and designed the study, completed statistical analysis and wrote the manuscript. Zi-Wei Yu, Chang-Wei Yang and Ming Hao participated in data collection and collation. Xin-Yuan Gao and Hong-Yu Kuang contributed to the preparation of the study and critically reviewed the manuscript. Xin-Yuan Gao gave final approval of the version to be submitted. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the Fund of Scientific Research Innovation of the First Affiliated Hospital of Harbin Medical University (grant number 2020 M27, China).
Availability of Data and Materials
The data are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no potential conflicts of interest regarding the publication of this paper.
References
- Dal Canto E, Ceriello A, Rydén L, Ferrini M, Hansen TB, et al. (2019) Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol 26: 25-32.
- Chooi Y, Ding C, Magkos F (2019) The epidemiology of obesity. Metab Clin Exp 92: 6-10.
- Wong T, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, et al. (2018) Guidelines on Diabetic Eye Care: The International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and Treatment Based on Resource Settings. Ophthalmol 125: 1608-1622.
- Wan H, Wang Y, Xiang Q, Fang S, Chen Y, et al. (2020) Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc Diabetol 19: 118.
- Zhao L, Huang G, Xia F, Li Q, Han B, et al. (2018) Neck circumference as an independent indicator of visceral obesity in a Chinese population. Lipids Health Dis 17: 85.
- Xia MF, Lin HD, Chen LY, Wu L, Ma H, et al. (2018) Association of visceral adiposity and its longitudinal increase with the risk of diabetes in Chinese adults: A prospective cohort study. Diabetes Metabol Res Rev 34: e3048.
- Huang Y, Gu L, Li N, Fang F, Ding X, et al. (2021) The product of waist and neck circumference outperforms traditional anthropometric indices in identifying metabolic syndrome in Chinese adults with type 2 diabetes: a cross-sectional study. Diabetol metab syndr 13: 35.
- Moh A, Neelam K, Zhang X, Sum CF, Tavintharan S, et al. (2018) Excess visceral adiposity is associated with diabetic retinopathy in a multiethnic Asian cohort with longstanding type 2 diabetes. Endocr Res 43: 186-194.
- Man RE, Sabanayagam C, Chiang PP, Li LJ, Noonan JE, et al. (2016) Differential Association of Generalized and Abdominal Obesity With Diabetic Retinopathy in Asian Patients With Type 2 Diabetes. JAMA Ophthalmol 134: 251-257.
- Lima-Fontes M, Barata P, Falcão M, Carneiro  (2020) Ocular findings in metabolic syndrome: A review. Porto Biomed J 5: e104.
- Alberti K, Zimmet P (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15: 539-553.
- Katsiki N, Anagnostis P, Kotsa K, Goulis DG, Mikhailidis DP, et al. (2019) Obesity, Metabolic Syndrome and the Risk of Microvascular Complications in Patients with Diabetes mellitus. Curr Pharm Des 25: 2051-2059.
- Zhu W, Wu Y, Meng Y, Xing Q, Tao JJ, et al. (2018) Association of obesity and risk of diabetic retinopathy in diabetes patients: A meta-analysis of prospective cohort studies. Medicine 97: e11807.
- Raman R, Rani P, Gnanamoorthy P, Sudhir RR, Kumaramanikavel G, et al. (2010) Association of obesity with diabetic retinopathy: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study (SN-DREAMS Report no. 8). Acta Diabetol 47: 209-215.
- Reilly S, Saltiel A (2017) Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol 13: 633-643.
- Després J, Lemieux I, Bergeron J, Pibarot P, Mathieu P, et al. (2008) Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 28: 1039-1049.
- Kahn R, Buse J, Ferrannini E, Stern M (2005) The metabolic syndrome. Lancet 366: 1921-1922.
- Genser L, Casella Mariolo J, Castagneto-Gissey L, Panagiotopoulos S, Rubino F, et al. (2016) Obesity, Type 2 Diabetes, and the Metabolic Syndrome: Pathophysiologic Relationships and Guidelines for Surgical Intervention. Surg Clin N Am 96: 681-701.
- Yang G, Yuan S, Fu H, Wan G, Zhu LX, et al. (2010) Neck circumference positively related with central obesity, overweight, and metabolic syndrome in Chinese subjects with type 2 diabetes: Beijing Community Diabetes Study 4. Diabetes Care 33: 2465-2467.
- Cui T, Yan B, Liu Z, Yang H, Gyan M, et al. (2018) Neck circumference: A valuable anthropometric measurement to detect metabolic syndrome among different age groups in China. Diabetes Metabol Res Rev 34.
- Xia M, Chen Y, Lin H, Ma H, Li XM, et al. (2016) A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep 6: 38214.
- Bonadonna R, Cucinotta D, Fedele D, Riccardi G, Tiengo A, et al. (2006) The metabolic syndrome is a risk indicator of microvascular and macrovascular complications in diabetes: results from Metascreen, a multicenter diabetes clinic-based survey. Diabetes Care 29: 2701-2707.
- Pang C, Jia L, Hou X, Gao X, Liu W, et al. (2014) The significance of screening for microvascular diseases in Chinese community-based subjects with various metabolic abnormalities. PloS one 9: e97928.
- Zhou Y, Wang C, Shi K, Yin X (2018) Relation of metabolic syndrome and its components with risk of diabetic retinopathy: A meta-analysis of observational studies. Medicine 97: e12433.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences