ISSN : 2393-8862
American Journal of Pharmacology and Pharmacotherapeutics
Antihypertensive and Anti-inflammatory Potential of the Edible Fruit of Artocarpus altilis (Breadfruit)
Omar E Christian1*, Keith E Jackson2 and Somnath Mukhopadhyaya1,3
1Department of Chemistry and Biochemistry, North Carolina Central University, Durham, USA
2Department of Basic Pharmaceutical Sciences, University of Louisiana, Monroe, USA
3Julius L. Chambers Biomedical/Biotechnology Research Institute, North Carolina Central University, Durham, USA
- *Corresponding Author:
- Omar E. Christian
Departmen t of Chemis tr y and Biochemistry, North Carolina Central University, Durham, NC 27540, USA
Tel: +919-530-5134
Fax: +919-530-5135
E-mail: ochristi@nccu.edu
Received Date: June 04, 2019; Accepted Date: June 21, 2019; Published Date: June 27, 2019
Citation: Christian OE, Jackson KE, Mukhopadhyaya S (2019) Antihypertensive and Anti-inflammatory Potential of the Edible Fruit of Artocarpus altilis (Breadfruit). Am J Pharmacol Pharmacother J Vol.6 No.1:1.
Abstract
Objective: Hypertension is a chronic disease which impacts about half of the US population, increasing their risk of death from heart disease and stroke. Although there is no cure hypertension can be controlled with diet and exercise. Nitric oxide and the inflammatory response are thought to play a critical role in the onset and progression of this disease. We herein hypothesize that due to the high phenolic content of breadfruits, the extracts will display anti-inflammatory, vasodilatory as well as antihypertensive activity.
Materials and Methods: Male Sprague-Dawley rats were used; the rats were anesthetized with a single injection of thiobutabarbital sodium and a tracheal tube was inserted. Following the surgical procedures, rats were allowed to stabilize for 45 minutes. After this initial stabilization period, a 15-minute baseline was collected, and animals treated with vehicle, angiotensin II or angiotensin II + breadfruit extract. The extracts were administered at a concentration of 0.5, 1, 2, and 2.5 mg/ml and an infusion rate of 1 ml/hr and mean arterial pressure (MAP) was recorded. The anti-inflammatory activities of the whole fruit extract were determined using a LPS-treated macrophage cells model at concentrations of 1 and 10 mg/ml.
Results: The whole fruit extracts produced significant attenuation (20-30 mmHg) of the angiotensin II mediated hypertension in Sprague-Dawley rats and displayed a dosedependent decrease in LPS-induced TNF-α and Il-1β. The whole fruits also caused an increase in nitric oxide (NO) production in endothelial cells.
Conclusion: The consumption of breadfruits as a part of a healthy diet may help to prevent the onset and progression of hypertension.
Keywords
Breadfruit; Anti-hypertensive; Anti-inflammatory; Nitric oxide; Pro-inflammatory cytokine
Introduction
Hypertension, otherwise known as high blood pressure, is a medical condition in which the blood pressure is chronically elevated. Blood pressure measurements are denoted in millimeters of mercury of a systolic over diastolic reading. Persons having a systolic reading of over 140 mmHg or diastolic of over 90 mmHg are classified as hypertensive [1]. Hypertension is often referred to as the “silent killer”, as it can remain asymptomatic for many years, manifesting later in life as a myriad of other diseases, cardiovascular diseases, diabetes, peripheral artery disease (PAD), cerebrovascular diseases (stroke) and kidney failure [2]. Current data suggests that well over 32% of all Americans are hypertensive [3].
Artocarpus altilis (Moreacae) known as breadfruit is widely distributed in tropical and subtropical regions. The yellowing leaves are brewed into a tea and used as a folk medicinal treatment for hypertension. In our preliminary studies, the brewed tea of the leaves displayed a significant attenuation of the mean arterial pressure in the angiotensin II induced hypertensive rat model (40 – 50 mmHg) [4]. The tea extracts also displayed moderate COX-2 inhibition and antioxidant activity. The antioxidant and anti-inflammatory activity observed is due to the presence of several flavonoid and other phenolic compounds [5]. More recently, the aqueous leaf extract was shown to have cardioprotective potential against isoproterenol induced myocardial damage in rats, displaying both negative chronotropic and inotropic effects [6]. This activity is similar to that of beta-adrenergic antagonist like propranolol [7]. The cardioprotection is theorized to be due to normalization of cell automatic sinus node function and sympathovagal balance [6,7].
While the current data suggests that consumption of leaf tea may be beneficial, the leaf tea is not widely consumed and is generally thought of as folk medicine. The fruits are consumed by a much wider population as a staple; they are roasted or boiled in the Caribbean, South Pacific Islands, and North America [8]. Additionally, breadfruit is high in carbohydrates, vitamin C, thiamine, and potassium and is a good source for several dietary minerals and micronutrients [9]. The fruit is also known to produce a range of antioxidant and anti-inflammatory flavonoids and other polyphenolics. We herein hypothesize that due to the high phenolic content of the whole fruit of Artocarpus altilis, the extracts will display anti-inflammatory, vasodilatory, as well as antihypertensive activity.
Materials and Methods
Plant material
Breadfruits (Artocarpus altilis) were purchased in Buff Bay Portland, Jamaica in July 2017, a voucher specimen (NCCU17-071) was deposited in the Department of Chemistry and Biochemistry, North Carolina Central University. The skins and hearts of the fruit were removed and the fruit flesh sliced into thin segments of approximately 2 × 3 cm. The segments were sundried for 7 days, then ground into a fine powder/flour (500 g) using a blender. The powder was stored in a sealed container at - 4°C until used.
Organic Extraction
The dried breadfruit samples (150 g) were sequentially extracted using hexane, ethyl acetate, and methanol on an Accelerate Solvent Extractor (ASE). During the ASE extraction process the samples were exposed to approximately 100 ml of solvent for 30 min at 100°C under an atmosphere of nitrogen at 1500 psi. The resulting extracts of hexane (FH), ethyl acetate (EAE), and methanol (ME) were collected and reduced in vaco to yield the crude organic extracts.
Nitric oxide production
Two different endothelial cells (Pulmonary Artery Endothelial (ATCC) and human umbilical vein endothelial (HUVEC; ATCC)) were grown to 80% confluence and serum starved for 6 h then treated with EAE and ME with varying dosing (0.3, 1, 3, 10, and 30 mg/ml) and time (0, 5, 10, 15, and 30 min). At the end of the treatment, cell lysates were prepared, and NO level was be measured using an OxiSelect™ Intracellular Nitric oxide assay kit [10].
Anti-inflammatory assay
Two different macrophage cell lines (murine MH-S alveolar macrophage cells (MH-S; ATCC) and murine macrophage cells (RAW 264.7; ATCC) were grown (5 × 104 cells/well) in triplicate in 6 well plates in RPMI media containing 10% FBS to 80% confluency. LPS-treated macrophage cells were treated with EAE or ME with different dose (0.3, 1, 3, 10, and 30 mg/ml) and time (0.1, 3, 6, 12, and 24 hr). At the end of the treatment, cell lysates, and conditioned media were collected, and the total RNA and protein lysates were isolated following manufacturer’s instructions. Conditioned media and cell lysates were used for ELISA assays. m-RNA expression of the same cytokines and Toll-like receptor (TLR; TLR 2, 3, 4, 7, 8, 9) were analyzed by q-PCR. The q-PCR and ELISA data from the dose-response study were analyzed to determine potency (EC50) and efficacy. Once the optimum dose and time for the maximum response of the individual extracts were obtained, we treated the above-mentioned macrophage cells with the submaximal dose of the extracts to determine if the component extracted from the two extracts had any additive and/or synergistic effects.
Antihypertensive assay
Male Sprague-Dawley rats (250-350 g; n=12, Harlan, Indianapolis, IN) were used. This protocol was approved by the University of Louisiana at Monroe Institutional Animal Care and Use Committee. Prior to experiments, rats were housed in a controlled environment and had free access to commercial rat chow and tap water. Rats were anesthetized with a single injection of thiobutabarbital sodium (120 mg/kg, IP) and a tracheal tube was inserted to maintain an open airway. Catheters (PE-50 tubing filled with heparinized saline) were implanted into a carotid artery and a jugular vein to allow for continuous monitoring of mean arterial pressure (MAP)/heart rate (HR) and for intravenous administration of drugs, respectively. The arterial catheter was connected to a pressure transducer (model TSD104A, Biopac Systems, Santa Barbara, CA) and the venous catheter was connected to a Sage micro infusion pump (Orion Research, Inc., model M361, Boston, MA) set at a 1ml/hour saline infusion rate. A bladder cannula was inserted to allow urine collection for determination of urinary volume and urinary concentrations of sodium/potassium (Flame Photometry; Instrumentation Laboratories, IL 943). Following the surgical procedures, rats were allowed to stabilize for 45 minutes. After this initial stabilization period, a 15-minute baseline was collected, and animals treated with vehicle, angiotensin II or angiotensin II + breadfruit extract. The extracts were administered at a concentration of 1 mg/ml and an infusion rate of 1 ml/hr and MAP was recorded.
Results
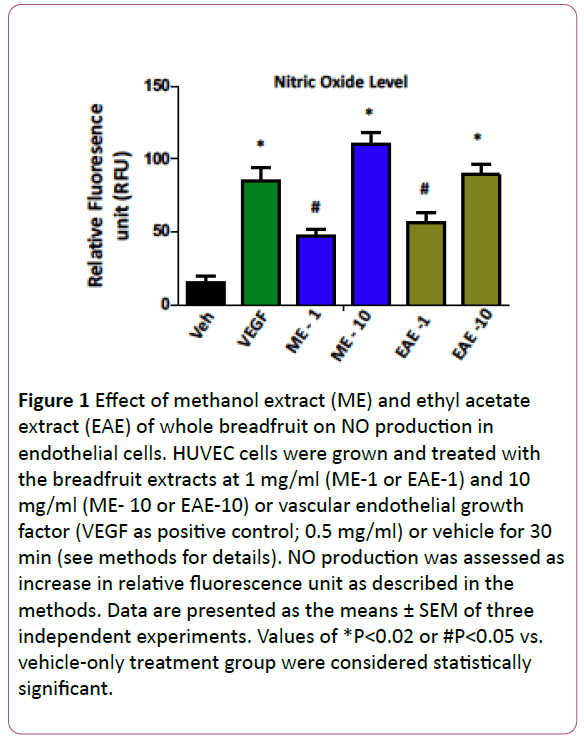
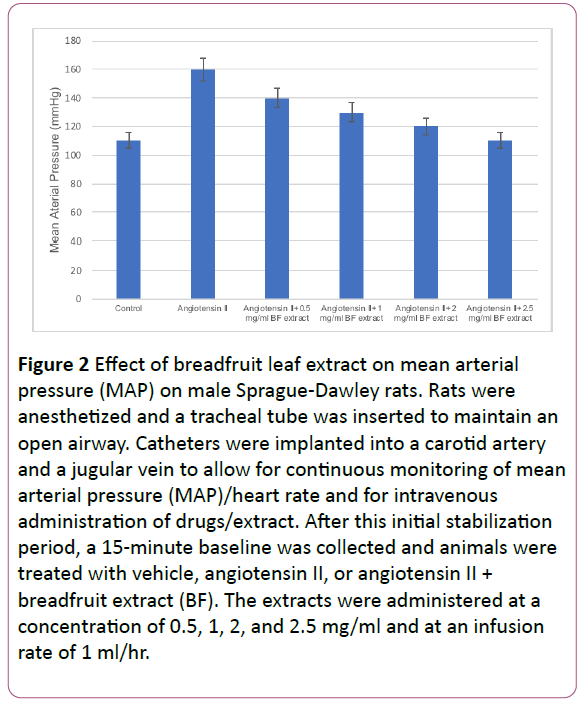
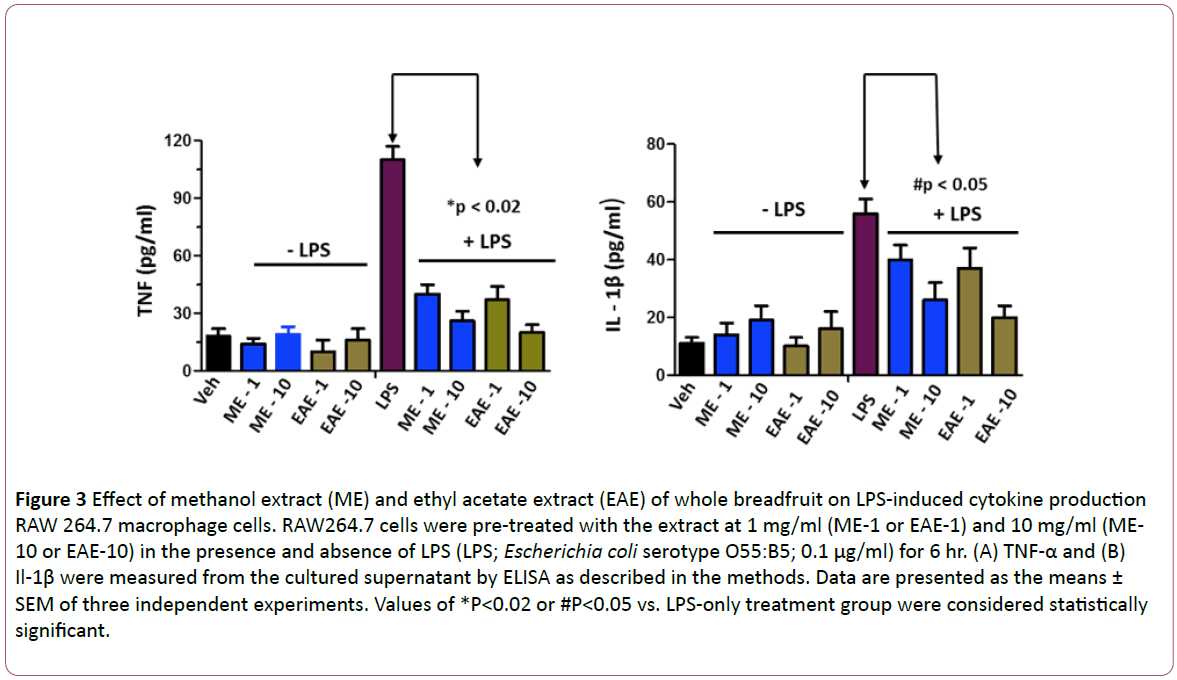
In order to determine the effects of the breadfruit extracts, we tested the effect of EAE and ME on in vitro NO production in endothelial (HUVEC) cells. As depicted in Figure 1, the ME and EAE both produced a significant dose-dependent increase in nitric oxide production in endothelial cells. Interestingly, the nitric oxide level produced is comparable with the robust vasodilator vascular endothelial growth factor (VEGF)-mediated nitric oxide production suggesting that these extracts also contains functionally active components that can stimulate significant nitric oxide production. Once we found that breadfruit extract is capable of nitric oxide production, we tested the efficacy of the methanolic extract of breadfruit for antihypertensive potential using Sprague-Dawley rats and measuring the blood pressure via mean arterial pressure (MAP) as described above. The hypertensive rats displayed a concentration dependent response to the extract with the maximum effect occurring at 2.5 mg/ml while inducing a 50- mmHg reduction in mean arterial pressure (Figure 2). The methanolic (ME) and ethyl acetate (EAE) extract produced a dose-dependent decrease in LPS-induced TNF-α and Il- 1β (Figure 3). These results suggest that components of the extracts can block the pathways leading to the pro-inflammatory cytokines suggesting that the consumption of the breadfruit may reduce inflammatory response.
Figure 1: Effect of methanol extract (ME) and ethyl acetate extract (EAE) of whole breadfruit on NO production in endothelial cells. HUVEC cells were grown and treated with the breadfruit extracts at 1 mg/ml (ME-1 or EAE-1) and 10 mg/ml (ME- 10 or EAE-10) or vascular endothelial growth factor (VEGF as positive control; 0.5 mg/ml) or vehicle for 30 min (see methods for details). NO production was assessed as increase in relative fluorescence unit as described in the methods. Data are presented as the means ± SEM of three independent experiments. Values of *P<0.02 or #P<0.05 vs. vehicle-only treatment group were considered statistically significant.
Figure 2: Effect of breadfruit leaf extract on mean arterial pressure (MAP) on male Sprague-Dawley rats. Rats were anesthetized and a tracheal tube was inserted to maintain an open airway. Catheters were implanted into a carotid artery and a jugular vein to allow for continuous monitoring of mean arterial pressure (MAP)/heart rate and for intravenous administration of drugs/extract. After this initial stabilization period, a 15-minute baseline was collected and animals were treated with vehicle, angiotensin II, or angiotensin II + breadfruit extract (BF). The extracts were administered at a concentration of 0.5, 1, 2, and 2.5 mg/ml and at an infusion rate of 1 ml/hr.
Figure 3: Effect of methanol extract (ME) and ethyl acetate extract (EAE) of whole breadfruit on LPS-induced cytokine production RAW 264.7 macrophage cells. RAW264.7 cells were pre-treated with the extract at 1 mg/ml (ME-1 or EAE-1) and 10 mg/ml (ME- 10 or EAE-10) in the presence and absence of LPS (LPS; Escherichia coli serotype O55:B5; 0.1 μg/ml) for 6 hr. (A) TNF-α and (B) Il-1β were measured from the cultured supernatant by ELISA as described in the methods. Data are presented as the means ± SEM of three independent experiments. Values of *P<0.02 or #P<0.05 vs. LPS-only treatment group were considered statistically significant.
Discussion
Recent research findings have shown that inflammatory processes are important participants in the pathophysiology of hypertension. Chronic inflammatory diseases (CIDs) are characterized by an increased risk of cardiovascular disease (CVD) related morbidity and mortality [11,12]. Pathogenic mechanisms underlying the hypertension in CID are multifactorial and still poorly understood. Current therapies for CID and hypertension/CVD are not fully effective and many promising pharmaceutical leads have failed at the clinical level. Nutraceuticals are emerging as promising alternatives or addons with current therapies. Unfortunately, research on nutraceuticals has not kept pace with that of the pharmaceutical industry. The identification of new nutraceuticals that can target for therapeutic prevention against CIDs and CVDs will be immensely important for those suffering from these diseases. Our findings suggest that breadfruit extracts reduce angiotensin-mediated MAP in mice along with enhanced NO production in a dose-dependent manner indicating that the breadfruit extract may exert this activity through interactions with NO receptors in the endothelium.
Soluble guanylate cyclase (sGC) is the major receptor for nitric oxide and a key signal-transducer in the nitric oxide-cyclic guanisine monophosphate (NO-cGMP) signaling pathway. Nitric oxide binds to the heme prosthetic group of sGC, which then activates the conversion of guanosine-5′-triphosphate to cGMP. cGMP then binds to and exerts effects on downstream targets including protein kinase G, cyclic nucleotide-gated ion channels, and phosphodiesterases (PDEs) [13]. Soluble guanylate cyclase is expressed in many cells and tissues [14], and its role in vascular smooth muscle is well known. In response to vascular shear stress, endothelial nitric oxide synthase (eNOS) produces nitric oxide which activates sGC to cause vasodilation and a concomitant increase in local blood flow [15].
During inflammatory condition, nitric oxide is known as a pro-inflammatory mediator and released from macrophages during early stage of inflammatory cascades. Excessive concentration of nitric oxide in macrophages is linked to inflammatory reactions [16-18]. However, in contrast to this pro-inflammatory response, several lines of evidence suggest that NO signaling through sGC plays a role in suppressing inflammation [19]. More recently, Tobin and colleagues [20] also showed that sGC stimulation via nitric oxide produced anti-inflammatory responses. All these studies are in agreement with our finding that the LPS-induced increase in pro-inflammatory cytokines were reduced by breadfruit extracts. These results also suggest that some components of breadfruit extract may act via the NO-sGC-cGMP pathway.
Conclusion
Essential or primary hypertension accounts for over 95% of the reported hypertension cases annually. Hypertension is a complex disorder that often presents with unidentified underlying causes, including inflammation. In spite of redundant systems including the baroreceptors, angiotensin II negative feedback that are present to maintain normal blood pressure, an individual can still become hypertensive. Thus, the need for identifying compounds that are also aimed at reducing the underlying causes of hypertension such as inflammation is essential and better if we can get it from our ingested food. The extracts used in this study were isolated from the edible breadfruit, suggesting that consumption of breadfruit may help to reduce the risk of hypertension and other cardiovascular diseases. This is the first report showing the dual activity of breadfruit extracts to reduce blood pressure and one of its underlying causes, e.g. inflammation.
References
- Chen ZY, Peng C, Jiao R, Wong YM, Yang N, et al. (2009) Anti-hypertensive nutraceuticals and functional foods. J Agric Food Chem 57: 4485-4499.
- Sá C, Pestana D, Calhau C, Faria A (2018) Unravelling the Effect of p,p '-Dichlorodiphenyldichloroethylene (DDE) in Hypertension of Wistar Rats. J Agric Food Chem 66: 12847-12854.
- Carretero OA, Oparil S (2000) Essential hypertension. Part I: Definition and etiology. Circulation 101: 329-335.
- Christian KR, Pothu S, Nair MG, Prathipati P, Jackson KE, et al. (2016) Antihypertensive, antiinflammatory and antioxidant activity of breadfruit leaf tea. Current topics in Phytochemistry 13: 29-33.
- Niu H, Limei M, Ke L, Wang N (2015) Geranyl Favonoid from Breadfruit Regulate Dyslipidemia in Hypercholesterolemic Rat. Journal of Food and Nutrition Research 3: 399-404.
- Nwokocha C, Palacios J, Simirgiotis MJ, Thomas J, Nwokocha M, et al. (2017) Aqueous extract from leaf of Artocarpus altilis provides cardio-protection from isoproterenol induced myocardial damage in rats: Negative chronotropic and inotropic effects. J Ethnopharmacol 203: 163-170.
- Nwokocha CR, Owu DU, McLaren M, Murray J, Delgoda R, et al. (2012) Possible mechanisms of action of the aqueous extract of Artocarpus altilis (breadfruit) leaves in producing hypotension in normotensive Sprague-Dawley rats. Pharm Biol 50: 1096-102.
- Soifoini T, Donno D, Jeannoda V, Rakotoniaina E, Hamidou S, et al. (2018) Bioactive compounds, nutritional traits, and antioxidant properties of Artocarpus altilis (Parkinson) fruits: Exploiting a potential functional food for food security on the Comoros Islands. Journal of Food Quality 2018: 1-11.
- De Souza CT, Soares SAR, Queiroz A, Ana MP, Dos Santos P, et al. (2016) Determination and evaluation of the mineral composition of breadfruit ( Artocarpus altilis ) using multivariate analysis technique. Microchemical Journal 128: 84-88.
- Mukhopadhyay S, Chapnick BM, Howlett AC (2002) Anandamide-induced vasorelaxation in rabbit aortic rings has two components: G protein dependent and independent. Am J Physiol Heart Circ Physiol 282: H2046-H2054.
- Ventura HO, Taler SJ, Strobeck JE (2005) Hypertension as a hemodynamic disease: the role of impedance cardiography in diagnostic, prognostic, and therapeutic decision making. Am J Hypertens 18: 26S-43S.
- Dinh QN, Drummond GR, Sobey CG, Chrissobolis S (2014) Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int 2014: 406960.
- Derbyshire ER, Marletta MA (2012) Structure and regulation of soluble guanylate cyclase. Annu Rev Biochem 81: 533-559.
- Budworth J, Meillerais S, Charles I, Powell K (1999) Tissue distribution of the human soluble guanylate cyclases. Biochem Biophys Res Commun 263: 696-701.
- Moncada S, Higgs EA (2006) Nitric oxide and the vascular endothelium. Handb Exp Pharmacol (176 Pt 1): 213-254.
- Nagy G, Koncz A, Telarico T, Fernandez D, Ersek B, et al. (2010) Central role of nitric oxide in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther 12): 210.
- Luo Z, Zheng B, Jiang B, Xue X, Xue E, et al. (2019) Peiminine inhibits the IL-1beta induced inflammatory response in mouse articular chondrocytes and ameliorates murine osteoarthritis. Food Funct 10: 2198-2208.
- Daiber A, Xia N, Steven S, Oelze M, Hanf A, et al. (2019) New therapeutic implications of endothelial nitric oxide synthase (eNOS) function/dysfunction in cardiovascular disease. Int J Mol Sci 20: E187.
- Sandner P, Becker-Pelster EM, Stasch JP (2018) Discovery and development of sGC stimulators for the treatment of pulmonary hypertension and rare diseases. Nitric Oxide 77: 88-95.
- Tobin JV, Zimmer DP, Shea C, Germano P, Bernier SG, et al. (2018) Pharmacological characterization of IW-1973, a novel soluble guanylate cyclase stimulator with extensive tissue distribution, antihypertensive, anti-inflammatory, and antifibrotic effects in preclinical models of disease. J Pharmacol Exp Ther 365: 664-675.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences