ISSN : 2393-8862
American Journal of Pharmacology and Pharmacotherapeutics
An Overview of Heavy Metals (Arsenic, Lead and Phosphorous) Analysis in Drinking Groundwater and its Cause of Cancer
Isha Talwar1*, Satish Sharma1 and Prabhat Kumar Verma2
1Department of Pharmacology, Glocal University, Uttar Pradesh, India
2Department of Pharmacology, National Cancer Research Welfare Society, Uttar Pardesh, India
- *Corresponding Author:
- Isha Talwar
Department of Pharmacology,
Glocal University,
Uttar Pradesh,
India,
Email: ishatalwar57@gmail.com
Received: March 13, 2023, Manuscript No. IPAPP-23-16505; Editor assigned: March 16, 2023, PreQC No. IPAPP-23-16505 (PQ); Reviewed: March 30, 2023, QC No. IPAPP-23-16505; Revised: April 14, 2023, Manuscript No. IPAPP-23-16505 (R); Published: May 12, 2023, DOI: 10.36648/2393-8862.10.3.152
Citation: Talwar I, Sharma S, Verma PK (2023) An Overview of Heavy Metals (Arsenic, Lead and Phosphorous) Analysis in Drinking Groundwater and its Cause of Cancer. Am J Pharmacol Pharmacother Vol.10 No.3: 152
Abstract
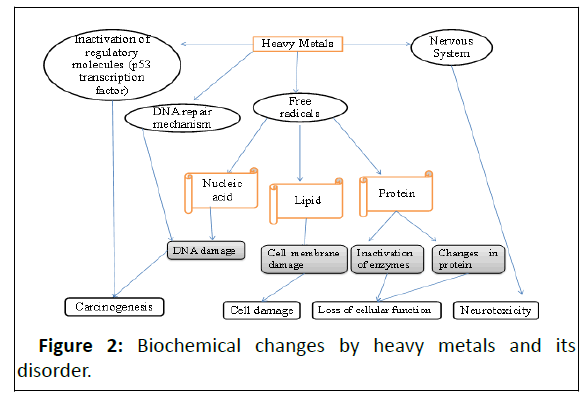
Globally, almost 2.5 billion people rely on groundwater for drinking, the concentration level of heavy metals in drinking water is a major challenge it may be either low or high and both conditions are the cause of diseases. A high level of heavy metals in the human body alters several biochemical pathways leads to the cause of several diseases and among them is cancer. It is necessary to know the accurate concentration level of heavy metals in drinking water before use. Precise estimation of metals ions presence can be done with an instrumental analytical technique like Atomic Absorption Spectrometry (AAS), Inductively coupled plasma atomic emission spectrometry (ICP-AES), Inductively coupled plasma mass Spectrometry (ICPMS), Capillary Zone Electrophoresis (CZE), HPLC/ICP-DRC-MS and Flame atomic absorption spectrometer, etc. By these instruments, the exact concentration of heavy metals like arsenic, lead and phosphorous can be determined. Heavy metals toxicity (As, Pb, and P) in the human body alter the biochemical pathways, and final it indicates cell damage, cellular membrane damage, inactivation of enzymes, and changes in protein structure these effects cause diseases such as carcinogenesis, cell damage, loss of cellular functions and neurotoxicity respectively. This review article was prepared from several sources for the articles like Pubmed, Google Scholar, Web of sciences, Scopus, and WHO website.
Keywords
Heavy metals; Toxicity; Drinking water; Analytical technique; Cancer
Introduction
In the ecosystem, the presence of heavy metals has been confirmed. Their presence is mainly due to daily activities like industrial and agriculture which is called anthropogenic. Pollution in water and soil is due to waste products of the industry that affect the growth of plants and aquatic life. Ultimately, the metals found in the consumable items such as dietary, supplements, cosmetics and various other commercial products. From literature, it confirmed that the heavy metal present in the soil, diet and natural medicine products.
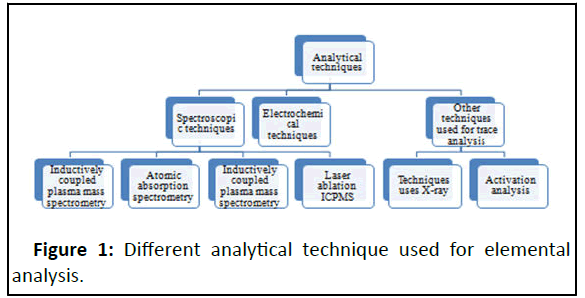
Continuous exposure to heavy metals ion in the body can cause many adverse health effects and even can cause toxicity in the body. A very minimum quantity of some heavy metals is essential to maintain the normal physiology of human body functions and a little excess of the heavy metals dangerous or even toxic. Accumulations of heavy metals such as Lead (Pb), Mercury (Hg), Cadmium (Cd) and Arsenic (As) are toxic a resulting in body organ disruption. Heavy metals interact with nuclear proteins and DNA resulting in oxidative deterioration of biological macromolecules [1,2]. It is necessary to measure the heavy metals in consumable items accurately and precisely. Several adverse effects were seen in the human body due to heavy elements but among these is carcinogenesis which is more serious. Qualitative and quantitative analysis of metal ions performed by several instruments in a laboratory. Low-level detection is very important and it can be only possible through the advanced analytical instrument. The Instrument generally employed for the analysis are Atomic Absorption Spectrometry (AAS), Atomic Emission/Fluorescence Spectrometry (AES/AFS), Inductively Coupled Plasma Mass Spectrometry (ICP-MS), Inductively Coupled Plasma Optical Emission Spectrometry (ICPOES), Neutron Activation Analysis (NAA), X-Ray Fluorescence (XRF) and Anodic Stripping Voltammetry (AVS) [3].
For example heavy metals arsenic is a cytotoxic element, it has been reported from the literature that exposure of this element to the human body can seriously risk for health. Generally, exposure to this element is from ingesting contaminated food and water, occupational exposure and environmental pollution. In pesticide industries, arsenic contact with people will cause several diseases. Contamination of arsenic in soil has the potential to enter the human food chain [4]. Arsenic is carcinogenic and has been detected in several malignant growths, from many research reports it had revealed that the development of cancer in the lung, bladder and skin is because of arsenic [5]. Mortality rates of cancer including colon, gastric, kidney, lung and nasopharyngeal is very high and lowlevel exposure to arsenic in the development of pancreatic cancer and non-Hodgkin’s lymphoma [6,10]. The mechanisms of cancer caused by this heavy metal are the production of Reactive Oxygen Species (ROS), change in DNA function and epigenetic alterations. With arsenic the epigenetic changes like alterations in DNA methylation, histones and miRNA that responsible for the malignant growth. This toxic element has a specific mechanism of action against the human lung epithelial cells, it alters the expression of the p53 protein that resulted in a decline in the expression of the p21 and another study reported that ability to reduce intracellular concentrations of glutathione, a heavy natural antioxidant [11]. The heavy metal lead is toxic and its exposure is a significant risk for health. The general source of this toxic metal is environmental pollution found in soil that can enter the human food cycle apart from this another source is aviation fuel mining [12-15]. In the human body, it has been observed that the exposure of lead maybe because of any cancer and is shown in research reports that it is not directly involved in cancer but it plays a supportive role, for example in glioma patients observed that along with cadmium, lead produce a highly toxic effect. Kidney cancer has a strong association with this toxic metal and also renal cancer [16-19]. Serious health complication has been reported in mercury toxic heavy metal, the source of mercury heavy metal is generally are in mineral form, a waste product of many industries. Mercury converts to vapor and enters the atmosphere or leech into soil or water systems, primary source of this toxic metal has been identified in populations that consuming in large quantities of seafood. From a research study, it was observed that increased mercury exposure is a strong cause of liver cancer and gastric cancer. A unique mechanism of this toxic metal is that it reduces the concentration of glutathione in the body [20-21].
Literature Review
In the twenty-first century, a large number of analytical techniques are available to perform the trace and ultra-trace analysis of elemental composition. The definition of the trace element is 100 ppm (parts per million) this concentration level is detected by the most widely used instrument that is Atomic Absorption Spectrometry (AAS) with flame atomization. For ultra-trace elements concentration level 1 ppm. Instruments with high sensitivity are required for the detection of such low levels. Common instruments like potentiometry, voltammetry, atomic spectrometry, X-ray and nuclear methods are used for the determination of trace elements[22].
An analytical technique like electrochemical methods is used for the estimation of free ions in solution (potentiometry) or free ions together with ions bound in labile complexes (voltammetry) and also gives the information of the oxidation state of some of the elements. For elemental analysis, the most common analytical technique used is Atomic Absorption Spectrometry (AAS) as it is very sensitive and can be determined the total element content within a sample. The sensitivity of AAS is affected by a matrix of the sample[23-25].
Two analytical techniques X-ray and nuclear employed for detections of very low quantity and matrix insensitivity and the result of the experiment of these instruments were used for comparison because their fundamental principles are different from those of other analytical techniques. The advantage or disadvantage of any analytical technique depends on several factors like the number of analytes, nature of analytes, use of techniques, occurrence of interference and difficulties, detection limits and expenses [26].
Estimation of trace elements and contaminants in complex matrices by instrumental analysis required substantial sample preparation and/or extraction. Quantity of sample needs in large for the estimation of trace elements in food environmental, clinical and biological samples. Elemental analysis is routinely carried out by Inductively Coupled Plasma Atomic Spectrometry (ICP-AES), Electrothermal Atomic Absorption Spectrometry (ETAAS) and Flame Atomic Absorption Spectrometry (FAAS)[27-29].
Presently, in the analytical field, several analytical techniques are available for the estimation of trace and ultra-trace elemental analysis[30]. An analytical technique like atomic absorption spectrometry with flame atomization is used for the analysis of trace elements that are present in parts per million ranges. But in the case of ultra trace elements where concentration ranges are in parts per billion and below a lack of analytical technique in the laboratory due to the required analytical sensitivity. For any analytical technique, there is always an advantage and disadvantage and it depends upon the use of technique, the occurrence of interferences and difficulties, detection limits, the throughput of samples, and expenses. The classification of analytical technique in Figure 1.
Discussion
Atomic absorption spectrometry
Flame Atomic Absorption Spectrometry (FAAS) is one of the conventional techniques for the quantification of trace metal ions. This technique compares to other techniques is very simple and inexpensive. The principle of the instrument is that the sample is introduced into a flame where it dissociated into constituent atoms. The flame is the source of electromagnetic radiation and is partially absorbed by the atoms. Based on Beer and Lambert's law quantification of trace elements was performed in various samples materials [31-35]. In many cases, the available analytical technique does not have desired sensitivity for the analysis of natural samples and suffers from matrix interferences. Before quantification, several procedures have required pre-concentration and separation of trace metals for the lower detection limits which improve the precision and accuracy of analytical results. General pre-concentration methods like solvent extraction, ion exchange, adsorption and co-precipitation are used in trace metals analysis [36-38].
Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES)
This analytical technique is also known as Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) and it is one of the best techniques for the determination of trace elements with high sensitivity. The uniqueness of this technique is the use of plasma which provides very high temperatures (up to 8,000 K) for atomization of the analyte. The principle of this technique is that ICP is created by argon gas and it is ionized in the intense electromagnetic field and flows in a particular rationally symmetrical pattern toward the magnetic field of the radiofrequency coil. The collision between neutral argon and the charged particles developed stable plasma, as the sample is introduced to the plasma it collides with the electrons and charged ions and is broken down into charged ions. Detects many elements simultaneously in dynamic range. Before quantification, an effective pre-concentration step is required, the factors like sample volume, the concentration of the eluent, sample and eluent flow rates [39-44].
Inductively Coupled Plasma Mass Spectrometry (ICPMS)
The determination of trace and ultra-trace elements in liquid samples has different matrix compositions by this technique.
The detection level of analytes is sub-μ g/L even to sub-pg/L. Because of high sensitivity and low detection limits, isotopic determination and small sample volume made this technique applied in a wide range, in the area like industrial analysis, geological, environmental clinical and biological and food [49-50]. Principle, sample ionized in the same type of argon plasma as in the ICP-AES technique. The first liquid sample is nebulized with an effective nebulizer transforming it into a fine aerosol and then it is transported with argon to ICP torch. The process in the plasma involved is a nebulized water matrix and chemical compounds are evaporated, molecules dissociated into atomic constituents and then ionized into positively single charged ions. Further, the separated ions are detected by photomultiplier or faraday cup (Table 1 and Figure 2) [51,53].
| S. No. | Objective | Method | Conclusion |
|---|---|---|---|
| 1 | Investigation of arsenic contaminant behavior in groundwater | Determination of arsenic speciation of oxyanions and thioanions is performed on the principle of comparing the pH of the sample with chromatographic elution pH. | The results of the experiment concluded that the presence of sulfide reacts with arsenite to form thioarsenite molecules S/As of 2:1 and 3:1. In the wide range of pH, the solid phase As2S3 dissolves into the sulfidic solution to form 3:1 thioarsenite with structural coordination |
| 2 | Phenyl arsenicals estimate in groundwater by an analytical technique | Determine the arsenic compounds like arsenite and arsenate and their degraded products like phenylarsonic acid, phenyl arsine oxide and diphenylarsinic acid by an analytical technique that is HPLC-ICP-MS | Low-level detection of degraded arsenic products is determined by an MS detector. Very accurately the quantity of diphenylarsinic acid (up to 2.1 mg/l) was determined in a water sample. An elevated concentration of inorganic arsenic up to 240 µg/l was founded. This method is suggested for the determination of arsenate and arsenite as well as phenylic arsenicals |
| 3 | Determination of arsenic speciation in the river and estuarine water | The analytical technique high-performance liquid chromatography-hydride generation-inductively coupled plasma-mass spectrometry (HPLC-HG-ICP-MS) used for the estimation of arsenic species like arsenite, arsenate, Monomethylarsonic (MMA) and Dimethyl arsenic (DMA) precisely | Analysis of water sample, taken from river crosses a mining site where As (III) presence is high. The accurate and precise quantity is determined. A low quantity of arsenite was detected very accurately |
| 4 | Estimation of arsenite, arsenate, and its derivatives by analytical technique | Instrument HPLC-ICP-MS was used to determine the arsenite, arsenate, mono-methylarsinic acid, dimethyalarsinic acid and arsenobetaine in various water samples | The developed method is highly sensitive; detect the analyte at a very low-level 0.4 pg. For analysis sample taken surface and well waters where the major analyte reported are arsenite and arsenate, but also AsBet and DMAA were found which never been reported before |
| 5 | Capillary Zone Electrophoresis (CZE) analytical technique used for the determination of aqueous soil extract | The CZE analytical technique to determine the content of arsenic in the sample of soil compared with the total content measured by Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES) is suited for the speciation of arsenic | The result of the analysis was indicated that the yield of phenyl arsenic compound derived from arsenic concentrations of the aqueous soil determined by ICP-AES after microwave digestion was founded 6%-32% of the total amount of arsenic content |
| 6 | Quantitative determination of arsenite As+3, As+5, Monomethylarsonate (MMA), Dimethyl Arsenate (DMA) and roxarsone | Analytical technique anion exchange chromatography to separate the arsenic species and inductively coupled plasma-mass spectrometry as an arsenic-specific detector | Analyzed more than 100 surface-water, groundwater and acid mine drainage samples and reference materials. Examine the total arsenic As+3, As+5, DMA and MMA. The quantity reported 13,000 µg As+3 L-1 and 3700 µg As+5 L-1 |
| 7 | To understand the toxicity level of As+3, As+5, Dimethyalarsinate (DMA) and Monomethylarsonate (MMA) in environmental water | A sensitive and robust method developed by an analytical technique coupled plasma-mass spectrometer with MS/MS mode. The column used PRP-X100 and nitrate/phosphate mobile phase. The developed method was applied for the investigation of surface water and groundwater matrices | The result of the analysis found mean recoveries range 87.2% to 108.7% and the relative standard deviation of replicates of all analytes ranged from 1.1% to 9.0% |
| 8 | Determination of compounds Dimethyl Arsenate (DMA), Mono-Methyl Arsenate (MMA), As+3 and As+5 in drinking water | Analytical technique capillary electrophoresis coupled to electrospray mass spectrometry used to determine the organic and inorganic arsenic compounds in drinking water | By this analytical technique first, optimize the composition and nature of both electrophoretic separation mediums using Hexafluoro-2-propanol (HFIP) as an additive. Based on Dispersive Liquid-Liquid Micro-Extraction (DLLME) intended for rapid determination of the total content of inorganic arsenic in the water sample and second based on partial evaporation of the water sample, to detect the arsenic four species |
| 9 | By analytical techniques, IEC/SEC-HPLC/ICP-DRC-MS and ESI-MS/MS performed the study of As, Cr and Sb in bottled flavored drinking water samples | Extended the developed analytical procedure for AsIII, AsV, CrVI, SbIII, and SbV analysis and arsenic speciation procedure for AsB, AsIII, DMA, MMA and AsV quantification by using analytical HPLC/ICP-DRC-MS and ESI-MS/MS to get a better result and it is called bespoke speciation analysis | A developed method by an individual analytical technique that is HPLC/ICP-DRC-MS and ESI-MS/MS gives a better result like the precision value range from 2.5% to 5.5% and from 3.6% to 7.2%, respectively and recoveries value ranged 97% to 106% and from 99% to 106%. These results indicate that the developed method was robust and use for the analysis of the sample |
| 10 | Advanced hyphenated technique HPLC/ICP-DRC-MS used for the analysis of multi-elemental speciation analysis having five toxic species: As (III), As (V), Cr (VI), Sb (III), and Sb (V) in drinking water | Chromatographic separation and quantification of As (III), As (V), Cr (VI), Sb (III) and Sb (V) in drinking water with hyphenated technique HPLC/ICP-DRC-MS used Dynamic Reaction Cell (DRC) with oxygen as a reaction gas was involved in the experiments | The result of the analysis confirmed the lack of interference influence on analytical signals as their values were in the range of 91%-110%. The developed method was tested on drinking water samples characterized by mineralization up to 650 mg L-1 |
| 11 | Micro determination of lead in drinking water by Atomic Absorption Spectrophotometer (AAS) | A simple, rapid, precise and reliable analytical method. Incorporates co-precipitation of lead on zirconium hydroxide followed by instrument Atomic Absorption Spectrophotometer (AAS) using 283.3 nm wavelength | First, by co-precipitation technique separate the trace metal ions. Easily trace metal ions separated then performed filtering, centrifuging and washing of the precipitate. Estimation of lead in water was performed by instrument flame atomic absorption spectrometry with a combination of 2-mercaptobenzo-thiazole as a chelating reagent and copper as a co-precipitate carrier |
| 12 | Analytical technique flotation-spectrophotometric used for the determination of trace amounts of lead | The principle of this method is flotation of a complex of Pb+2 ions and Alizarin yellow between aqueous and n-hexane interface at pH=6. It is a very simple and highly selective method for separation, pre-concentration and determines extremely low concentrations of lead | The advantage of this method is it very simple and easily performed. The quantification of Pb (II) in the range of 8 ngmL-1-170 ngmL-1 in various environmental samples |
| 13 | Analytical technique Atomic Absorption Spectrometry (AAS) with dithizone extraction to determine lead in drinking water | First extractions performed, take 10 ml of water sample and adjusted to alkaline pH extracted with 5 ml of 0.01% dithizone in chloroform. Washed the chloroform phase and evaporated to dryness. Dithizone chelated lead is reextracted into 2 ml of 5% HNO3 the analyzed in instrument Perkin Elmer Atomic Absorption Spectrometer (AAS) for its quantification | For quantitative analysis first prepared calibration of the curve in range 0 μg/L to 40 μg/L, then determine lead in spiked water samples and recovery founded 97% |
| 14 | Determination of lead in water samples by graphite furnace atomic absorption spectrometry after cloud point extraction | Lead formed a complex with 2-(5-Bromo-2-pyridylazo)-5 (diethylamino)-phenol (5-Br-PADAP) then require a Cloud Point Extraction (CPE) for the preconcentration after this step estimation performed by instrument Graphite Furnace Atomic Absorption Spectrometry (GFAAS) using octylphenoxypolyethoxy ethanol (TritonX-114) as surfactant | Separation of the two phases was accomplished by centrifugation for 15 min at 4000 rpm. Under the optimum conditions i.e., pH 8.0, cloud point temperature 40°C, [5-Br-PADAP]=2.5 × 10-5 mmol l-1, [Triton X-114]=0.05%, added methanol volume=0.15 ml, pre-concentration of only 10 ml sample permitted an enhancement factor of 50-fold |
| 15 | Determinations lead in wastewater by flame atomic absorption spectrometer | The principle for this analysis is determining lead in wastewater and effluent by flame atomic absorption spectrometry after preconcentration of lead by the rapid co-precipitation technique with gallium phosphate | The determination of lead in the sample does not affect by gallium phosphate in flame atomic absorption spectrometry at 250.0 nm in final concentration. Used co-precipitation technique, which does not require the complete collection of the precipitate |
| 16 | By the flame atomic absorption spectrometry determine trace lead in water and milk tea powder samples with organic co-precipitation | The method applied for the determination of trace lead with flame atomic absorption spectrometry after pre-concentration of lead by rapid co-precipitation technique with PAR-Fe (III) at pH 6.0. Examined the other analytical parameters such as pH, amount of reagent, the standing time of the precipitate | Performed analysis of lake water and the milk tea powder samples. The Detection Limits (DL) were founded 18.7 microg x L-1 for Pb (II). RSD's and the standard addition recovery of this method were in the ranges of 1.03%-2.24% and 94.2%-98.3% respectively |
| 17 | Precipitate sulfate by improved precipitation technique and the used chelatometric titration with HEDTA for the determination of lead | Estimation of lead by selective titrimetric analysis performed, prior have done modified method of precipitation | Back titration method adopted, precipitate collected and transferred more than HEDTA solution, the excess of the sample back titrated with Zn (II) at pH 5.0-5.5. Used catechol violet and xylenol orange as a mixed indicator give a sharper end-point. This method was successfully used to estimate Pb in non-ferrous alloys |
| 18 | Analytical technique atomic-absorption spectrophotometry with electro-thermal atomization used for the estimation of lead in drinking water | The use of electro-thermal atomization to determine concentrations of lead in water, although sensitive, generally suffers from suppressive interference effects that can produce large and variable negative bias | The pre-treatment technique can be either impregnation of the furnace tube with lanthanum or the addition of lanthanum (as lanthanum chloride) to each sample |
| 19 | Quantification of lead in Industrial samples using 1,3-Benzenediamine N, N'-bis (2-furanylmethylene) in presence of surfactant by direct spectrophotometric method | For spectrophotometric analysis, Schiff’s base is one of the excellent chelating agents for the determination of metal ions. Lead (II) with 1,3-Benzenediamine, N, N'-bis (2-furanylmethylene) (BDFM) formed a colored complex | The colored complex of lead has a maximum absorbance at 620 nm in presence of Sodium Lauryl Sulfate (SLS) as a surfactant with molar absorptivity 10.16 × 103 L mol-1cm-1 the complex was stable at room temperature and for a long time |
| 20 | A Flow Injection Analysis (FIA) system with on-line pre-concentration and spectrophotometric detection for the determination of lead in aqueous samples | Developed a method for the analysis by FIA system followed spectrophotometric detection. FIA systems based on the reaction of lead (II) with 4-(2-pyridylazo) resorcinol (PAR) in buffer pH 9 with an adsorption maximum at 523 nm | The detection limit of this developed method 11 μg L-1 from aqueous lead solution at a sampling rate of 12 h-1. Linear detection range is 0.10 to 0.90 mg L-1. Evaluated precision and accuracy with a standard synthetic solution and a real sample and the result indicated 2.4% RSD and 90%-117% recovery |
| 21 | Determination of phosphorus by the spectrophotometric method by using bismuth-phosphomolybdate complex | Based on the formation of the blue Bismuth-Phosphomolybdate complex (BiPMo), estimated phosphorus for the P-PO4-3 ion natural waters by spectrophotometric analysis which is very simple, easy and sensitive | This method is very simple and easily performed. The developed method is the sensitive, quick and simple determination of trace amounts of phosphorus as orthophosphate in natural waters even in laboratories equipped with simple instruments |
| 22 | Based on the reaction of vanadomolybdophosphate with malachite green determine phosphorus in river water by spectrophotometric | The development of an ion-associate between vanadomolydophosphate and malachite green in aqueous acidic solution (0.5 M sulphuric acid); determine 0-1 × 10-5 M of phosphate | The developed complex was stabilized by adding polyvinyl alcohol and estimated at 620 nm with molar absorptivity 1.05 × 105 l mol-1 cm-1. The other ions like silicate and arsenate do not interfere in the analysis, interference of arsenate is avoided by reduction with thiosulfate |
| 23 | Estimation of trace amounts of phosphate in water and soil by spectrophotometric analysis | The principle of this method is the formation of phosphomolybdate by reaction between ammonium molybdate and hydrazine by a reduction in acidic medium produce blue of uncertain composition | System followed the Lambert-Beer's law at 830 nm in concentration range 0.5 μg/mL-5 μg/mL of phosphate, complex showing blue colour with maximum absorption and have Relative Standard Deviation (RSD) of 0.1% and correlation coefficient of 0.99 and at this wavelength molar absorptivity 2.9 × 10⁴ L mol⁻¹ cm⁻¹ |
| 24 | Spectrophotometric simultaneous estimation of phosphate and silicate ions in river water by using ion-exchange chromatography separation and post-column derivatization | Phosphate and silicate ions were separated by the ion-exclusion column packed with a polymethacrylate-based weakly acidic cation-exchange resin in the H+ form (TSKgel Super IC-A/C) by using ultrapure water as eluent | Examine the effects of sulphuric acid, sodium molybdate and ascorbic acid concentration and reaction coil length, which form the reduced complexity of molybdate and ions. The detector response of phosphate and silicate ions was examined. The concentration under the optimized condition color-forming reactant 50 mM sulfuric acid, 10 mM sodium molybdate reducing agent, 50 mM ascorbic acid and length of 6 m. The linearity of the calibration curves of phosphate and silicate ions were 50 microg L-1-2000 microg L-1 and 250 microg L-1-10,000 microg L-1 respectively |
| 25 | Environmental water containing silicate and phosphate determined by analytical technique pre-column derivatization ion-pair liquid chromatography | By this analytical technique estimate, the soluble silicates and phosphate in an environmental sample of waters preceded by the formation of their yellow alpha-heteropolymolybdates | The pre-column coloring reactions at moderate-pH were reproducible for both silicate and phosphate in all quantification ranges with RSD less than 2% and 5% respectively. The linear calibration lines between concentrations mg-SiO2/L and mg-PO4/L and peak area intensities were obtained for silicate and phosphate both with acceptable determination coefficients r (2) of 0.9999. The limits of determination for both analytes were 0.007 mg-SiO2/L and 0.003 mg-PO4/L, which were calculated theoretically using 10 sigma/ slope |
| 26 | Determination of phosphate in water sample by a simple spectrophotometric method | Formation of phosphomolybdate complex followed by its reduction with thiourea in an aqueous sulphuric acid medium | The result of the analysis is based on Beer’s Law at 840 nm in linearity range 0.5 μg/ml-10.0 μg/ml. Molar absorptivity, correlation coefficient, and Sandell's sensitivity values are found to be 1.712 mol-1 cm-1, 0.9769 μg cm-2 and 0.0555 μg cm-2 respectively |
| 27 | Determination of phosphate in a water sample by a simple spectrophotometric method | Determine phosphorus as its ion associate with a quaternary ammonium salt and molybdoantimonophosphoric acid immobilized on silica gel in the concentration range 1.9 μg P/L -124 μg P/L | The developed method was tested in the determination of various phosphorus forms in natural waters |
| 28 | Factorial design for multivariate optimization of the pre-concentration system for spectrophotometric phosphorus determination | Extraction of phosphorus as phosphomolybdenum blue was performed on to Ambulate XAD-4 polymeric adsorbent. Quantification performed in ultraviolet-visible spectrophotometer based on the formation phosphomolybdate and reduction to molybdenum blue | By using two-level full factorial design optimized the method taking three variables (resin amount, sample volume, flow rate). At 0.08 microg mL-1 the relative standard deviation was 2% and limit of detection was 2.23 microg L-1 (N=15) |
| 29 | Estimation of phosphate in sugarcane juices, water and detergent samples by simple spectrophotometric analytical technique | The principle of this method is the formation of phosphomolybdate with added molybdate followed by its reduction with sodium sulfide in aqueous sulphuric acid | Developed a calibration curve at 715 nm based on Lambert-Beer’s law in the concentration range 0.3 ppm-12.24 ppm. The results of the analysis were molar absorptivity, correlation coefficient and Sandell’s sensitivity 6.1 x 3 mol-1 cm-1, 0.999 µg cm-2 and 0.0156 µg cm-2 respectively |
Table 1: Estimation of heavy metals (arsenic, lead and phosphorus) by various analytical techniques.
Arsenic
Molecular mechanism of arsenic-induced cancer: The power of carcinogenic capacity of this toxic metal is usually linked with its biotransformation. The arsenic metal in drinking water is easily absorbed by the gastrointestinal tract[54-58]. As this toxic metal is ingested its oxidation state is a pentavalent form (As+5) and enters the cells through the membrane transporter-like Inorganic Phosphate Transporters (PiT) and aquaporins. Inside the cell, it reduces to more toxic that is in trivalent form (As+3) in a glutathione-dependent reaction that proceeds by polynucleotide phosphorylase and mitochondrial ATP synthase. Further for the detoxification process As+3 and its methylated conjugates are transferred to hepatocytes into bile as glutathione conjugates. In the liver, the availability of mono- and dimethylated As+3 species are highly reactive and it induces to damage different organs including the lungs. The damage of different organs occurs primarily because of the generation of Reactive Oxygen Species (ROS)[59-64].
Arsenic biotransformation as a toxicity activation mechanism: During the metabolism of arsenic a series of redox reactions happened, pentavalent convert to trivalent species. Oxidative methylation gives methylated tri- and pentavalent metabolites. Apart from the detoxification methylation can activate the toxic and carcinogenic potential of arsenic. Research reported that the mono/dimethylated arsenical species can change the gene transcription and are more potent enzyme inhibitors and cytotoxins than nonmethylated species [65,66]. SAdenosylmethionine (SAM) functioned as a methyl group donor; the cellular processes can interfere with the arsenic metal ion that altered epigenetic mechanism that participates in–induced carcinogenesis [67,68]. Purine nucleoside phosphorylase reduced As+5 to As+3, with SAM a methyl group donor As+3 is methylated via an As+3 methyltransferase producing mono and dimethylated trivalent species. As+3 could be carcinogenic for the skin. The toxicity of arsenic metal results from its ability to interact with proteins sulfhydryl functional groups and substitute phosphorous in a variety of biochemical reactions. In vitro studies reported the inactivation of enzymes through sulfhydryl groups like dihydrolipoyl dehydrogenase and thiolase [69].
Lead
The heavy metal leads toxicity induced cancer by damaging and disrupting the DNA along with the repair system of cellular tumor regulatory genes through the generation of ROS. Several research studies have been reported that the generation of ROS by lead change the structure and sequence of chromosome and alter the transcription processes by replacing zinc in certain regulatory proteins [70]. Excessive exposure to lead causes toxicity and it is called lead poisoning, the effects of poisoning are generally related to the gastrointestinal tract and central nervous system disorder. In the human body, lead poisoning might be acute or chronic. Diseases because of acute exposure such as loss of appetite, abdominal pain, fatigue, sleeplessness, renal dysfunction, hypertension, arthritis, hallucinations and due to chronic exposure mental retardation, birth defects, autism, psychosis, allergies, paralysis, weight loss, dyslexia, muscular weakness, kidney damage, coma and may even cause of death. The acute and chronic condition is preventable but the diseases that happened due to lead toxicity are very dangerous as it can affect most of the body organs [71]. A condition of edema has been found in excess levels of lead in the body in which the plasma membrane of the blood-brain barrier moves into the interstitial spaces and also alters the function of the central nervous system by disrupting the intracellular second messenger systems [72].
Lead is a divalent element and it can easily substitute the divalent metal cations and such substitutions have been seen in cellular function, the substitution of an essential element with divalent lead inhibits the cellular functions by binding with various proteins and in some cases, it has been observed that lead can change the functional activity of metal-chelating proteins by substituting the normal metal cation. The synthesis of heme is also inhibited by deactivating the d-aminolevulinic acid dehydrase zinc-containing enzyme. The disorder in the nervous system is generally due to interference of lead with calcium-dependent processes related to neuronal signaling and intracellular signal transduction [73]. In many in vitro and in vivo studies found that lead compounds cause genetic damage through many indirect mechanisms which include inhibition of DNA synthesis and repair, oxidative damage and interaction with DNA-binding proteins and tumor suppressor proteins.
Phosphorus
Drinking water inorganic phosphate is a source of phosphorus, for cellular function an optimum concentration of its required as the concentration increases in an intracellular fluid becomes toxic. The toxic level of phosphorus causes cancer in the body. Inorganic phosphate is incorporated into the cells which regulate cellular function. This element is present in nucleic acid, Adenosine Triphosphate (ATP) and other metabolites. Many biochemical pathways alter as the concentration reach in toxic level, membrane transport of minerals disturb and some damage the membrane of some cells. Thyroid, breast and lung cancer are because of the high concentration of NaPi2b in normal tissue [74]. A high concentration of phosphate enhanced the expression of the gene in forkhead box protein C2 (FOXC2), osteopontin and vascular endothelial growth factor in dosedependent manners that alter the synthesis of nucleic acid, a wrong formation of the nucleic acid cause of cancer [75]. High concentrations of phosphate directly damage the mitochondrial membrane, produce Reactive Oxygen Species (ROS), reduce nitric oxide production and induce apoptosis and also, activate the PKC pathway these events are responsible for the cause of cancer [76]. High phosphate level in serum indicates the stage of cancer and can predict the survival of patients. The researcher reported that a high level of phosphate in cells stimulates tumorigenesis it is due to exerting mitogenic effects on tumor cells, enhancing angiogenesis, inducing chromosome instability and finally metastasis [77,78].
Conclusion
This review article concludes that drinking water with heavy metals like arsenic, lead, and phosphorus is present in the toxic level cause of several diseases. Cancer disease due to the toxic level of heavy metals in the human body induces the generation of free radicals which interfere in the nucleic acid synthesis and finally damage the DNA. Providing safe drinking water in a population is very necessary and it can be possible if the estimation of heavy metals has been performed with advanced analytical techniques. In the human body, the requirement of heavy metals is very low as it crosses the level of concentration it starts to accumulate in the body. That’s it is necessary to know the metal ions concentration in the drinking water before use.
Funding
The authors declare that no funding was obtained from any government or private sources.
Conflict of Interest
The authors declare no conflicting interest.
References
- Ray SA, Ray MK (2009) Bioremediation of heavy metal toxicity with special reference to chromium. Al Ameen J Med Sci 2: 57-63.
- Leonard SS, Harris GK, Shi X (2004) Metal-induced oxidative stress and signal transduction. Free Radic Biol Med 37: 1921-1942.
[Crossref] [Google Scholar] [PubMed]
- Helaluddin ABM, Khalid, RS, Alaama M, Abbas SA (2016) Main analytical techniques used for elemental analysis in various matrices. Tropical J Pharma Res 15: 427-434.
- Pershagen G (1981) The carcinogenicity of arsenic. Environ Health Perspect 40: 93-100.
[Crossref] [Google Scholar] [PubMed]
- Nachman KE, Ginsberg GL, Miller MD, Murray CJ, Nigra AE, et al. (2017) Mitigating dietary arsenic exposure: Current status in the United States and recommendations for an improved path forward. Sci Total Environ 581-582: 221-236.
[Crossref] [Google Scholar] [PubMed]
- Amaral AF, Porta M, Silverman DT, Milne RL, Kogevinas M, et al. (2012) Pancreatic cancer risk and levels of trace elements. Gut 61: 1583-1588.
[Crossref] [Google Scholar] [PubMed]
- Chen K, Liao QL, Ma ZW, Jin Y, Hua M, et al. (2015) Association of soil arsenic and nickel exposure with cancer mortality rates, a town-scale ecological study in Suzhou, China. Environ Sci Pollut Res Int 22: 5395-5404.
[Crossref] [Google Scholar] [PubMed]
- Hall MN, Niedzwiecki M, Liu X, Harper KN, Alam S, et al. (2013) Chronic arsenic exposure and blood glutathione and glutathione disulfide concentrations in Bangladeshi adults. Environ Health Perspect 121: 1068-1074.
[Crossref] [Google Scholar] [PubMed]
- McCumber A, Strevett KA (2017) A geospatial analysis of soil leads concentrations around regional Oklahoma airports. Chemosphere 167: 62-70.
[Crossref] [Google Scholar] [PubMed]
- Southard EB, Roff A, Fortugno T, Richie JP Jr, Kaag M, et al. (2012) Lead, calcium uptake, and related genetic variants in association with renal cell carcinoma risk in a cohort of male Finnish smokers. Cancer Epidemiol Biomarkers Prev 21: 191-201.
[Crossref] [Google Scholar] [PubMed]
- Rice KM, Walker EM Jr, Wu M, Gillette C, Blough ER (2014) Environmental mercury and its toxic effects. J Prev Med Public Health 47: 74-83.
[Crossref] [Google Scholar] [PubMed]
- Thiers RE (1957) Contamination in trace analysis and its control. Methods Biochem Anal 5: 273-337.
[Crossref] [Google Scholar] [PubMed]
- Nowak S, Gesell M, Holtkamp M, Scheffer A, Sperling M, et al. (2014) Low gas flow inductively coupled plasma optical emission spectrometry for the analysis of food samples after microwave digestion. Talanta 129: 575-578.
[Crossref] [Google Scholar] [PubMed]
- Butler O, Cairns WRL, Cook J, Davidson CM (2013) Atomic spectrometry update. Environmental analysis. J Anal At Spectrom 28: 177-216.
- El Ati-Hellal M, Hellal F, Hedhili A (2014) Application of Plackett-Burman and Doehlert designs for optimization of selenium analysis in plasma with electrothermal atomic absorption spectrometry. Clin Biochem 47: 95-100.
[Crossref] [Google Scholar] [PubMed]
- Zawisza B, Pytlakowska K, Feist B, Polowniak M, Kita A, et al. (2011) Determination of rare earth elements by spectroscopic techniques: A review. J Anal Atomic Spectrom 26: 2373-2390.
- Waheed S, Rahman S, Siddique N (2014) Calcium supplements as source of trace elements: Adequacy and safety of supplements with vitamin C, vitamin D and phosphate formulations. Appl Radiat Isot 89: 134-140.
[Crossref] [Google Scholar] [PubMed]
- Rahman S, Waheed S (2011) Blood-cooper and zinc levels and consequences of cardiovascular complications: A study by INAA and FAAS. J Radioanal Nucl Chem 287: 657-664.
- Sidique N, Majid A, Chaudhry MM, Tufail M (2012) Determination of heavy metals in air conditioner dust using FAAS and INAA. J Radioanal Nucl Chem 292: 219-227.
- Korn MGA, de Andrade JB, de Jesus DS, Lemons VA, Bandeira MLSF, et al. (2006) Separation and preconcentration procedures for the determination of lead using spectrometric techniques: A review. Talanta 69: 16-24.
[Crossref] [Google Scholar] [PubMed]
- Rezvani M, Ebrahimzadeh H, Aliakbari A, Khalilzadeh A, Kasaeian M, et al. (2014) Novel modified carbon nanotubes as a selective sorbent for preconcentration and determination of trace copper ion in fruit samples. J Sep Sci 37: 2559-2565.
[Crossref] [Google Scholar] [PubMed]
- Ozdemir C, Sacmci S, Kartal S, Sacmaci M (2014) Determination of gold and palladium in environmental samples by FAAS after dispersive liquid-liquid microextraction pretreatment. J Ind Eng Chem 20; 4059-4065.
- Pourjavid MR, Arabieh M, Yousefi SR, Jamali MR, Rezaee M, et al. (2015) Study on column SPE with synthesized grapheme oxide ad FAAS for determination of trace amount of Co (II) and Ni (II) ions in real samples. Mater Sci Eng C 47; 114-122.
[Crossref] [Google Scholar] [PubMed]
- Mendil D, Karatas M, Tuzen M (2015) Separation and pre-concentration of Cu(II), Pb (II), Zn(II), Fe(III) and Cr(III) ions with co-precipitation method without carrier element and their determination in food and water samples. Food Chem 177: 320-324.
[Crossref] [Google Scholar] [PubMed]
- Ohtsu N, Ashino T, Kimura H, Takada K, Hanawa T (2007) Investigation for analytical procedure for determination of trace metallic ions in simulated body fluids by inductively coupled plasma atomic emission spectrometry. J Mater Sci Mater Med 18: 429-433.
[Crossref] [Google Scholar] [PubMed]
- Nomngongo PN, Ngila JC, Msagati TAM, Moodley B (2013) Preconcentration of trace multi-elements in water sample using Dowex 50W-x8 and Chelex-100 resins prior to their determination using inductively coupled plasma atomic emission spectrometry. Phys Chem Earth 66: 83-88.
- Garcia-Otero N, Barciela-Alonso MC, Dominguez-Gonzalez R, Herbello-Hermello P, Moreda-Pineiro A, et al. (2015) Evaluation of gel electrophoresis, electrothermal atomic absorption spectroscopy and inductively coupled plasma optical emission spectroscopy for trace metal analysis in marine plankton protein. Microchem J 119: 51-57.
- Simpson LS, Hearn R, Catterick T (2004) The development of a high accuracy method for the analysis of Pd, Pt and Rh in auto catalysts using a multi-collector ICP-MS. J Anal At Spectrom 19: 1244-1251.
- Krachler M, Mohl C, Emons H, Shotyk W (2002) Influence of digestion procedures on the determination of rare earth elements in peat and plant samples by USN-ICP-MS. J Anal At Spectrom 17: 844-851.
- Siemianowski O, Barabasz A, Kendziorek M, Ruszczynska A, Bulska E, et al. (2014) HMA4 expression in tobacco reduces Cd accumulation due to the induction of the apoplastic barrier. J Exp Bot 63: 1125-1139.
[Crossref] [Google Scholar] [PubMed]
- Barabasz A, Wilkowska A, Ruszczynska A, Bulska E, Hanikenne M, et al. (2012) Metal response of transgenic tomato plants expressing P1B-ATPase. Physiol Plant 145: 315-331.
[Crossref] [Google Scholar] [PubMed]
- Barbaste M, Robinson K, Guilfoyle S, Medina B, Lobinski R (2002) Precise determination of the strontium isotope ratios in wine by inductively coupled plasma sector field multicollector mass spectrometry (ICP-SF-MC-MS). J Anal At Spectrom 17: 135-137.
- Ammann AA (2007) Inductively coupled plasma mass spectrometry (ICPMS): A versatile tool. J Mass Spectrom 42: 419-427.
[Crossref] [Google Scholar] [PubMed]
- Becker JS (2005) Trace and ultratrace analysis in liquids by atomic spectrometry. Trends Anal Chem 24: 243-254.
- Wilkin RT, Ford RG, Costantino LM, Ross RR, Beak DG, et al. (2019) Thioarsenite detection and implications for arsenic transport in groundwater. Environ Sci Technol 53: 11684-11693.
[Crossref] [Google Scholar] [PubMed]
- Daus B, Mattusch J, Wennrich R, Weiss H (2008) Analytical investigations of phenyl arsenicals in groundwater. Talanta 75: 376-379.
[Crossref] [Google Scholar] [PubMed]
- Sanchez-Rodas D, Luis Gomez-Ariza J, Giraldez I, Velasco A, Morales E (2005) Arsenic speciation in river and estuarine waters from southwest Spain. Sci Total Environ 345: 207-217.
[Crossref] [Google Scholar] [PubMed]
- Ronkart SN, Laurent V, Carbonnelle P, Mabon N, Copin A, et al. (2007) Speciation of five arsenic species (arsenite, arsenate, MMAAV, DMAAV and AsBet) in different kind of water by HPLC-ICP-MS. Chemosphere 66: 738-745.
[Crossref] [Google Scholar] [PubMed]
- Kutschera K, Schmidt AC, Kohler S, Otto M (2007) CZE for the speciation of arsenic in aqueous soil extracts. Electrophoresis 28(19): 3466-3676.
[Crossref] [Google Scholar] [PubMed]
- Bednar AJ, Garbarino JR, Burkhardt MR, Ranville JF, Wildeman TR (2004) Field and laboratory arsenic speciation methods and their application to natural-water analysis. Water Res 38: 355-364.
[Crossref] [Google Scholar] [PubMed]
- Stetson SJ, Lawrence C, Whitcomb S, Kanagy C (2020) Determination of four arsenic species in environmental water samples by liquid chromatography-inductively coupled plasma-tandem mass spectrometry. MethodsX 8: 101183.
[Crossref] [Google Scholar] [PubMed]
- Dominguez-Alvarez J (2020) Capillary electrophoresis coupled to electrospray mass spectrometry for the determination of organic and inorganic arsenic compounds in water samples. Talanta 212: 120803.
[Crossref] [Google Scholar] [PubMed]
- Lorenc W, Markiewicz B, Kruszka D, Kachlicki P, Baralkiewicz D (2019) Study on speciation of As, Cr, and Sb in bottled flavored drinking water samples using advanced analytical techniques IEC/SEC-HPLC/ICP-DRC-MS and ESI-MS/MS. Molecules 24: 668.
[Crossref] [Google Scholar] [PubMed]
- Marcinkowska M, Komorowicz I, Barałkiewicz D (2016) New procedure for multielemental speciation analysis of five toxic species: As(III), As(V), Cr(VI), Sb(III) and Sb(V) in drinking water samples by advanced hyphenated technique HPLC/ICP-DRC-MS. Anal Chim Acta 920: 102-111.
[Crossref] [Google Scholar] [PubMed]
- Shrivastava AK, Khaladkar HS (2013) Novel method for micro determination of lead in water samples. Sci Revs Chem Commun 3: 43-53.
- Shiri S, Delpisheh A, Haeri A, Poornajaf A, Golzadeh B, et al. (2011) Determination of trace amounts of lead using the flotation-spectrophotometric method. Anal Chem Insights 6: 15-20.
[Crossref] [Google Scholar] [PubMed]
- Mao GD, Foreback CC, Chu JW (1997) Measurement of lead in drinking water by atomic absorption spectrometry with dithizone extraction. Toxicol Environ Chem 63: 163-170.
- Chen J, Xiao S, Wu X, Fang K, Liu W (2005) Determination of lead in water samples by graphite furnace atomic absorption spectrometry after cloud point extraction, Talanta 67: 992-996.
[Crossref] [Google Scholar] [PubMed]
- Kagaya S, Araki Y, Hasegawa K (2000) Flame atomic absorption spectrometric determination of lead in waste water and effluent after preconcentration using a rapid coprecipitation technique with gallium phosphate. Fresenius J Anal Chem 366: 842-845.
[Crossref] [Google Scholar] [PubMed]
- Lin JM, Yao JX, Zhao WY (2013) Determination of trace lead in water and milk tea powder samples with organic coprecipitation-flame atomic absorption spectrometric. Guang Pu Xue Yu Guang Pu Fen Xi 33: 1357-1359.
[Crossref] [Google Scholar] [PubMed]
- Nan Z, Yao XZ, Gu YX, Yu RQ (1990) Determination of lead by selective chelatometric titration with HEDTA after separation as its sulphate by an improved method of precipitation. Talanta 37: 1021-1024.
[Crossref] [Google Scholar] [PubMed]
- Bertenshaw MP, Gelsthorpe D, Wheatstone KC (1981) Determination of lead in drinking water by atomic-absorption spectrophotometry with electrothermal atomisation. Analyst 1258.
[Crossref] [Google Scholar] [PubMed]
- Zaky M, Amin AS, Elgendy KH, Gomaa A (2017) Direct spectrophotometric determination of lead (II) in industrial samples using 1, 3-Benzenediamine, N, N'-bis(2-furanylmethylen) in presence of surfactant. BFSZU 39: 134-148.
- Klamtet J, Sanguthai S, Sriprang S (2007) Determination of lead in aqueous samples using a flow injection analysis system with On-line preconcentration and spectrophotometric detection. NU Science J 4: 122-131.
- Mihajlovic RP, Kaljevic VM, Vukasinovic MP, Mihajlovic LV (2007) Pantic ID Spectrophotometric method for the determination of phosphorus in natural waters using the bismuthphosphomolybdate complex 33: 513-518.
- Motomizu S, Oshima M, Hirashima A (1988) Spectrophotometric determination of phosphorus in river water based on the reaction of vanadomolybdophosphate with malachite green. Analytica Chimica Acta 211: 119-127.
- Ganesh S, Khan F, Ahmed MK, Velavendan P, Pandey NK, et al. (2012) Spectrophotometric determination of trace amounts of phosphate in water and soil. Water Sci Technol 66: 2653-2658.
[Crossref] [Google Scholar] [PubMed]
- Nakatani N, Kozaki D, Masuda W, Nakagoshi N, Hasebe K, et al. (2008) Simultaneous spectrophotometric determination of phosphate and silicate ions in river water by using ion-exclusion chromatographic separation and post-column derivatization. Anal Chim Acta 619: 110-114.
[Crossref] [Google Scholar] [PubMed]
- Yokoyama Y, Danno T, Haginoya M, Yaso Y, Sato H, et al. (2009) Simultaneous determination of silicate and phosphate in environmental waters using pre-column derivatization ion-pair liquid chromatography. Talanta 79: 308-313.
[Crossref] [Google Scholar] [PubMed]
- Shyla B, Mahadevaiah, Nagendrappa G (2011) A simple spectrophotometric method for the determination of phosphate in soil, detergents, water, bone and food samples through the formation of phosphomolybdate complex followed by its reduction with thiourea. Spectrochim Acta A Mol Biomol Spectrosc 78: 497-502.
[Crossref] [Google Scholar] [PubMed]
- Zaporozhets OA, Zinko LS, Kachan IA (2007) Solid-phase-spectrophotometric and test determination of simultaneously present phosphorus forms (phosphorus speciation) in water. J Anal Chem 62: 1146-1150.
- Divrikli U, Akdogan A, Soylak M, Elci L (2009) Factorial design for multivariate optimization of preconcentration system for spectrophotometric phosphorus determination. Talanta 77: 1287-1291.
[Crossref] [Google Scholar] [PubMed]
- Mahadevaiah, Kumar MSY, Galil MSA, Suresha MS, Sathish MA, et al. (2007) A Simple Spectrophotometric Determination of Phosphate in Sugarcane Juices, Water and Detergent Samples. J Chem 4: 12.
- Cullen WR, McBride BC, Reglinski J (1984) The reaction of methylarsenicals with thiols: Some biological implications. J Inorg Biochem 21: 179-194.
- Styblo M, Drobna Z, Jaspers I, Lin S, Thomas DJ, et al. The role of biomethylation in toxicity and carcinogenicity of arsenic: A research update. Env Health Perspect 110: 767-771.
[Crossref] [Google Scholar] [PubMed]
- Thomas DJ, Styblo M, Lin S (2001) The cellular metabolism and systemic toxicity of arsenic. Toxicol App Pharm 176: 127-144.
[Crossref] [Google Scholar] [PubMed]
- Simeonova PP, Luster MI (2011) Mechanisms of arsenic carcinogenicity: Genetic or epigenetic mechanisms? J Env Pathol Toxicol Oncol 19: 281-286.
[Google Scholar] [PubMed]
- Wang Y, Fang J, Leonard SS, Rao KM (2004) Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic Biol Med 36: 1434-1443.
[Crossref] [Google Scholar] [PubMed]
- Dilda PJ, Hogg PJ (2007) Arsenical-based cancer drugs. Cancer Treat Rev 33: 542-564.
[Crossref] [Google Scholar] [PubMed]
- Valko M, Morris H, MTD C (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12: 1161-1208.
[Crossref] [Google Scholar] [PubMed]
- Martin S, Griswold W (2009) Human health effects of heavy metals. Env Sci Technol Briefs Citizens 15: 1-6.
- Daneman R, Prat A (2015) The blood-brain barrier. Cold Spring Harb Perspect Biol 7: 020412.
[Crossref] [Google Scholar] [PubMed]
- Seaquaris JM, Swiatek J (1991) Interaction of DNA with Pb2+ voltammetric and spectroscopic studies. Bio Bioenerget 26: 15-28.
- Belton JC, Benson NC, Hanna ML, Taylor RT (1985) Growth inhibition and cytotoxic effects of three arsenic compounds on cultured Chinese hamster ovary cells. J Environ Sci Health 20: 37-72.
- Brown RB, Razzaque MS (2018) Phosphate toxicity and tumorigenesis. Biochim Biophys Acta Rev Cancer 1869: 303-309.
[Crossref] [Google Scholar] [PubMed]
- Lin Y, McKinnon KE, Ha SW, Beck GR Jr (2015) Inorganic phosphate induces cancer cell mediated angiogenesis dependent on forkhead box protein C2 (FOXC2) regulated osteopontin expression. Mol Carcinog 54: 926-934.
[Crossref] [Google Scholar] [PubMed]
- Faratian D, Um I, Wilson DS, Mullen P, Langdon SP, et al. Phosphoprotein pathway profiling of ovarian carcinoma for the identification of potential new targets for therapy. Eur J Cancer 47: 1420-1431.
[Crossref] [Google Scholar] [PubMed]
- Shuto E, Taketani Y, Tanaka R, Harada N, Isshiki M, et al. (2009) Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol 20: 1504-1512.
[Crossref] [Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences