ISSN : 0976-8505
Der Chemica Sinica
Abattoir Wastewater Treatment by Electrocoagulation Using Iron Electrodes
Joseph T Nwabanne* and Christopher C Obi
Department of Chemical Engineering, Nnamdi Azikiwe University, Awka, Nigeria
Abstract
This paper presents the results of the treatment of abattoir wastewater by electrocoagulation (EC) process using ironiron electrodes. The electrolytic cell used had a working volume of 500 ml equipped with magnetic stirrer operating at 150 rpm. The effect of various process variables such as initial pH, current intensity, electrolysis time, settling time and operating temperature on removal efficiency of turbidity was investigated. The removal efficiency of 93.69% was achieved at optimum condition of solution pH 2, current intensity 2.5A, electrolysis time of 30 minutes, settling time of 60 minutes, and operating temperature of 60oC at 0.55 kWh/L power consumption. The study of the reaction kinetics showed conformity to a pseudo second order process. The findings were favorable and showed that EC technique using Fe-Fe electrodes is effective in abattoir wastewater treatment.
Keywords
Electrocoagulation, Wastewater, Abattoir, Iron, Turbidity.
Introduction
Environmental pollution is one of the greatest problems that the world is facing presently. This has adversely affected the humans and other species of our biosphere directly or indirectly.
One of the major sources, which wastes the environment are the wastewaters produced from human activities [1]. Wastewater can originate from combination of domestic, industrial, commercial or agricultural activities, surface runoff or storm water, and from sewer inflow or infiltration [2]. Certain wastewater components can degrade water quality and endanger public health. Wastewater is sewage, storm water, and water that have been used for various purposes around the community. Unless properly treated, wastewater can harm public health and the environment.
Abattoir wastewater exhibit high organic and inorganic load, high suspended solids content, dark color and offensive odour. The main sources of organic materials in the wastewater include faeces, urine, blood, fat oil and grease, washings from carcasses/floors/utensils, undigested food from paunch, waste water from cooking/curing/pickling of meat and condensate from rendering of offal and other processing by-products [3]. When red meat processing industry wastewater is discharged to a water course/underground it may lead to a depletion of dissolved oxygen, odour release and sludge deposits and floating scum [4]. Equally, improper disposal systems of wastes from slaughter houses could lead to transmission of pathogens to humans [5].
A variety of wastewater treatment techniques including physical or physicochemical, chemical and conventional biological process are available for the treatment of effluent. The commonly used physico-chemical treatment process is chemical coagulation in which a coagulant is added to the wastewater and allows the formation of flocs for the removal of pollutants. The sludge is separated from the clear water using sedimentation. But chemical coagulation leads to generation of large amount of sludge and sludge disposal becomes a problem. Further, chemical coagulation leads to an increase in total dissolved solids. Electrocoagulation (EC) is an attractive technique for the treatment of effluent in which there is an in-situ generation of coagulant by the dissolution of sacrificial anode. The application of current leads to the generation of hydroxide ions at the anode and hydrogen gas at the cathode. The generation of the hydroxide ions leads to the formation of flocs, which settles the pollutant and can be easily removed by sedimentation. There is formation of gas bubbles, which entrains the lighter pollutants and brings to the surface of the effluent where they can be removed by flotation [6]. This method (EC) is characterized by simple equipment and easy operation.
The EC processes have lesser amount of sludge and it has been successfully used in removal of pollutants from abattoir wastewater. Cast iron (Fe) and aluminum (Al) cylinder were used as anode/cathode pair in the treatment of abattoir wastewater by EC process [7]. 90% COD removal has been reported by Eryuruk et al. [8] in a plug flow electrocoagulation reactor for the treatment of cattle abattoir wastewater. Aluminium electrodes have also been successfully applied in the EC process for the treatment of slaughterhouse wastewater [9].
The present work aims to investigate the performance of the EC process using iron electrodes in the treatment of turbid abattoir wastewater in a batch process. The effect of various process variables such as pH, current intensity, operating time and temperature where also studied.
Mechanism of EC process
The mechanism of EC is extremely dependent on the chemistry of the aqueous medium, especially its conductivity [10]. When a potential is applied from an external power source, the anode material undergoes oxidation, while the cathode is subjected to reduction or reductive deposition of elemental metals [11]. Electrodes which produce coagulants into water are mostly made from either iron or aluminium. In the iron electrode used in the present work, two mechanisms have been proposed [12,13].
Mechanism 1:
Anode: 4Fe(s) → 4Fe+2(aq)+8e (1)
4Fe+2(aq) +10H2O(l)+O2(g) → 4Fe( OH)3(s)+8H+(aq) (2)
Cathode: 8H+(aq)+8e → 4H2(g) (3)
Overall: 4Fe(s)+10 H2O(l)+O2(g) → 4Fe(OH)3(s)+4H2(g) (4)
Mechanism 2:
Anode: Fe(s) → Fe+2(aq)+2e- (5)
Fe+2(aq)+2OH- (aq) → Fe(OH)2 (s) (6)
Cathode: 2H2O(l)+2e- → H2(g) + 2OH-(aq) (7)
Overall: Fe(s)+2H2O(l) → Fe(OH)2(s)+H2(g) (8)
In the aqueous solution the Fe(OH)n(s) formed remains as a gelatinous suspension, which aid the removal of the pollutants from wastewater either by complexation or by electrostatic attraction followed by coagulation.
Materials and Methods
Abattoir wastewater used in this study was collected from a local an abattoir located at Anambra State, Nigeria. The effluent was characterized and the result is presented in Table 1. The characterization of the sample was carried out using standard method [14].
| Parameter | Value |
|---|---|
| pH | 6.8 |
| Conductivity (µs/cm) | 522 |
| Turbidity (NTU) | 330 |
| COD (mg/l) | 1814 |
| BOD5 (mg/l) | 920 |
| TS (mg/l) | 2660 |
| TSS (mg/l) | 1080 |
| Total hardness (mg/l) | 60.31 |
| Sulphate (mg/l) | 13.85 |
| Iron (mg/l) | 2.09 |
| Potassium (mg/l) | 0.1 |
| Magnesium (mg/l) | 57 |
| DO (mg/l) | 2.38 |
| Colour | Dark red |
| Odour | Objectionable |
Table 1: Physico-Chemical properties of abattoir wastewater.
The electro-coagulator has two aluminium plates of dimension (100 mm × 11 mm), one serving as a cathode and the other as anode. The electrode pair previously washed with distilled water was dipped in the wastewater to a depth of 8.0 cm. The total effective area for each electrode was 59.0 cm2 and the spacing between electrodes was 1.0 cm. The Direct Current (DC) power supply of constant 220 V and (0-3 A) capacity was used. For each run, 500 ml of the wastewater sample were placed into the electrolytic cell and a gentle stirring rate of about 150 rpm was applied to allow the chemical precipitate to grow large enough for removal. Conductivity of the solution was increased by the addition of 0.001M Na2SO4. The effect of various process variables such as the initial wastewater pH, current intensity, electrolysis time, settling time and operating temperature was investigated. The pH of the wastewater was adjusted by adding HCl/NaOH. During settling, samples were withdrawn from 2 cm depth and analysed for turbidity which was used for monitoring the removal efficiencies. The efficiency of pollutant removal, R was evaluated using Equation 9.
% Removal=(T0-T/ T0) × 100 (9)
T0 is the turbidity of raw wastewater; T is the turbidity of wastewater after treatment.
Results and Discussion
Characterization of Abattoir Wastewater
The result of the characterization of the wastewater is presented in Table 1. The result showed that the COD and BOD5, which measure the organic load of wastewater, are high. This can be attributed to the fact that abattoir wastewater contains blood and other body fluids, which are mainly organic molecules like proteins, fats and glucose. TS and TSS were also quite high. The colour and the odour of the raw effluent were dark red and objectionable respectively. Hence, the need for the treatment of the effluent prior its discharge. Conductivity of the solution was increased by the addition of 0.001 M Na2SO4 salt to minimize energy consumption in the EC process.
Effect of Initial Solution pH
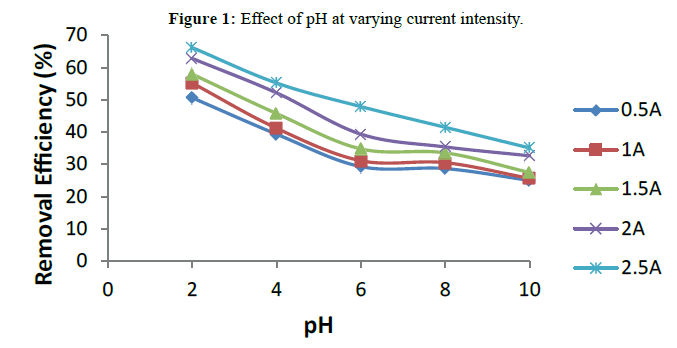
Initial pH of the solution is an important operating factor influencing performance of the EC process. The influence of initial pH on turbidity removal efficiency was studied between initial pH 2-10 at electrolysis time of 15 min, settling time of 30 min and varying current intensity. The pH was adjusted to a desirable value using HCl and NaOH. Figure 1 shows the removal efficiency of turbidity as a function of initial solution pH. The result shows that better removal efficiency is obtained when the solution is in acidic medium as the pollutant removal efficiency increased by decreasing the pH. When the pH was decreased to 2 the removal efficiency increased up to 62.01% under the condition at which pH was investigated and current intensity of 2.5 A. The high percentage of pollutant removal at acidic medium is in agreement with the findings of Bayar et al. [15] and Bayar et al. [9] in similar studies. However, the effluent pH after electro-coagulation treatment was found to increase. The increase of pH at acidic condition was attributed to hydrogen evolution and the generation of OH- ions at the cathodes [7].
Effect and Current Intensity and electrolysis Time
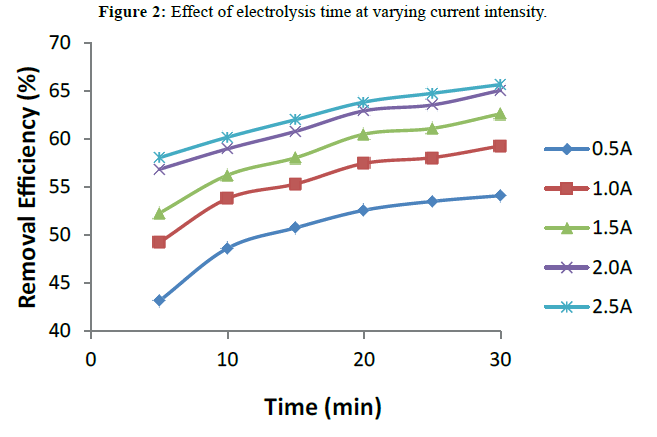
Current intensity and electrolysis time are among the most important parameters that must be considered in the quest to determine the optimum condition for the performance of EC process. The result is shown in Figure 2 Current intensity is important for controlling the reaction rate in most electrochemical processes. It is known that the amount of current density determines the coagulant dosage, and size of the bubble production, and hence affects the growth of flocs [16]. It can be seen in Figure 2 that 2.5A current intensity gave the highest turbidity removal efficiency. Similarly, tt can be noticed that turbidity removal efficiency increases with an increase in electrolysis time though not much significant increase in removal efficiency was witnessed after 20 mins. This is due to the fact that with an increase in the electrolysis time, more ions will be dissolved in the wastewater leading to an increase in flocs formation [6]. It should be noted that the higher the reaction time the more power is consumed. An optimal removal efficiency of 65.65% was obtained at 30 minutes operating time and current density of 2.5 A as shown in Figure 2.
Effect of Settling Time
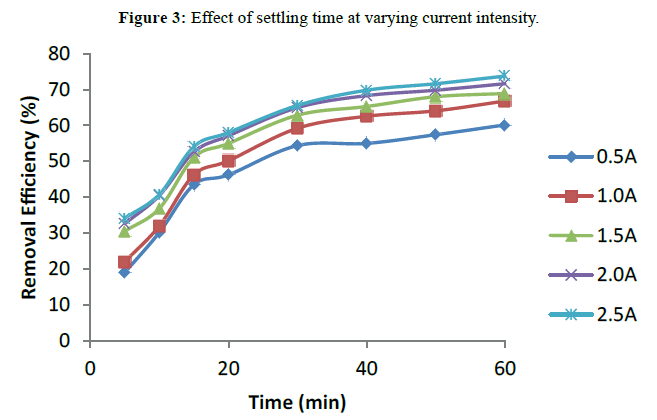
Settling time was examined at varying current intensity to determine the optimum level. The settling time ranged from 5-60 minutes. The time given for the settlement of the flocs was analysed by varying time at optimum pH and electrolysis time and various current intensities. The result is presented in Figure 3. It can be shown in the graph that removal efficiency increased with settling time. The highest degree of settling was witnessed within the first 30 minutes. The optimal removal efficiency of about 73.72% was achieved at current intensity of 2.5 A and settling time of 60 minutes. The sharp increase in removal efficiency with time at the early stage of settling is in accordance with the theory of rapid coagulation proposed by Smoluchowski et al. [17].
Effect of Operating Temperature
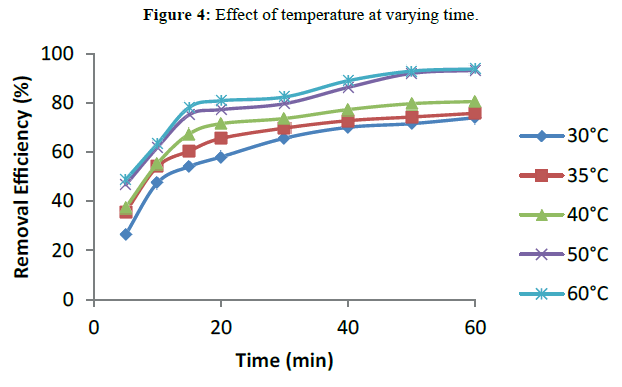
Temperature is another important operating condition that can affect pollutant removal efficiency in wastewater treatment generally. The effect of solution temperature was studied at the range of 30-60ºC. Figure 4 shows that the efficiency of turbidity removal from abattoir wastewater in the EC process increased by increasing solution temperature though the increase between 40 ºC and 60ºC was not very significant. The increase in removal efficiency with temperature in the EC process can be explained by the fact that increasing solution temperature can improve ion transfer from the anode and/or cathode surface to the solution bulk resulting in a decrease in solution viscosity and consequent increase in the ion diffusivity. At the optimum of all other factors, the optimum removal efficiency of 93.69% was obtained at 60ºC.
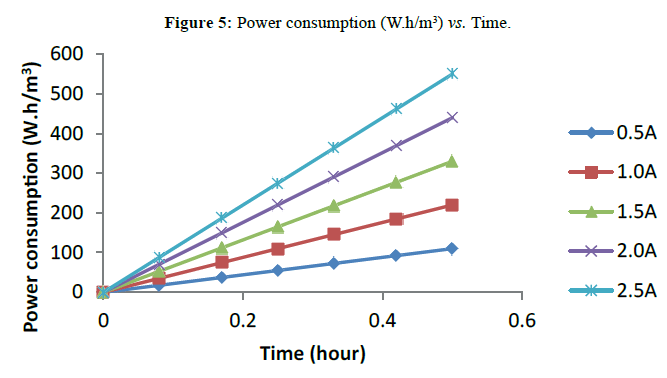
Electrical Power Consumption for the EC Process
The electric power consumption of the process was calculated per m3 of the effluent solution using equation (10);
P=EIt/V (10)
P is the specific power consumption (W.h/m3). E is the cell voltage in volt (V). I is the current in ampere (A), t is the time of electrocoagulation in hour (h) and V is the solution volume in cubic meter (m3). The time of operation is varied between 5 and 30 minutes. It is clear in Figure 5 that increasing the current intensity will increase power consumption. The increase in power consumption can be ascribed to the increased polarization on the two electrodes by increasing the current intensity [18]. At the optimum condition for turbidity removal efficiency, 0.55 kWh/L power consumption was recorded.
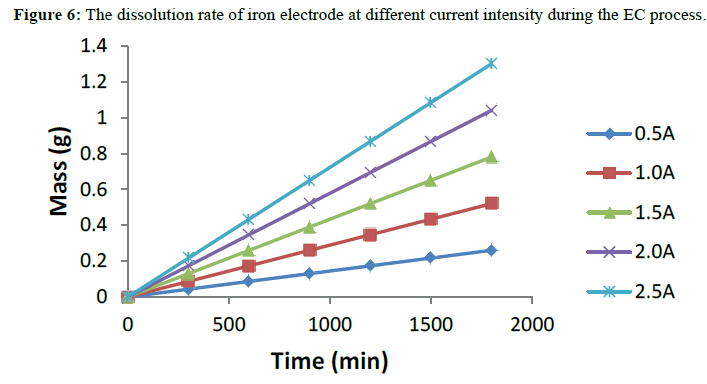
Mass of the Electrodes Dissolved during the Electrocoagulation Process
The theoretically maximum dissolved mass of iron that occur during electrocoagulation process from the sacrificial anode for a specific electrical current flow in an electrolytic cell was calculated using equation (11), [11]
M=I.t.Mr/z.F (11)
m is the amount of the dissolved anode material (g); I is the current (A); t is the electrolysis time (s); Mr is the specific molecular weight of the anode electrode (Fe). The anodic dissolution of iron led to the species Fe2+ (and thus z for iron is 2), this species oxidizes rapidly in air to Fe (III) which has significant coagulation properties [19].
The Mr for iron is 55.85 gmol-1. The data were subsequently fitted in the appropriate kinetic model for performance evaluations. The dissolution rate of the electrode is presented in Figure 6. The result shows that an increase in both time and current intensity resulted in an increased rate of electrode dissolution at the anode for the EC process. The higher dissolution rate is ascribed to the higher molecular weight of iron when compared to other metals like aluminium.
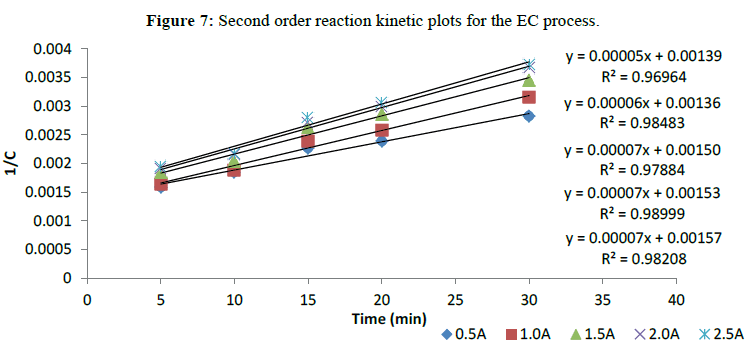
Pseudo-Second-Order Reaction Kinetics for the EC Process
The Pseudo-Second order reaction kinetics for the EC process was evaluated from the removal efficiency of turbidity with respect to time using linear-pseudo second order equation [20-22]. The results obtained for turbidity of sample in NTU, by use of the turbiditimeter, were converted to TSDP (Total Suspended and Dissolved Particles) concentrations (mg/l) by multiplying with factor of 2.35 [20]. Table 2 presents the functional parameters for a linear pseudo–second order reaction obtained in this study. The kinetic parameters such as R2, Co and K contained in the Table 2 were determined from plots I/C versus time as shown in Figure 7. The results presented in Table 2 indicate that 1.5 A, 2.0 A and 2.5 A current intensity has the highest K value while 0.5 A has the least K value. The values of R2 being greater than 0.9000 are satisfactory. It can be observed from the table that the K values didn’t change remarkably as the current intensity varies. This may be attributed to the equal aggregation rate achieved by the varying current intensities.
| Current Intensity | K(l.mg/min) | Rate Equation(mg/l.min) | Co (mg/l) | R2 |
|---|---|---|---|---|
| 0.5A | 5.00E-05 | -r=5 ´ 10-5C2 | 719.42 | 0.9696 |
| 1.0A | 6.00E-05 | -r=6 ´ 10-5C2 | 735.29 | 0.9848 |
| 1.5A | 7.00E-05 | -r=7 ´ 10-5C2 | 666.67 | 0.9788 |
| 2.0A | 7.00E-05 | -r=7 ´ 10-5C2 | 653.59 | 0.99 |
| 2.5A | 7.00E-05 | -r=7 ´ 10-5C2 | 636.94 | 0.9821 |
Table 2: Second order reaction kinetic parameters for the EC process.
The performance of EC process using iron electrodes in treating turbid abattoir wastewater was studied in the present work. The result obtained in the study showed that EC technology can be used to treat abattoir wastewater for safe discharge. Under the operating conditions studied, the performance of the process was improved by lowering the initial solution pH and increasing the electrolysis time, current intensity, settling time and operating temperature.
The removal efficiency of 93.69% was achieved under optimum condition in which solution pH was 2, current intensity 2.5 A, electrolysis time of 30 minutes, settling time of 60 minutes, and operating temperature of 60oC. At the optimum condition for turbidity removal efficiency, 0.55 kWh/L power consumption was recorded. The reaction kinetics study showed that the process conformed to a pseudo-second order kinetic model. The findings of the study show that electrocoagulation technique using Fe-Fe electrodes is effective in abattoir wastewater treatment.
References
- Abdelaal AM (2004) Using a natural coagulant for treating wastewater. 8th International Water Technology Conference, Alexandria, Egypt.
- Tilley E, Ulrich LC, Reymond Ph, Zurbrugg C (2014) Compendium of sanitation systems and technologies, 2nd Revised edn, Swiss Federal Institute of Aquatic Science and Technology (Eawag), Duebendorf, Switzerland, p. 175.
- Hamawand I (2015) Anaerobic digestion process and bio-energy in meat industry: A Review and a Potential. Renew Sust Energ Rev 44: 37-51.
- Kroyer G (1995) Impact of food processing on the environment an overview. Lebensm-Wiss Technol 28: 547-552.
- www.fao.org/docrep/010/ai407e/AI407E26.htm
- Mahajan R, Khandegar V, Saroha AK (2013) Treatment of hospital operation theatre effluent by electrocoagulation. IJCEE 4: 104-107.
- Budiyono, Widiasa IN, Johari S (2010) Study on treatment of Slaughterhouse wastewater by Electro-coagulation technique. Internat J of Sci and Eng 1: 25-28.
- Eryuruk K, Tezcan Un U, Ogutveren UB (2011) Treatment of cattle-slaughterhouse wastewater using tubular electrocoagulator. 2nd International Conference on Chemical Engineering and Applications IACSIT Press, Singapore.
- Bayar S, Yildiz YŞ, Yilmaz AE, Koparal AS (2014) The effect of initial pH on treatment of poultry slaughterhouse wastewater by electrocoagulation method. Desalin Water Treat 52: 3047-3053.
- Ilhan F, Kurt U, Apaydın O, Gonullu T (2008) Treatment of Leachate by electrocoagulation using aluminum and iron electrodes. J Hazard Mater 154: 381-389.
- Vlachou M, Hahladakis J, Gidarakos E (2013) Effect of various parameters in removing Cr and Ni from model wastewater by using Electrocoagulation. Global Nest J 15: 494-503.
- Chaturvedi SI (2013) Electrocoagulation: A novel waste water treatment method. Int J Mod Eng Res Technol 3: 93-100.
- Un TU, Koparal AS, Ogutveren UB (2008) Treatment of slaughterhouse wastewater with iron electrodes. WIT Trans Ecol Envir 111: 545-555.
- APHA (1998) Standard methods for the examination of water and wastewater. 20th edn, USA.
- Bayar S, Yıldız YS, Yılmaz AE, Irdemez S (2011) The effect of stirring speed and current density on removal efficiency of poultry slaughterhouse wastewater by electrocoagulation method. Desalination 280: 103-107.
- Akyol A (2012) Treatment of paint manufacturing wastewater by electrocoagulation. Desalination 285: 91-99.
- Smoluchowski M (1917) Verscuheiner Mathematischen Theory der koagulations kinetic kolloider lousungen. Z Phys Chem 92:129-168.
- El-Shazly, Danous MA (2013) Kinetics and performance of phosphate removal from hot industrial effluent using a continuous flow electrocoagulation reactor. Int J Electrochem. Sci 8:184-194.
- Zongo I, Merzouk B, Palm K, Wethe J, Hama Maiga A, et al. (2012) Study of an electrocoagulation (EC) unit for the treatment of industrial effluent of Ouagadougou, Burkina Faso. Adv Appl Sci Res 3: 572-582.
- Menkiti MC, Igbokwe PK, Ugodulunwa FXO, Onukwuli OD (2008) Rapid coagulation/flocculation inetics of coal effluent with high organic content using blended and unblended chitin derived coagulant (CSC). Research Journal of Applied Sciences 3: 317-323.
- Babayemi KA, Onukwuli OD, Okewale AO (2013) Coag-flocculation of phosphorus containing waste water using Afzella-Africana biomass. Int J Appl Sci Technol 3: 43-50.
- Ani JU, Menkiti MC, Onukwuli OD (2011) Coagulation-flocculation performance of snail shell biomass for waste water purification. N Y Sci J 4: 81-90.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences