ISSN : 0976-8505
Der Chemica Sinica
L-Tyrosine Catalysed Mild and Efficient Synthesis of Dihydropyrano[2,3c] pyrazole under Microwave Irradiation
Balaji D Rupnar1*, Vijay P Pagore2, Sunil U Tekale2, Suresh U Shisodia2 and Rajendra P Pawar2
1Department of Chemistry, R.B.Attal College, Georai Dist. Beed, Maharashtra, India
2Department of Chemistry, Deogiri College, Aurangabad, Maharashtra, India
Abstract
A highly efficient and environmentally benign protocol has been developed for the construction of dihydropyrano[2,3-c] pyrazole derivatives through one-pot, multi-component cascade reaction of various aldehydes, acetoacetic ester, hydrazine hydrate and malononitrile under microwave irradiation using L-Tyrosine as catalyst in the presence of green solvent medium (ethanol-water) is described. The significant feature of this protocol is the use of ethanol-water as a green solvent system.
Keywords
Multicomponent reaction, L-Tyrosine, Microwave, Green solvent, Pyranopyrazole
Introduction
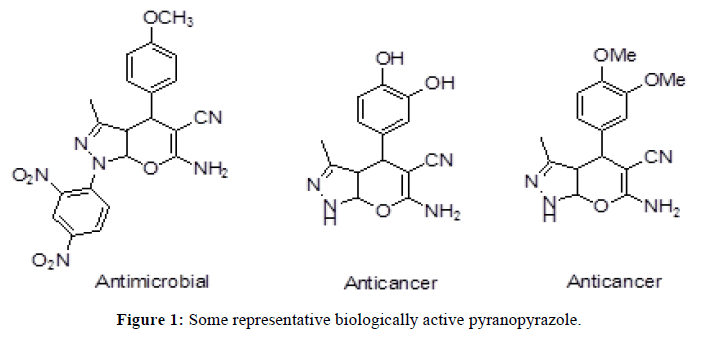
Oxygen and Nitrogen containing heterocycles, pyrans and pyran-annulated derivatives, pyrazoles and pyrano [2,3- c]pyrazoles are commonly found as essential subunits of a wide range of biologically active natural compounds. Functionalized pyran is an important class of heterocyclic compounds due to their wide range of biological activity. Among these, 4H-pyran has been associated with a wide range of pharmacological properties, such as anticancer, antioxidant, antibacterial, anti-inflammatory and anticholinesterase activities [1]. The pyranopyrazole derivative is source of biologically important scaffolds because of their wide application in pharmaceuticals and in organic synthesis. These moieties showed anti-cancer [2], anti-inflammatory [3], anti-microbial [4], fungicidal and molluscicidal [5] and analgesic [6] properties. They are also find applications in biodegradable agrochemicals [7]. They also serve as potential inhibitors of human Chk1 kinase [8-10]. Apart from this, pyrano[2,3-c]pyrazoles have been used as potential insecticide [1]. Some representative biologically active pyranopyrazole are shown in Figure 1.
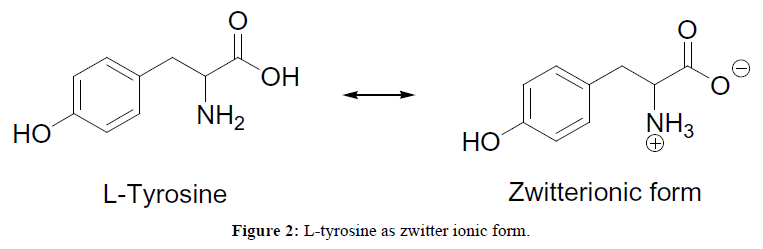
Microwave irradiation (MW) has emerged as effective heating source for organic synthesis due to shorter reaction times, uniform and selective heating, higher yields, cleaner reactions, easy work up [11]. Recently, Microwave-assisted organic synthesis has become a significant tool for accelerating drug discovery and development processes [12-14]. For this process, we have selected L-tyrosine organo catalyst. The choice of L-tyrosine is based on a fact that it is an efficient, bi-functional, zwitter ionic (Figure 2) and eco-friendly organo-catalyst; capable in playing multiple catalytic roles as an acid and base. Though L-tyrosine used in psychiatric disorder, schizophrenia [15], its catalytic activity in various organic transformations is till unnoticed. Very few report of the catalytic activity of L-tyrosine was reflected in its application such as Bigenelli reaction and Knoevenagel condensation reaction under grindstone condition [16]. Recently Khaskel et al. [17] used L-Tyrosine loaded nanoparticle for the synthesis of Bis-coumarin and Hantzsch dihydropyridines.
Multicomponent reactions (MCRs) significantly accelerate chemical synthesis, converting three or more components into complex molecules via simple one-pot routes. Multicomponent reactions are advantageous over multistep reaction such as high atom economy, short reaction times, high yields, low costs and minimization of waste, labor, energy and avoidance of complex or tedious processes. These reactions are valuable assets in organic synthesis and pharmaceutical chemistry due to their wide range of usage in the preparation of various scaffolds and discovery of new drugs [18]. With the growing concern over environment pollution and related societal health problems, green chemistry concept is emerging as one of the important tools in the development of environmentally benign chemical processes and clean technologies. Considering the great importance of pyranopyrazoles, various multicomponent reactions for the synthesis of 6-amino-5-cyano dihydro-pyranopyrazoles has been reported [19-22].
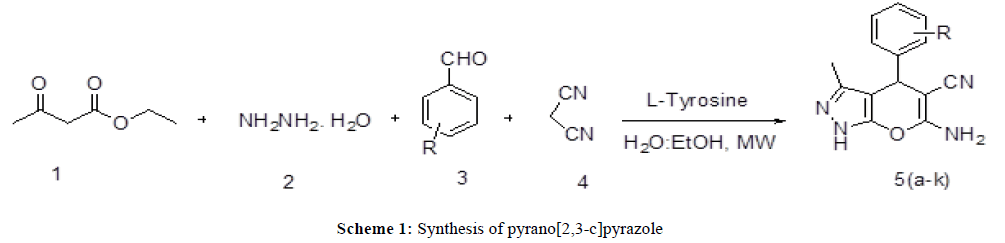
The present study describes a mild, efficient and environmentally friendly L-Tyrosine catalyzed one-pot fourcomponent condensation of acetoacetic ester, hydrazine hydride, aldehydes, and malononitrile in water:ethanol medium to afford pyrano[2,3-c]pyrazole derivatives in high yields (Scheme 1).
Materials and Methods
FTIR spectra were recorded on a Perkin-Elmer FTIR spectrophotometer 65 as KBr pellets and the absorption expressed in cm-1. 1H NMR spectra were recorded in CDCl3 or DMSO on 400 MHz FT-NMR spectrometer at 25oC with tetramethylsilane (TMS) as the internal standard, and resonances (δ) are given in ppm. Data are reported as follows: chemical shift (d), multiplicity (s=singlet, d=doublet, t=triplet, m=multiplet), coupling constants (Hz), and integration. Melting points of the products were recorded in capillaries open at one end and were uncorrected using an Electrothermal Mk3 apparatus. All experiments under microwave irradiation were carried out in microwave synthesis system 700W model manufactured by RAGA’s Scientific Microwave Synthesis System Pvt. Ltd; Pune, India has a maximum power output of 700W and 2450 MHz frequency. Thin layer chromatography (TLC) was performed using Merck pre-coated silica gel and the components were visualized under a UV or an iodine chamber.
Experimental study
General Procedure for the synthesis of pyrano[2,3-c]pyrazole (Scheme 1): In a capped 10 mL MW-vessel charged with aromatic aldehyde (1 mmol), malononitrile (1 mmol), acetoacetic ester (1 mmol), hydrazine hydrate, L-Tyrosine and water:ethanol (5 ml). The tube was positioned in irradiation cavity and the mixture was irradiated in monomode microwave oven (210 W) for specific time. The progress of reaction was monitored by TLC in ethyl acetate: hexane (1:4). After completion of reaction, the mixture was cooled to room temperature and poured onto 5 mL ice cold water. The separated solid was filtered and washed with water several times. The residue was dried and recrystallized from ethanol to afford corresponding pyrano [2,3-c]pyrazole (5a-k). The products were confirmed by comparisons of their melting points with authentic samples and spectral data such as IR, 1H NMR. The filtrate was heated over burner to evaporate water:ethanol content and the residual catalyst was used for second cycle of reaction.
Spectral Data
6-amino-1,4-dihydro-3-methyl-4-(4-nitrophenyl)-1-phenylpyrano[2,3-c]pyrazole-5-carbonitrile (5b): Melting point: 250-252oC. IR (KBr) cm-1: 3380 (NH2), 3183 and 2958 (C-H), 2188 (CN), 1674, 1635, 1604 (C=C), 1491, 1405, 1364, 1246, 1216. 1H NMR (DMSO-d6, 400 MHz, δ ppm): 12.10 (s, 1H, NH), 8.11 (d, 1H, J=8.2 Hz, Ar- H), 8.01 (s, 1H, Ar-H), 7.64 (m, 2H, J= 8.4 Hz, Ar-H), 6.84 (s, 2H, NH2), 4.80 (s, 1H, CH), 1.83 (s, 3H, CH3).
6-amino-4-(4-bromophenyl)-1,4-dihydro-3-methyl-1-phenylpyrano[2,3-c]pyrazole-5-carbonitrile (5e).:
Melting point: 178-180oC. IR (KBr) cm-1: 3405 and 3332 (NH2), 3203 (NH), 2199 (CN), 1692, 1650, 1607 (C=C), 1519, 1346, 1210. 1H NMR (DMSO-d6, 400 MHz, δ ppm): 11.97 (s, 1H, NH), 7.45 (d, 2H, J=8.6 Hz, Ar-H), 7.12 (d, 2H, J=8.4 Hz, Ar-H), 6.59 (s, 2H, NH2), 4.55 (s, 1H, CH), 1.82 (s, 3H, CH3).
6-amino-3-methyl-4-(4-chloro-phenyl)-2,4-dihydro-pyrano-[2,3-c]pyrazole-5-carbonitrile (5d).
Melting point: 230-232oC. IR (KBr): ν 3408, 3234, 3178, 2190, 1642, 1597, 1508, 1490, 1401, 1264, 1091, 1014, 806, 745; 1H NMR (300, CDCl3): δ 1.82 (s, 3H), 4.59 (s, 1H), 6.80 (s, 2H), 7.88 (s, 1H), 8.21 (d, J=8.4 Hz, 1H), 7.20-7.53 (m, 2H), 12.06 (s, 1H).
6-amino-3-methyl-4-(3-nitro-phenyl)-2,4,dihydro-pyrano-[2,3-c]pyrazole-5-carbonitrile (5c)
Melting point: 2214-212oCIR (KBr): ν 3473, 3224, 3117, 2195, 1652, 1525, 1491, 1400, 1349, 805, 733 cm−1; 1H NMR (300, CDCl3): δ 1.82 (s, 3H), 4.82 (s,1H), 6.92 (s, 2H), 7.59-7.81 (m, 2H), 8.01 (s, 1H), 8.09(d, J=8.4 Hz, 1H), 12.13 (s, 1H).
Results and Discussion
Initially for optimization of reaction we chose p-nitro benzaldehyde, acetoacetic ester, malononitrile and hydrazine hydrate as model substrates. Firstly reactions performed by stirring at room temperature and catalyst-free condition in ethanol gave unsatisfactory results (Table 2, entry 1). Although in presence of catalytic amount of L-Tyrosine gave very poor yield of product (Table 2, entry 2). Keeping in mind the green chemistry principle in synthesis, microwaveassisted organic synthesis has become a significant tool for accelerating drug discovery and development processes. Microwaves have ability to couple directly with the reacting molecules leads to rapid rise in the temperature. In this regard we able to carry out this transformation in microwave irradiation.
To optimize the reaction condition, the model reaction irradiate under microwave without catalyst in ethanol gave low yield of desired product (Table 2, entry 3). Moderate yield observed when L-tyrosine was used as catalyst in ethanol. The solvent effect was also examined by using protic solvent such as ethanol, methanol and water. It was found that reaction in water gave better yield than ethanol and methanol medium (Table 2, entry 4-7). Excellent result was observed when water: EtOH (1:1) medium solvent system was used (Table 2, entry 8).
With this result, we optimized the catalyst load which was finally arrived at 0.05 g giving maximum yield of 94 %, since further increase in the amount of catalyst beyond 0.05 g did not improve the yield of products (Table 3, entries 1-3).
The reusability of the L-Tyrosine catalyst was further studied through a model reaction under optimized conditions. After completion of reaction the mixture was cooled to room temperature and poured onto ice cold water, product was separated by simple filtration. The filtrate was heated over burner to evaporate water:ethanol content and the residual catalyst was washed with diethyl ether and dried in a hot oven and reused. It was observed that the reusability of L-Tyrosine was considerably effective for four consecutive runs.
In a subsequent investigation for the substrate scope using the optimized reaction conditions, we found that various aromatic aldehydes (electron donating as well as electron withdrawing) offered the corresponding products in high yields (Table 1, entries a-k).
| Entry | R | Product | Time (min) | Yield % | Melting Point | |
|---|---|---|---|---|---|---|
| Found | Reported | |||||
| a | 4-H | 5a | 7 | 92 | 226-228 | 228-230 [18] |
| b | 4-NO2 | 5b | 5 | 94 | 250-252 | 251-252 [18] |
| c | 3-NO2 | 5c | 5 | 93 | 212-214 | 214-216 [18] |
| d | 4-Cl | 5d | 6 | 94 | 230-232 | 233-234 [18] |
| e | 4-Br | 5e | 4 | 91 | 178-180 | 179-180 [19] |
| f | 4-F | 5f | 4 | 92 | 243-245 | 240-242 [19] |
| g | 4-Me | 5g | 6 | 88 | 174-175 | 176-177 [18] |
| h | 4-OMe | 5h | 7 | 86 | 244-246 | 240-242 [19] |
| i | 4-OH | 5i | 6 | 89 | 220-222 | 224-226 [19] |
| j | 3,4-OMe | 5j | 8 | 89 | 175-177 | 170-172 [18] |
| k | 3-OMe, 4-OH | 5k | 6 | 90 | 238-240 | 234-236 [20] |
Table 1: Synthesis of pyranopyrazole by using L-Tyrosine under microwave.
| Entry | Condition | Solvent | Time (min) | Yield % |
|---|---|---|---|---|
| 1 | Cat. Free, RT | EtOH | 60 | - |
| 2 | L-Tyrosine, RT | EtOH | 60 | 30 |
| 3 | Cat.free, MW | EtOH | 20 | 40 |
| 4 | L-Tyrosine, MW | EtOH | 20 | 60 |
| 5 | L-Tyrosine, MW | MeOH | 20 | 55 |
| 6 | L-Tyrosine, MW | H2O | 20 | 75 |
| 7 | L-Tyrosine, MW | H2O | 40 | 80 |
| 8 | L-Tyrosine, MW | H2O:EtOH (1:1) | 5 | 94 |
| 9 | L-Tyrosine, MW | H2O:EtOH (2:1) | 15 | 85 |
Table 2: Optimization of reaction condition.
| Entry | Amount of Catalyst (g) | Time (min) | Yield % |
|---|---|---|---|
| 1 | 0.01 | 20 | 60 |
| 2 | 0.03 | 20 | 75 |
| 3 | 0.05 | 2 | 94 |
| 4 | 0.06 | 5 | 90 |
| 5 | 0.07 | 5 | 90 |
Table 3: Electronic spectra of free ligand and its complexes.
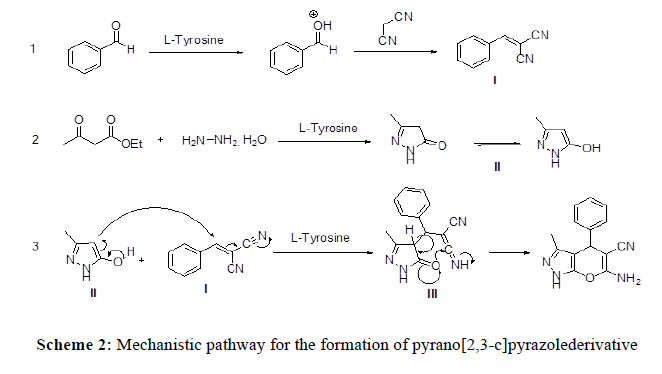
A proposed mechanistic pathway for the formation of pyrano[2,3-c]pyrazolederivatives is shown in Scheme 2. The reaction initiated knoevenagel condensation of benzaldehyde and malononitrile in presence of catalyst to provide the 2-benzylidene malononitrile (I). Simultaneously formation of pyrazolone (II) from acetoacetic ester and hydrazine hydrate has been also takes place. Michael addition of pyrazolone (II) onto α,β-unsaturated compound (I) facilitates the formation of intermediate (III), which further undergoes cyclisation to give the desired product pyrano[2,3-c] pyrazole (Scheme 2).
Conclusion
We reported an atom-economical multicomponent reaction, using energy-efficient microwave irradiation; L-Tyrosine as mild, cost effective and ‘greener’ catalyst along with eco-friendly green solvent water: ethanol for the synthesis of pyranopyrazoles. The attractive features of this protocol are the mild reaction conditions, high conversions, operational simplicity and inexpensive and ready availability of the catalyst; which makes it a useful and attractive strategy for the preparation of dihydropyrano[2,3-c] pyrazoles.
Acknowledgement
The authors express appreciation to the principal Dr. S. N. Thore Deogiri College, Aurangabad for providing laboratory facility. We also thank to SAIF Chandigarh for providing spectral data.
References
- El-Tamany ES, El-ShahedF A, Mohamed BH (1999) Synthesis and biological activity of some pyrazole derivatives. J Serb Chem Soc 64: 9.
- Wang JL, Liu D, Zheng ZJ, Shan S, Han X, et al. (2009) Structure-based discovery of an organic compound that bind Bc1-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci USA 97: 7124-7129.
- Zaki MEA, Saliman HA, Hickal OA, Rashad AEZ (2006) Pyrazolopyranopyrimi- dines as a class of anti-inflammatory agents. Z Naturforsch C 61: 1-5.
- Smith WP, Sollis LS, Howes DP, Cherry CP, Starkey DI, et al. (1998) Dihydropyrancarboxamides related to Zanamivir: A new series of inhibitors of influenza virus sialidases. discovery, synthesis, biological activity, and structure-activity relationships of 4-guanidino-and 4-amino-4h-pyran-6-carboxamides. J Med Chem 41: 787-797.
- Abdelrazek FM, Metz P, Metwally NH, El-Mahrouky SF (2006) Synthesis and molluscicidal activity of 5-oxo-5,6,7,8-tetrahydro-4H-chromene derivatives. Arch Pharm Med Chem 339: 456-460.
- Kuo SC, Huang LJ, Nakamura H (1984) Studies on heterocyclic compounds. 6. Synthesis and analgesic and anti-inflammatory activities of 3,4-dimethylpyrano[2,3-c]pyrazol-6-one derivatives. J Med Chem 27: 539-544.
- Foloppe N, Fisher LM, Howes R, Potter A, Robertson AGS, et al. (2006) Identification of chemically diverse Chk1 inhibitors by receptor-based virtual screening. Bioorg Med Chem 14: 4792-4802.
- Liu Z, Zhang R, Meng Q, Zhang X, Sun Y (2016) Discovery of new protein kinase CK2 inhibitors with 1,3-dioxo-2,3-dihydro-1H-indene core. Med Chem Comm 7: 1352-1355.
- Zhang W (2009) Green chemistry aspects of fluorous techniques-opportunities and challenges for small-scale organic synthesis. Green Chem 11: 911-920.
- Candeias NR, Branco LSC, Gois PMP, Afonso CAM, Trindade AF (2009) More Sustainable Approaches for the Synthesis of N-Based Heterocycles. Chem Rev 109: 2703-2802.
- Joshi VM, Pawar RP (2013) Microwave Assisted Expeditious Synthesis of Bioactive Polyhydroquinoline Derivatives. Eur Chem Bull 2: 679-682.
- Reddy LV, Suman A, Beevi SS, Mangamoori LN, Mukkanti K, et al. (2010) Design and synthesis of 1-Aroyl-2-ylidene Hydrazines under Conventional and Microwave Irradiation conditions and their Cytotoxic Activities. J Braz Chem Soc 21: 98-104.
- Pal S, Mareddy J, Suneetha Devi N (2008) High Speed Synthesis of Pyrazolones using Microwave-Assisted Neat Reaction Technology. J Braz Chem Soc 19: 1207-1214.
- Bera R, Dhananjaya G, Singh SN, Kumar R, Mukkanti K, et al. (2009) Microwave-accelerated witting olefination of β-Chloroacroleins. Tetrahedron 65: 1300-1305.
- Young SN (2007) How to increase serotonin in the human brain without drugs. J Psychiatry Neurosci 32: 394-399.
- Khaskel A, Gogoi P, Barman P, Bandyopadhyay B (2014) L-Tyrosine loaded nanoparticles: an efficient catalyst for the synthesis of dicoumarols and Hantzsch 1,4-dihydropyridines, RSC Adv 4: 35559.
- Thirupathi G, Venkatanarayana M, Dubey PK, Bharathi Kumari Y (2012) L-tyrosine catalyzed Knoevenagel condensation: Facile synthesis of cyanoacrylonitriles cyanoacrylates and cyanoacrylamides in solvent free condition under grindstone method. Der Pharma Chemica 4: 1897-1901.
- Syamala M (2009) Recent Progress in Three-Component Reactions. An Update. Org Prep Proc Int 41: 1-68.
- Tayade YA, Padvi SA, Wagh YB, Dalal DS (2015) β-Cyclodextrin as a supramolecular catalyst for the synthesis of dihydropyrano[2,3-c]pyrazole and spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] in aqueous medium. Tetrahedron Letters 56: 2441-2447.
- Shi D, Mou J, Zhung Q, Niu L, Wu N, et al. (2004) Three Component one pot synthesis of dihydropyrano[2,3-c] pyrazole Derivatives in aqueous medium. Synth Commun 34: 4557-4563.
- Sohal HS, Goyal A, Sharma R, Khare R, Kumar S (2013) Glycerol mediated, one pot, multicomponent synthesis of dihydropyrano[2,3-c] pyrazoles. Euro J Chem 4: 450-453.
- Imene AK, Wassima G, Raouf B, Taous B, Abdelmadjid D (2015) Phenylboronic acid-catalysed a four component synthesis of Pyrano[2,3-c] pyrazole derivatives in aqueous media: an ecofriendly method. Der Pharma Chemica 7: 175-180.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences