Side Effects of Sodium Valproate in a Tunisian Population
Makhlouf S1*, Mansour M1, Msselmeni M1, Bedoui I1, Zaouali J1, Mrissa R1, Siala K2, Btiri S2 and Kacem W3
1Department of Neurology, Military Hospital ot Tunis, Tunis, Tunisia
2Deparment of Pharmacology, Military Hospital ot Tunis, Tunis, Tunisia
3Department of Physiology, Faculty of Medicine of Tunis, Tunis, Tunisia
- *Corresponding Author:
- Makhlouf S
Military Hospital of Tunis
Department of Neurology, Tunis, Tunisia
Tel: +21671391133
E-mail: sinda.makhlouf@yahoo.fr
Received Date: November 04, 2017; Accepted Date: April 09, 2018; Published Date: April 19, 2018
Citation: Makhlouf S, Mansour M, Msselmeni M, Bedoui I, Zaouali J, et al. (2018) Side Effects of Sodium Valproate in a Tunisian Population. J Nerv Syst Vol.1 No.2:8
Abstract
The epilepsy care was marked in 1967 by the discovery of Sodium Valproate, a broad spectrum molecule, which finds its indication both in focal and generalized epileptic seizures. The objective of our work was to highlight and study the clinical side effects inducted by Sodium Valproate in a Tunisian population. A questionnaire was filled for each patient. This permitted us to collect the following information: age, sex, pathological history, therapeutic modalities, the other associated medicines and the different adverse effects reported by patients. We included the epileptic patients treated by Sodium Valproate in monotherapy since at least one year with a good patient’s compliance and a rate of Sodium Valproate in the therapeutic range. We excluded the patients taking other antiepileptic medicines in association with Sodium Valproate; children and newborns unable to respond individually to the questionnaire and patients whose files are incomplete. Seventy four patients were included in the study. The average age was 38.9 years. Sex ratio was 1:7. The side effects observed were: Behavioral disorders (58%) Headache (55%) Weight gain (43%) Slowness of execution of movements (20%) Tremor (34%) Gastrointestinal disorders (35%) Memory disorder (34%) Endocrine disorders (12%). Sodium Valproate remains the reference molecule in the treatment of epilepsy especially in developing countries. Although it is a previously used molecule, the side effects of Sodium valproate still attract interest today. Medical profession should focus its attention on the potentially embarrassing or serious side effects of Sodium Valproate.
Keywords
Sodium valproate; Epilepsy; Side effects
Introduction
Epilepsy is a disease of the brain defined by any of the following conditions: at least two unprovoked (or reflex) seizures occurring >24 h apart or one unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (atleast 60%) after two unprovoked seizures, occurring over the next 10 years/or diagnosis of an epilepsy syndrome [1]. The epilepsy care was marked in 1967 by the discovery of Sodium Valproate (VPA), a broad spectrum molecule, which finds its indication both in focal and generalized epileptic seizures, both in adults and children. The effectiveness of Valproate Sodium is not limited to epilepsy since it is also indicated for the treatment of other pathologies such as migraine, neuropathic pain or mood disorders. The most common adverse effects of Sodium Valproate consist mainly of gastrointestinal effects, weight gain and hair loss. The objective of our work was to highlight and study the clinical side effects inducted by Sodium Valproate in a Tunisian population.
Methods
We realized a prospective and descriptive study in the neurology department of the military hospital of Tunis over a period of six months.
A questionnaire was filled for each patient. This permitted us to collect the following information: age, sex, pathological history, habits, therapeutic modalities (duration of treatment and its dosage), the other associated medicines and the different adverse effects reported by patients.
We included the epileptic patients treated by Sodium Valproate in monotherapy since at least one year with a good patient’s compliance and a rate of VPA in the therapeutic range (50- 100 mg/l). We excluded the patients taking other antiepileptic medicines in association with Sodium Valproate; children and newborns unable to respond individually to the questionnaire and patients whose files are incomplete.
Ethics
Oral informed consent was obtained from each patient and all procedures performed in the study were in accordance with the ethical guidelines of the "World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects" adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964 and amended by the 59th WMA General Assembly, Seoul, South Korea, October 200.
Statistics
Data entry and its statistical analysis were made thanks to SPSS software and excel. The corresponding results and graphs were generated by this same software.
Result
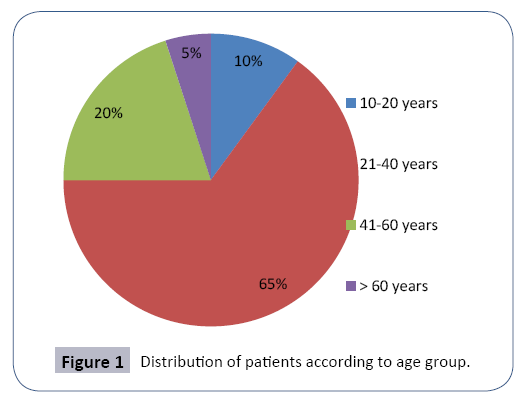
Seventy four patients were included in the study. The average age was 38.9 years. The distribution of patients according to age groups of 20 years is shown in Figure 1. Sixty four percent were aged between 21 and 40 years whereas only 4 patients were older than 60 years. We noted a male predominance with a sex ratio of 1.7 (47 men and 27 women).The treatment duration by Sodium Valproate in our patients varied from 1 to 18 years. The dosage of Sodium Valproate varied from 1000 mg to 3000 mg/ day.
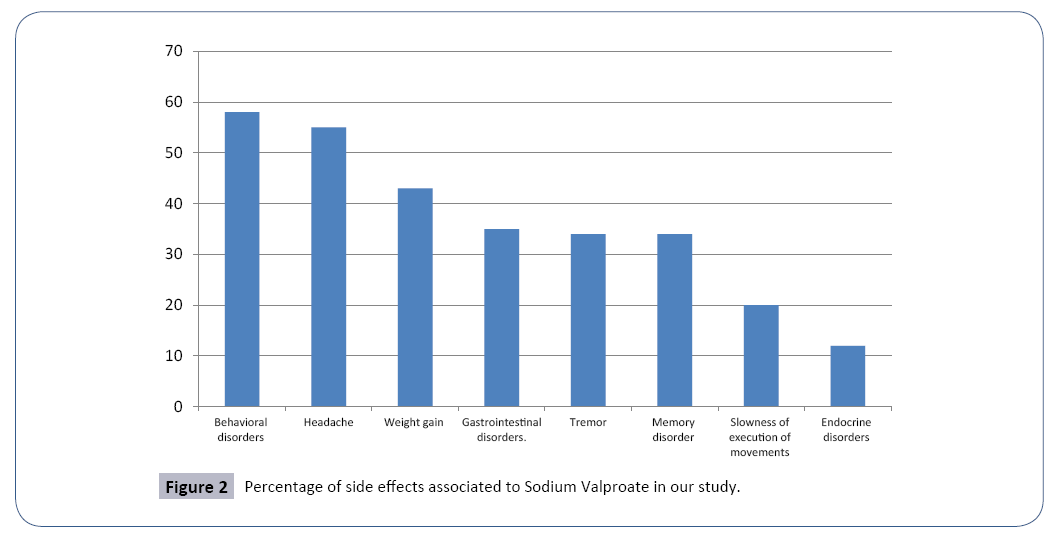
The side effects observed in the studied population were: Behavioral disorders (aggressiveness and agitation) (58%); Headache (55%); Weight gain (43%); Gastrointestinal disorders (35%); Tremor (34%). Memory disorder (34%); Slowness of execution of movements (20%); endocrine disorders (dysmenorrhea, hirsutism) (12%) (Figure 2).
Side effects such as behavioral disorders, headache and gastrointestinal troubles were more important during the first years of treatment in particular the first four years following the initiation of the treatment and tend to diminish thereafter; in contrast to memory disorders, tremor, weight gain and slowness of execution of movements that worsened in parallel with the duration of treatment.
Discussion
Sodium Valproate acts by blocking voltage-gated calcic and sodic ion channels. The blocking of these channels allows raising the threshold of excitability of the cell and prevents thus the release of excitatory neuromodulators such as glutamate. Sodium Valproate has a quick digestive absorption, an extensive plasma protein binding (90%) and a half-life going from 8 to 10 hours. It follows a hepatic metabolism via the cytochrome P450. Although it is a previously used molecule, the side effects of VPA still attract interest today. VPA remains the reference molecule in the treatment of epilepsy especially in developing countries such as Tunisia.
In our series; behavioral disorders were found in 58% of our patients, which represent the most frequent side effect. This concurs with the study performed in 2013 by Glauser et al.: Behavioral and/or psychiatric disorders were the most frequent side effects in a pediatric population treated by Sodium Valproate for newly diagnosed absence epilepsy. These behavioral disorders even led to treatment discontinuation in certain patients [2].
But it seems, however, that Sodium Valproate has a good behavioral profile compared to other antiepileptics in particular Phenobarbital and carbamazepine.
Paroxysmal headache moderate in severity was found in 55% of our patients. This headache tended to diminish or disappear in parallel with the treatment duration. The headaches were reported by some authors in association with the treatment by Sodium Valproate but they don’t seem to be one of the most important side effects [3,4]. The weight gain, observed in 43% of our patients, is indeed one of the principal side effects linked to Sodium Valproate. Many mechanisms are mentioned in order to explain this weight gain: [5,6].
• Direct stimulation of pancreatic beta cells.
• Insulin resistance by the suppression of the absorption of peripheral glucose.
• Increased levels of postprandial insulin.
• Increased levels of LDL cholesterol with decrease in the levels of HDL.
Tremor was also objectified in almost one-third of our patients. It was postural tremor. This side effect was identified by several studies, especially after the long-lasting treatment by Sodium Valproate (>6 month).
Twenty percent of our patients reported slowness of execution of movements with an extrapyramidal syndrome at the examination [7-9].
In a literature review published by Brugger in 2016, a total of 116 patients presenting a secondary parkinsonism associated to Sodium Valproate were identified [10]. The prevalence of Parkinsonism varied from 1.4 to 75% of patients treated by Sodium Valproate. The Parkinsonian syndrome was reversible in the majority of patients. A dopaminergic deficit was found in 3 patients.
Several pathophysiological mechanisms, including altered gene expression and neurotransmitter signaling, enhanced neurodegeneration or epileptic unmasking subclinical dopaminergic degeneration could explain parkinsonism in patients treated with Sodium Valproate [10].
VPA can alter cognitive function [11,12] when its concentrations increase, it can induce attentional or memory disorders, damage to visuo-spatial functions, to decision-making or speed of information processing. However, VPA seems better tolerated cognitively than phenobarbital and carbamazepine [12].
Gastrointestinal disorders due to VPA are manifested especially through ulcerous pains in one-third of the cases; followed by nausea and/or vomiting which are often transitory and appear especially at the beginning of treatment. At a lower frequency, we report disturbance of bowel mobility of constipation type or rarely diarrhea [13].
In our study; 7% of female patients presented dysmenorrhea and 5% presented hirsutism. A prospective study, led over two years by Hadassa Goldberg-Sternr et al. [14,15], was interested in endocrine side effects linked to VPA. In fact, they found that a long-term treatment by VPA in boys has no effects on the endocrine system; while in girls, it induces an increase the levels of androgens leading to hormonal disorders of dysmenorrhea type and hirsutism. This is explained by pharmacokinetic properties of VPA. This is indeed an enzyme inhibitor; it reduces the conversion of testosterone into estradiol increasing thus blood concentration in testosterone [16].
The goal of our work was to highlight the side effects of VPA and their frequency in the Tunisian population. with the aim that these adverse effects are systematically sought. The limits of our study are principally the limited number of patients and the fact that we looked for clinical and not biological side effects.
Conclusion
Despite the discovery of numerous new generation antiepileptic molecules, VPA remains the reference molecule in the treatment of epilepsy in developing countries. However, the medical profession should focus its attention on the potentially embarrassing or serious side effects of VPA.
References
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, et al. (2014) ILAE official report: a practical clinical definition of epilepsy. Epilepsia 55: 475-482.
- Glauser TA, Cnaan A, Shinnar S, Hirtz DG, Dlugos D, et al. (2013) Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy: initial monotherapy outcomes at 12 months. Epilepsia 54: 141-155.
- Nanau RM, Neuman MG (2013) Adverse drug reactions induced by valproic acid. Clin Biochem 46: 1323-1138.
- Viteri C, Codina M, Cobaleda S, Lahuerta J, Barriga J, et al. (2010) Quality of life and treatment satisfaction in Spanish epilepsy patients on monotherapy with lamotrigine or valproic acid. Seizure 19: 432-438.
- Stephen LJ, Kwan P, Shapiro D, Dominiczak M, Brodie MJ (2001) Hormone profiles in young adults with epilepsy treated with sodium valproate or lamotrigine monotherapy. Epilepsia 42: 1002-1006.
- Verrotti A, la Torre R, Trotta D, Mohn A, Chiarelli F (2009) Valproate-Induced Insulin Resistance and Obesity in Children. Horm Res 71: 125-131.
- Yu P, Zhu G, Wu X, Li T, Xu L, et al. (2011) A 6-month prospective study on efficacy safety and QOL profiles of extended-release formulation of valproate in patients with epilepsy. Seizure 20: 23-26.
- Terbach N, Williams RSB (2009) Structure-function studies for the panacea, valproic acid. Biochem Soc Trans 37: 1126-1132.
- Arbaizar B, Gómez-Acebo I, Llorca J (2008) Postural induced-tremor in psychiatry. Psychiatry Clin Neurosci 62: 638-645.
- Brugger F, Bhatia KP, Besag FMC (2016) Valproate-Associated Parkinsonism: A Critical Review of the Literature. CNS Drugs 30: 527-540.
- Masmoudi K, Gras-Champel V, Bonnet I, Pannier M, Masson H, et al. (2000) [Dementia and extrapyramidal problems caused by long-term valproic acid. Therapie 55: 629-634.
- Aldenkamp AP, Baker G, Mulder OG, Chadwick D, Cooper P, et al. (2000) A multicenter, randomized clinical study to evaluate the effect on cognitive function of topiramate compared with valproate as add-on therapy to carbamazepine in patients with partial-onset seizures. Epilepsia 41: 1167-1178.
- Charfi R, Lakhal M, Klouz A, Trabelsi S, Salouage I (2015) Therapeutic Drug Monitoring of Valproic Acid in Children: A Prospective Study of The Effect of The Compliance and The Economic Level on the Trough Plasmatic Concentrations and Epileptic Seizures. Therapie 70: 415-424.
- Goldberg-Stern H, Yaacobi E, Phillip M, De Vries L (2014) Endocrine effects of valproic acid therapy in girls with epilepsy: A prospective study. Eur J Paediatr Neurol 18: 759-765.
- Goldberg-Stern H, Itzhaki T, Landau Z, De Vries L (2015) Endocrine Effects of Valproate versus Carbamazepine in Males with Epilepsy: A Prospective Study. Horm Res Paediatr 83: 332-339.
- Striano P, Belcastro V (2017) Update on pharmacotherapy of myoclonic seizures. Expert Opin Pharmacother 18: 187-193.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences