A Case of Broken Heart Syndrome after an Intrathecal Pump Refill
Kanchana Gattu* and Luis Chabla-Penafiel
Pain Medicine and Anesthesia, University of Maryland, USA
- *Corresponding Author:
- Kanchana Gattu Pain Medicine and Anesthesia University of Maryland, USA Tel: 410-448-6629 E-mail: kgattu@som.umaryland.edu
Received date: November 30, 2017; Accepted date: February 7, 2018; Published date: February 14, 2018
Citation: Gattu K, Chabla-Penafiel L (2018) A Case of Broken Heart Syndrome after an Intrathecal Pump Refill. J Anaesthesiol Crit Care. Vol 1 No.1:4
Copyright: ©2018 Gattu K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Abstract
We report a case involving a 57 year old female with history of HTN, depression, chronic left knee pain secondary to CRPS (Complex regional pain syndrome type I) since 2000. She had failed conservative therapy with pain medications, physical therapy, acupuncture, lumbar sympathetic blocks, and spinal cord stimulator. She had a Medtronic intrathecal pump implanted in 2005 with a continuous infusion of intrathecal solution, containing bupivacaine 40 mg/mL, Dilaudid 80 mg/mL and clonidine 2 mg/mL. The intrathecal infusion was titrated over time to 14.99 mg/day in combination with oxycodone 10 mg PO every 6 h PRN, Topamax 50 mg every 8 h and intermittent femoral nerve blocks for flare-ups.
Keywords
Takosubo cardiomyopathy; Intrathecal pump; Pain management
Introduction
Takotsubo cardiomyopathy (TTC) is a transient cardiac condition that involves left ventricular (LV) apical akinesis and mimics acute coronary syndrome (ACS). It was first described in 1990 in Japan. “Tako-subo” means “fishing pot for trapping octopus,” because the LV of a patient diagnosed with this condition resembles that shape. A transient entity typically precipitated by acute emotional stress, Takotsubo cardiomyopathy is also called “stress cardiomyopathy” or “broken-heart syndrome.” Although the exact etiology of TTC is still unknown, the syndrome appears to be triggered by a significant emotional or physical stressor with catecholamine release, leading to toxicity and subsequent stunning of the myocardium. It is accompanied by reversible LV apical ballooning in the absence of angiographically significant coronary artery stenosis. Patients often present with chest pain and new ECG abnormalities (either ST-segment elevation and/or T-wave inversion) or modest elevation in the cardiac troponin levels. On cardiac angiography, LV apical ballooning is present, without significant coronary artery stenosis or evidence of acute plaque rupture. On echocardiogram TTC presents with transient hypokinesis, dyskinesis or akinesis of the LV mid segments, with or without apical involvement. The regional wall-motion abnormalities extend beyond a single epicardial vascular distribution [1].
A study by Wittstein et al. [2] looked at 19 patients who presented with chest pain or symptomatic heart failure after experiencing emotional stress, and underwent neurohumoral assessment. Thirteen of the nineteen patients showed that on hospital day 1 or 2, plasma levels of epinephrine, norepinephrine and dopamine were elevated, with levels elevated as much as 7 to 34 times normal values. Plasma levels of catecholamine remained elevated for up to 9 days. This showed that emotional stress can cause a surge in catecholamine for about one week and may not be short-lived [2].
The mechanism of how a sympathetic surge leads to myocardial stunning has not been well described. It has been suggested that sympathetic stimulation can cause epicardial arterial spasms, microvascular spasms or coronary vasoconstriction. Another possible mechanism may involve direct myocyte injury by catecholamines. Catecholamines can cause cyclic AMP-mediated calcium overload in myocytes and are a source of free radicals. These free radicals may then interfere with sodium and calcium transporters [2].
Postmenopausal women over age of 50 are at the highest risk of developing Takotsubo cardiomyopathy. Common stressors that cause TTC include death of a loved one, relationship conflicts, fear, anger and anxiety [3]. Patients usually present with chest pain or general weakness. Diagnosis of TTC can start by an ECG showing ST segment elevation and/or T-wave inversions. Most patients show elevated concentrations of myocardial damage. These include an elevation in brain natriuretic peptide, creatinine kinase MB fraction, and troponin I. When a transthoracic echocardiogram (TTE) is performed about 94% of patients will show the classical pattern of TTC. However, about 6% of patients can show an “inverted” pattern [3]. When diagnosed, the mean left ventricular ejection fraction (LVEF) is between 30 and 35%, but improves to greater than 50%. With a decreased LVEF, TTC can also cause mild mitral valve regurgitation. In considering a diagnosis of TTC, it is important to note that about 85% of patients may only show at most mild coronary artery disease on a coronary angiogram. The prognosis in TTC is typically excellent, with nearly 95% of patients experiencing complete recovery within 4-8 weeks. There are many reports of different inciting events that have led to TTC.
A case report by Sarcon et al. [4] describes TTC after opiate withdrawal. He presents a situation where a 60-year old woman with chronic pain presented with signs and symptoms of opioid withdrawal after she ran out of her MS Contin for several days. In the emergency department, she developed chest pain and an ECG was performed that showed ST-segment elevation. After a negative cardiac catherization, and an echocardiogram with findings of left ventricle apical ballooning pattern, basal hypercontractility, and reduced LV ejection fraction, she was diagnosed with TTC. It was suspected that opioid withdrawal caused a hyperadernergic state that led to TTC. Furthermore, Sarcon et al. [4] identified seven more cases of drug-withdrawal associated TTC and suggest that it is more common than reported and underdiagnosed.
Patients with chronic pain are not only managed with oral opioids, but they can also have intrathecal pumps that deliver opioids. If the intrathecal pump malfunctions, there is the potential for opioid withdrawal or overdose. There is lack of evidence or peer reviewed literature investigating the occurrence of Takotsubo cardiomyopathy after an intrathecal pump refill or malfunction. We present a case that appears to be the only report of Takotsubo cardiomyopathy after an intrathecal pump refill.
Case Report
The patient had successful, uneventful refill cycles for the intrathecal pump at the clinic every 3 months for 10 years. Pump refill was done using a Medtronic refill kit and filled in the same manner as the previous years. However, on a subsequent refill, the patient had complained of itching at the site of injection. On examination, no hives were observed, vital signs were stable, and patient was sent home on an antihistamine and advised to go to the ER if symptoms worsened. After leaving the clinic, she called to inform us that following the pump refill she had to be taken to the hospital for complaints of drowsiness. In the ER, they treated her with naloxone for presumed opioid overdose, which caused her to go into withdrawal.
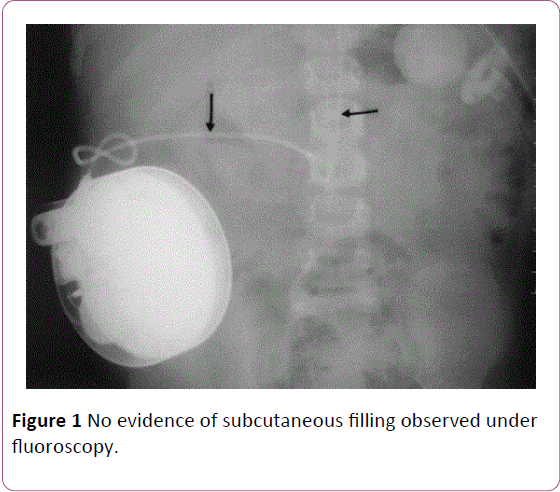
After the patient recovered she was bought back to our practice for reprogramming. A shuntogram was performed, which did not show any evidence of leak. The pump contents were also aspirated, which correlated with the amount of injectate used. We started weaning the patient off of the pump by decreasing therapy by 10% every visit. However, on subsequent refills, the patient experienced similar symptoms of mild pruritus and dizziness. To further investigate the cause, we went back to aspirate the contents under fluoroscopy, which showed no evidence of subcutaneous filling (Figure 1). She was observed and discharged once stable and without symptoms.
After her third weaning session, the patient reported that on the following day she went back to the ER, complaining of chest pain and fatigue. In the ER, she was worked up for ACS. She underwent cardiac catheterization and echocardiogram, which led to a diagnosis of TTC. She was then treated with medications that reversed myocardial changes. However, her pain complaints were unchanged, including a slight increase in pain level.
We continued to wean the patient off the opiate medications by 10% at each refill, followed by explanation of the pump. During the removal of the intrathecal pump, a small tear was found in the catheter, proximal to the entry into the intrathecal space; and which had not been detected during previous studies. This may have caused the pump to malfunction. After the removal of the intrathecal pump, the patient's pain was managed with fentanyl patch 50 mcg/h, tramadol 50 mg every 8 h and oxycodone 10 mg every 6 h PRN. Patient continues to do well without further admissions for Takotsubo cardiomyopathy and continues to be monitored by her cardiologist. The changes in the myocardium were also reversible without any permanent sequelae.
Discussion
Patient is a 57 year old with insignificant past medical history, undergoing a series of intrathecal pump refills for several years, complicated by development of MI secondary to diagnosis of TTC during one of her routine refills for her intrathecal pump [5]. Although the exact mechanism is unclear, the possibility of stress induced at the time of refill and naloxone in the ER may have predisposed the patient to an MI. The stress during pump refill and down titration was not initially considered significant because it was a routine procedure that the patient had undergone several times before. However, in this case it may have been emotionally significant as the patient anticipated the changes to her intrathecal medication dose. It is well known that significant emotional or physical stressors with catecholamine release can lead to toxicity and subsequent stunning of the myocardium, and development of an MI.
It is not clear why our patient experienced pruritus and dizziness after intrathecal pump refill. It may have been a sign of overdose. However, after the patient returned to our pain practice, studies were conducted to rule out incorrect filling volume, subcutaneous filling, and damage to pump mechanisms. There are several possible complications with intrathecal pumps. These complications maybe due to human error, medication error, or mechanical system failure. Other causes of pump complications are an inflammatory mass, microfracture, kinking, pinhole leakage, migration into the epidural space or out of the spine, and tip fibrosis or granuloma. The most common causes of intrathecal pump failure are a change in performance (battery depletion, motor failure) and failure of catheter. Of catheter malfunction, catheter kinking and perforation represented 50% of cases. This can result in delivering lower amounts of the drug, or depositing drug into a pocket or subcutaneous tissue. If this were to happen, the patient may show signs or symptoms of withdrawal, but in some cases it may be subtle. Overall, the frequency of intrathecal pump complications is decreasing [6].
There are several important issues to note when investigating pump failures. A kink in the catheter may decrease the total infusion volume and increase the reservoir volume. Therefore, at the time of pump re-fill, it is important to investigate the expected residual volume. A fractured, dislodged, or disconnected catheter can be seen on plain x-ray or fluoroscopy. Some pumps may be MRI compatible and an MRI study can detect masses at the catheter tip, epidural hematomas, abscess, while also verifying the location of the catheter in the intrathecal space. If an MRI study is conducted, the physician should be aware that the MRI magnet does stop the roller function and drug withdrawal precautions would need to be taken.
Another method of testing the pump integrity is with the catheter access port aspiration. It is recommended that one first aspirate 1.5 ml to ensure that the catheter is free of medications. If 2-3 ml of CSF follows, then this maybe a sign that the catheter top is in the CSF. If aspiration is difficult, it may be a sign that there is a clot or a kink in the catheter. A small hole in the catheter will allow CSF to flow without resistance; a larger hole will cause interruptions in CSF flow. After aspiration of CSF, one can further evaluate the catheter by injecting contrast. A pitfall of this method is that the contrast will not be able to detect small holes. The current methods of testing the integrity of intrathecal pumps are not without fault. In our patient we performed several of the above tests to investigate the etiology of her symptoms, but were negative [7].
It is also unclear why this patient developed repeated dizziness after subsequent refills. It may be due to the fact that the studies that were conducted failed to detect any pump malfunction in this particular case or there was a change in drug pharmacodynamics or pharmacokinetics. All of these events could have caused great emotional stress, with the igniting event of opioid withdrawal after naloxone. These repeated stressors associated with intrathecal pump refill may have led to TTC.
This case may show that the development of TTC after intrathecal pump malfunction. There are no previous case reports, which limit our knowledge on the incidence and pathophysiology of TTC and intrathecal pump malfunction. Moreover, there is very little literature that has studied the association of opioid withdrawal and the development of TTC. Therefore, there is no reported incidence and morbidity related to opioids and TTC. There is a need to investigate the relationship of opioids and TTC.
Conclusion
Without a prior history of CAD or chronic comorbidities, a MI after an intrathecal pump refill can be an unanticipated event. Development of Takotsubo cardiomyopathy (TTC) after a pump refill is not well known. It may be associated with significant emotional or physical stressors with catecholamine release, resulting in toxicity and subsequent stunning of the myocardium.
TTC has important implications because it may cause a decrease in ejection fraction and it can lead to other morbidities. Its clinical presentation mimics that of an acute coronary syndrome and the diagnosis is not straightforward. Identification of TTC in patients with intrathecal pumps may be facilitated with close monitoring and patient education of adverse events and signs and symptoms of ACS. This may prompt the patient seek assistance earlier, and may prevent adverse sequelae.
The prognosis after a diagnosis of TTC is good, with a mortality rate of 17.2% [6]. Increased awareness of Takotsubo cardiomyopathy will likely increase the frequency of diagnosis. However, more studies are needed in order to make more accurate determination of the incidence of Takotsubo cardiomyopathy, and its association with intrathecal pump therapy and opioid withdrawal. Studies are also needed to elucidate the specific pathophysiologic mechanisms responsible for this cardiomyopathy and its long-term outcomes [8].
References
- Marmoush FY, Barbour MF, Noonan TE, Al-Qadi MO (2015) Takotsubo cardiomyopathy: A new perspective in asthma. Case Rep Cardiol, p: 640795.
- Wittstein IS, Thiemann DR, Lima JAC, Baughman KL, Schulman SP, et al. (2005) Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 352: 539-548.
- Glaveckaite S, Serpytis P, Peciuraite D, Puronaite R, Valevicine N (2016) Clinical features and three-year outcomes of Takotsubo (stress) cardiomyopathy: Observational data from one center. Hellenic J Cardiol 57: 428-434.
- Sarcon A, Ghadri JR, Wong G, Luscher TF, Templin C, et al. (2014) Takotsubo cardiomyopathy associated with opiate withdrawal. QJM 107: 301-302.
- Virani SS, Khan AN, Mendoza CE, Ferreira AC, de Marchena A (2007) Takotsubo cardiomyopathy or broken-heart syndrome. Tex Heart Inst J 34: 76-79.
- Jones RL, Rawlins PK (2005) The diagnosis of intrathecal infusion pump system failure. Pain Physician 8: 291-296.
- Kurisu S, Kihara Y (2014) Clinical management of Takotsubo cardiomyopathy. Circ J 78: 1559-1566.
- https://emedicine.medscape.com/article/1513631-overview
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences