ISSN : 2393-8862
American Journal of Pharmacology and Pharmacotherapeutics
Antioxidant Effect of Curcumin Iron Complex of U87 Cells Induced by Beta-Amyloid
Guluzar Ozlobat1* and Ares Alizade2
1Department of Pharmacology, Sinop University, Sinop, Turkey
2Department of Pharmacology, Mustafa Kemal University, Hatay, Turkey
- *Corresponding Author:
- Guluzar Ozlobat
Department of Pharmacology
Sinop University
Sinop, Turkey
E-mail: guluzarozbolat@gmail.com
Received Date: August 06, 2021; Accepted Date: August 20, 2021; Published Date: August 27, 2021
Citation: Ozlobat G, Alizade A (2021) Antioxidant Effect of Curcumin Iron Complex of U87 Cells Induced by Beta-Amyloid. Am J Pharmacol Pharmacother Vol.8 No.3:12.
Abstract
Curcumin iron complex could be utilized effectively for the treatment of Alzheimer's disease and anticipated that it could be evaluated among alternative treatment strategies in the field of medicine. The present study was conducted to investigate the antioxidant activities of iron with curcumin complex. In the study; three groups were formed as the control group, the Aβ group, and the Aβ+ curcumin iron complex group. Firstly, the cytotoxic potential of curcumin iron complex in U87 cells was investigated by the colorimetric MTT (3-4, 5-dimethylthiazolyl- 2,5 diphenyltetrazolium bromide) test. To determine the antioxidant status in the cell line treated with curcumin iron complex, examine the effects of superoxide dismutase (SOD) and catalase (CAT) activities were measured by ELISA method. The SOD enzyme level increased significantly in the curcumin iron complex group after curcumin was applied to the U87 cell (p<0.05). The results herein illustrated that CAT enzyme level (pg/ml) decreased after curcumin iron complex administration in all the groups except for the control group (p<0.05). When compared to the control group, the SOD and CAT levels were increased in the U87 cell line exposed to curcumin. Curcumin iron complex has a protective effect by increasing antioxidant parameters in the U87 cell lines.
Keywords
Superoxide dismutase; Catalase; Drug; Iron; Curcumin
Introduction
Alzheimer's disease (AD) is one of the leading causes of dementia affecting millions of people worldwide [1]. Clinical manifestations including cognitive and neuro-psychiatric disturbances or behavioural disorder that eventually lead to severe functional disability. Alzheimer's disease is the most common cause of dementia a continuous decline in thinking, behavioural and social skills that affects a person's ability to function independently. The early signs of the disease include forgetting recent events or conversations. As the disease progresses, a person with Alzheimer's disease will develop severe memory impairment and lose the ability to carry out everyday tasks. Older age does not cause Alzheimer’s, but it is the most important known risk factor for the disease. The number of people with Alzheimer’s disease doubles about every 5 years beyond age 65. About one-third of all people age 85 and older may have Alzheimer's disease. Alzheimer’s disease can range from mild to severe. The scale ranges from a state of mild impairment, through to moderate impairment, before eventually reaching severe cognitive decline. [2].
Amyloid-β (Aβ) plaques and neurofibrillary tangles composed of hyper phosphorylated tau are among the neuropathological complications of Alzheimer's disease. AD, abnormal beta-amyloid (Aβ) accumulation in brain tissues, death of cholinergic neurons, tangles caused by hyper phosphorylation of microtubuleassociated tau protein, dyshomeostasis of metals, such as copper, iron, zinc and aluminium, metal-induced oxidative stress, and various other factors. It is multifactorial with an unknown etiology, characterized by various pathological symptoms, including [3,4].
Currently, the most prominent targets for therapeutic intervention include the inhibition of Amyloid Precursor Protein (APP) and Aβ production by blocking Aβ aggregation and the resulting inflammatory response and inhibiting Aβ-induced neurotoxicity. Age-related memory impairments have been depicted to be associated with decreased antioxidant mechanisms in the brain and plasma. The interaction of Aβ42 plaques with free radicals and the oxidative stress as a result of it may play a pivotal role in AD pathogenesis [5,6].
Curcumin is a polyphenol compound responsible for the bright yellow color of turmeric. It is a natural phenolic compound with antibacterial, antioxidant, and anti-inflammatory properties [7,8]. Curcumin is also promising because it can bind iron. It is thought that curcumin iron complex could be utilized effectively for the treatment of Alzheimer's disease and we anticipated that it could be evaluated among alternative treatment strategies in the field of medicine.
Materials and Methods
Control group
50 μl of saline was added to the medium of differentiated U87 cells and incubated for 48 hours. Di-Methyl SulfOxide (DMSO) was added to the medium for 24 hours in the incubator.
Aβ group
Aβ1-42 was added to the medium of the differentiated U87 MG cells at a concentration of 5 μM and incubated for 48 hours. At the end of this period, DMSO was added to the medium of the cells in a final concentration of 0.1% and the cells were incubated for another 24 hours in the incubator.
Aβ+ Curcumin iron complex group
The differentiated U87 cells were added to the culture medium at a final concentration of 5 μM, 48 hours after Aβ1-42 application. 300 μmol/L curcumin iron was added and the mixture was incubated for another 24 hours.
Cell culture
Cell media: Dulbecco's Modification of Eagle's Medium (DMEM) with glutamine was used as the cell medium in our study. In addition to the medium, HEPES, FBS, and PSA were added. The final volume of the cell medium contained 10% FBS (v/v), 1% PSA, and 25 mM HEPES. The cell line was propagated in flasks using this prepared medium. The density of the U87 cell line in the flask kept in the CO2 incubator for 48 hours was later examined under light microscope and the cells from the dense visible flasks were propagated via passaging.
Cell viability: Cell count was carried out for the U87 cell line and the viability of the cells was measured with trypan blue excretion test. By placing 10 μL of the cells+10 μL of trypan blue on a slide, the viability was determined counting the cells that did not receive dye under the light microscope. The percentage of nonstaining cells was calculated by counting 100 cells in the area.
(Enzyme Linked Immunosorbent Assay) test: To evaluate the antioxidant status in the cell line treated with DPA, SOD, CAT activities were measured based on ELIZA method. Experimental protocols of ELIZA kits vary for each kit.
Results
MTT Test
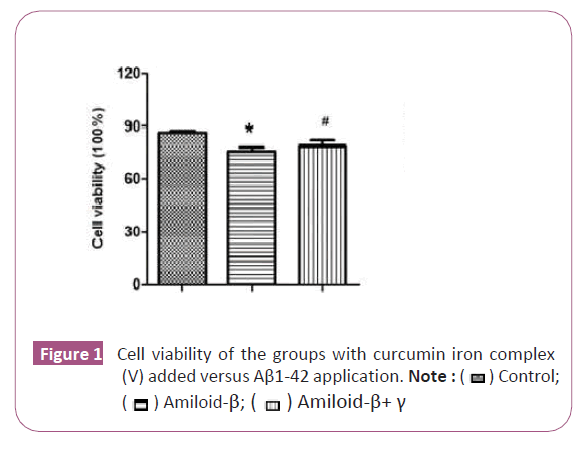
The effect of curcumin iron complex U87 (glioblastoma astrocytoma) on cell viability and proliferation was also investigated. After the application of curcumin+iron complex on U87 cells, there was a significant statistical increase in the number of cells in the curcumin+iron complex group (p<0.05) (Figure 1).
Superoxide dismutase activity of the complex
The SOD enzyme level increased significantly in the curcumin iron complex group after curcumin was applied on the U87 cell (p<0.05) (Figure 2). It has been determined that complex triggers the antioxidant enzyme mechanism.
Catalase activity of the complex
We observed a significant statistical increase in CAT enzyme level in the curcumin iron complex group after curcumin was applied on the U87 cell (p<0.05) (Figure 3). Moreover, the results implied that complex triggers the antioxidant enzyme mechanism [14-21].
Discussion and Conclusion
Reactive Oxygen Species (ROS), including hydroxyl radicals (OH), Superoxide anion and hydrogen peroxide (H2O2), are considered to be important in the formation of cancer and various diseases by acting on cell metabolism [11-12]. Excessive ROS production and increased oxidative stress have been reported to be associated with various diseases. Reactive Oxygen Species (ROS) are created during aerobic cellular reactions and are effectively cleansed by the detoxification defence system of the ROS cell [13].However, when ROS production exceeds cell detoxification capacity, overproduced ROS can directly replace lipid, protein, or DNA and signal transduction pathways, resulting in an irreversible oxidative modification. Many studies today also have shown that curcumin has a potential role in the prevention and treatment of AD and due to its antioxidant, anti-inflammatory, and lipophilic effects, it improves cognitive functions [14-18].
A number of therapeutic agents have been shown to increase intracellular ROS generation by regulating antioxidant enzymes such as catalase and SOD. However, the activity of SOD and CAT of the curcumin and curcumin iron complex complex of U87 cells has not been explored. This study was, therefore, conducted to examine whether curcumin can increase the level of SOD and CAT. In the study, aimed to examine the effects of curcumin and iron complex on the U87. According to the obtained data herein, curcumin iron complex administration significantly reduced SOD activity on U87 cells compared to the controls. Liu et al. (2020) tested that human glioma U87 cells were treated with curcumin of the oxidative stress indexes total antioxidant capacity (T-AOC), malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione (GSH). The results showed that curcumin promoted ROS production by increasing cell NADPH oxidase activity, increased MDA content, decreased GSH content, and increased SOD activity. Catalase is an important enzyme that acts to dissociate hydrogen peroxide into molecular oxygen and water. Catalase activity is always directly proportional to the rate of dissociation of hydrogen peroxide. We found that complex application significantly augmented this decrease and implied an antioxidant effect. The results herein illustrated that CAT enzyme level (pg/ml) decreased after curcumin administration in all the groups except for the control group (p<0.05). Moreover, we observed a significant statistically increase in CAT enzyme level in the curcumin group after curcumin iron complex was applied on the U87 cell p<0.05). It has been determined that curcumin triggers the antioxidant enzyme mechanism. To our knowledge, the present study is the first study in the literature to show the decreased in of SOD and CAT in U87 cell lines after curcumin iron complex treatment. In conclusion, the present work confirmed the previous reports on the therapeutic potential of curcumin.
Authors' Contributions
All funding of the study was supported by the authors.
References
- Prince MJ, Wimo A, Guerchet MM (2015) World alzheimer report-the global impact of dementia: An analysis of prevalence, incidence, cost and trends 8(1).
- Jack Jr CR, Bennett DA, Blennow K (2018) NIAâ?AA research framework: Toward a biological definition of alzheimer's disease. Alzheimers Dement Dement 14(4):535-562.
- Hippius H, Neundörfer G. The discovery of Alzheimer's disease. Dialogues clin. neuro sci. 5(1):101-108.
- Crimins JL, Pooler A, Polydoro M, Luebke JI, Spires-Jones TL (2013) The intersection of amyloid beta and tau in glutamatergic synaptic dysfunction and collapse in Alzheimer's disease. Ageing research reviews 12(3):757-763.
- Dinamarca MC, Cerpa W, Garrido J, Hancke JL, Inestrosa NC (2006) Hyperforin prevents β-amyloid neurotoxicity and spatial memory impairments by disaggregation of Alzheimer's amyloid-β-deposits. Mol. psychiatry 11(11):1032-1048.
- Talib WH, Zarga MH, Mahasneh AM (2012) Antiproliferative, antimicrobial and apoptosis inducing effects of compounds isolated from Inula viscosa. Molecules 17(3):3291-3303.
- Ireson CR, Jones DJ, Orr S, Coughtrie MWH, Boocock DJ, et al. (2002) Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiology and Prevention Biomarkers 11(1):105-111.
- Esatbeyoglu T, Huebbe P, Ernst IM (2012) Curcumin-from molecule to biological function. Angew. Chem. Int. Ed 51(22):5308-5332.
- Lee J, Lim KT (2013) Normalizing effect of SJSZ glycoprotein (38 kDa) on proliferating cell nuclear antigen and interferonâ?γ in diethylnitrosamineâ?induced mice splenocytes. J Cell Biochem 114(4):808-815.
- Halliwell B, Gutteridge JM (1986) Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Archives of biochemistry and biophysics 246(2):501-514.
- Lovell MA, Ehmann WD, Butler SM (1995) Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer's disease. Neurology 45(8):1594-1601.
- Gsell W, Conrad R, Hickethier M, Sofic E, Frolich L, et al. (1995) Decreased catalase activity but unchanged superoxide dismutase activity in brains of patients with dementia of Alzheimer type. J. Neurochem 64(3):1216-1223.
- Guo-an LI, Ya-dong JI, Miao-miao RA, Lı Gui-chen, Yang Qi-li, et al. (2020) Curcumin inducing apoptosis of U87 cells by promoting ROS production. Nat Prod Res 32(4):541.
- Camaschella C, Nai A, Silvestri L (2020) Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 105(2):260.
- Papanikolaou G, Pantopoulos K (2005) Iron metabolism and toxicity. Toxicol Appl Pharmacol.202(2):199-211.
- Shander A, Cappellini MD, Goodnough LT (2009) Iron overload and toxicity: The hidden risk of multiple blood transfusions. Vox sanguinis 97(3):185-197.
- Meydani M, Hernandez-Rodriguez J, Youdim MB (2001) Antioxidants and cognitive function. Nutrition Reviews 59(8):75.
- Pietrangelo A (2002) Mechanism of iron toxicity. Iron Chelation Therapy 19-43
- Guo-an LI, Ya-dong JI, Miao-miao RA (2020) Curcumin inducing apoptosis of U87 cells by promoting ROS production. Natural product research and development. 32(4):541.
- Jenkins RR (1981) Catalase activity in skeletal muscle of varying fibre types. Experientia. 37(1):67-68.
- Olson KR, Gao Y, DeLeon ER, Arif F, Arora N, et al. (2017) Catalase as a sulfide-sulfur oxido-reductase: An ancient (and modern?) regulator of Reactive Sulfur Species (RSS). Redox Biology. 12:325-339.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
 ) Control;(
) Control;( ) Amiloid-β; (
) Amiloid-β; ( ) Amiloid-β+ γ
) Amiloid-β+ γ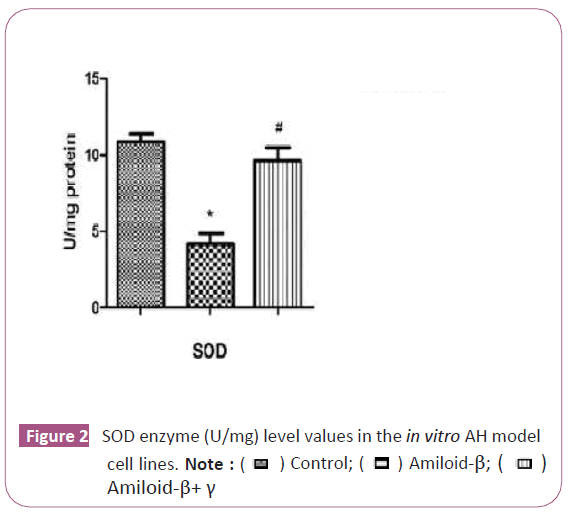
 ) Control; (
) Control; ( ) Amiloid-β; (
) Amiloid-β; ( )Amiloid-β+γ
)Amiloid-β+γ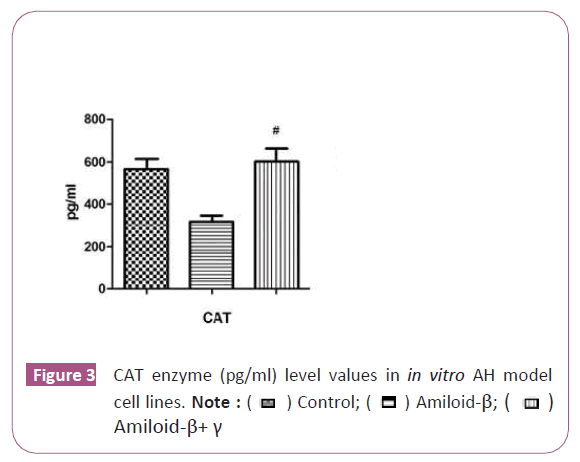
 ) Control; (
) Control; ( ) Amiloid-β; (
) Amiloid-β; ( )Amiloid-β+ γ
)Amiloid-β+ γ