ISSN : 2575-7725
Journal of Stem Cell Biology and Transplantation
Light and Electron Microscopic Study of the Anti-Inflammatory Role of Mesenchymal Stem Cell Therapy in Restoring Corneal Alkali Injury in Adult Albino Rats
Eman Mohammed Faruk1*, Aisha El Mansy2, Amal Mohmmod Al-Shazly2 and Neama Mahmoud Taha3
1Histology and Cell Biology Department Benha Faculty of Medicine, Benha University, Egypt
2Anatomy Department Benha Faculty of Medicine, Benha University, Egypt
3Physiology Department, College of Medicine, 3Umm Al-Qura University, Saudi Arabia
- *Corresponding Author:
- Eman Mohammed Faruk
Histology and Cell Biology
Department Benha Faculty of Medicine
Benha University, Egypt
Tel: 00966596640779
E-mail: faruk_eman@yahoo.com
Received Date: May 25, 2017; Accepted Date: May 29, 2017; Published Date: June 06, 2017
Citation: Faruk EM, Mansy AE, Al-Shazly AM, et al. Light and Electron Microscopic Study of the Anti-Inflammatory Role of Mesenchymal Stem Cell Therapy in Restoring Corneal Alkali Injury in Adult Albino Rats. J. stem cell Bio. transplant. 2017, 1:1.
DOI: 10.21767/2575-7725.100008
Abstract
Keywords
Word multipotent; Stem cells; Autologous mesenchymal stem cells transplantation
Introduction
The cornea is highly specialized organ, so healing of corneal injury is characterized by fibrosis [1] and neovascularization [2] all of these lead to defective in corneal clearance and impaired vision which are common complications of alkali burn. Most of corneal injury is caused by chemical burns in young age [3].
Corneal injury lead to release of progenitor cells as neutrophils, epithelial cells, [4] fibroblasts, [5] endothelial cells [6] so; reendothelialization and neovascularization were occur which lead to defective in corneal clearance [7].
So, the basis of treatment depends on the inhibition of inflammation but, the anti-inflammatory drugs available are not sufficient to inhibit neovascularization.
Also, corneal injury stimulates epithelial stem cells in limbal area which can change into endothelial cells [8]. But, LSCs transplantation are not easily available and have high immunorejection rates [9].
Mesenchymal stem cells (MSC) have better repair treatment because they have less or no immunogenic potential and are easy to isolate from bone marrow, expanded in vitro with the ability to differentiate into epithelial cells [10].
Mesenchymal stem cells (MSCs) are undifferentiated cells that can proliferate, and differentiate into mature cells [11,12]. The main sources of this undifferentiated cells is the bone marrow [13]. They have immunoregulatory properties so, they are better in treatment than LSCs transplantation [14,15].
When transplanted MSCs systemically, they had the ability to identify and travel to sites of injury and finally differentiate into the same tissue cells and act as one member of this tissue [16].
One of transmembrane protein that was used as a marker to identify the mesenchymal cells is CD44 [17].
So, that we aimed to study the histological and ultrastructure changes occur in the ability of mesenchymal stem cells to help in corneal healing when induced by corneal alkali injury.
Materials and Methods
Animals and grouping
Thirty adult male albino rats weighing between 170 and 230 mg were used in this study and were imported from the animal house, faculty of Veterinary Medicine, Benha University Egypt.
Under normal atmospheric conditions male albino rats were putted in clean cages and given standard food and water ad libitum.
In this study all ethical protocols for animals conform to the principles established by the Institutional Animal Care Committee for the Experimental Animals. Randomly animals were divided into three equal groups: Group I as a control, group II (bilateral alkali-burnt corneas using NaOH) as normal bone marrow function and divided into: subgroup II a, normal bone marrow function, without MSCs transplantation; subgroup IIb, normal bone marrow function, with MSCs transplantation and group III (bilateral alkali-burnt corneas) as a bone marrow-suppression model received 200 mg/kg BW, Intra Peritoneal IP cyclophosphamide (CP) (endoxan ampoule of 200 mg (AstaMedica, Germany) a vial was dissolved in 10 mL saline [18] and divided into: subgroup IIIa, bone marrow suppressed without MSCs; sub group III b, bone marrow suppressed with MSCs.
Experimental procedure
Corneal ulcer induction: 100-200 mg/kg body weight of thiopental sodium (Thiopental; Eipico) was used as intraperitoneal injection for anesthetized the animals. Alkali burn was done in widely opened eyes by using soaked round filter paper (4 mm in diameter) in 1 mol/l NaOH for 5 s and then putted on the cornea of eyes of the two and third groups for 30 seconds. Then rinsing the eyes with 30 mL sterile saline. The control eyes (group I) were similarly treated with topical saline [19,20]. Two days after experiment animals from subgroup IIa and subgroup IIIa were sacrificed [21], while animals of subgroup IIa and subgroup IIIb were maintained for 2 weeks after stem cell injected and then sacrificed [22].
Mesenchymal stem cells isolation and preparation: The plastic adherence method is method was used in MSCs cultivation. 10 mL heparinized bone marrow was aspirated with complete aseptic condition and diluted with phosphate buffered saline (PBS) 1:2 (Sigma-Aldrich, St. Louis, MO, USA) and centrifuged at 450 g for 30 min. Then, the middle layers of mononuclear cells were collected and washed with PBS three times. Then were washed in Dulbecco’s modiï¬ÂÂed Eagle’s medium DMEM (Gibco/ BRL; Gel Company Inc., San Francisco, California, USA) containing antibiotics (penicillin 10 000 U⁄mL, streptomycin 10 000 U⁄mL,), 10% fetal bovine serum (FBS; USDA, Gibco, Grand Island, NY, USA), The cells were cultured and incubated in a humidiï¬ÂÂed atmosphere containing 5% CO2 for two weeks at 37°C [23]. Detection of cell growth and changes in their morphology was checked daily in the cultures , then when formation of adherent spindle-shaped fibroblastoid cell colonies the cultures were washed twice with PBS with 0.25% trypsin in 1 mmol/L EDTA (Gibco/BRL) for cells trypsinization for 5 min at 37°C. Then centrifugation was done at (2400 rpm for 20 min), and cells were suspended with serum supplemented medium and put in a 50 cm2 culture flask for incubation (Falcon, Becton, Dickinson and Company, Lovetone Circle, Sparks, Maryland, USA). After two weeks the adherent colonies of cells were trypsinzed, counted and re suspended in 5 mL of PBS at a density of 1.4–1.6 × 106 cells/mL, prior to intramuscular (IM) or intra-venous (IV) transplantation [24].
Cultured MSCs were identified by morphology (adhesive fibroblast like cells), Florescent Analysis Cell Sorting (FACS), negativity of CD34+ and positivity of CD29+ (specific for MSCs) by flow cytometers.
Flow cytometric analysis: In order to detect the characterization of the MSC preparations and to characterize the phenotype of in vitro expanded MSCs we used the flow cytometry [25]. This was done on a fluorescence activated cell sorter flow cytometer (Coulter Epics Elite, Miami, Florida, USA), as MSC were taken, trypsinized then washed twice with PBS for evaluation presence of MSCs markers. After that incubation of cells were taken in dark with the mAb for 30 min at 4°C and then washed twice with PBS before cytometry analysis CD34FITC labeled mouse anti rat IgG was used as the negative control. At room temperature for 10 min 1 million cells per 5 mL conical tube were incubated with labeled monoclonal antibodies with phycoerythrin again st one of CD29 or CD34 [26].
Analysis of cell viability: it was assessed using the trypan blue dye test, where viable cells do not take up certain dyes as dead cells do.
Labeling of MSCs: Labeled cells are important to know biological and multiplication activity, also for in-vivo cell identification. We use green fluorescent protein (GFP) for labeling MSCs and this done by using monster green fluorescent protein vector and lipofectamin transfast transfection reagent kit (Promega, Madison, WI, USA). Twenty four hours before labeling, 0.5–1 x 106 cells were placed in 500 mL of growth medium. Then cells were incubated into wells (6 well-plates) for 24 h in culture medium, and then were subjected to 2 μg GFP plasmid/well of cells. Before exposure to the cells GFP plasmid was incubated with lipofectamin for 15-20 min. The cells were incubated at 37°C in 5% CO2 for 2 h after transfection. Then removed the transfection medium and incubated the cells for an additional 48 h in growth medium (2 mL per well) [27]. Intravenous injection of the cells (into the tail vein). After two weeks, to detect the cells stained with GFP corneal tissue was examined with a fluorescence microscope.
Histological study: By a lethal dose of ether we sacrificed all rats and corneas were dissected from each eye for histological light microscopic (LM) and transmission electron microscopic (TEM) studies.
Light microscopic study
Fixation of Right Corneas in 10% formalin to be processed for paraffin blocks. 5 μm sections were cut and stained with hematoxylin and eosin (H&E) for histological study [28].
Transmission electron microscopic study: The left corneas were taken and putted rapidly in 2.5% phosphate-buffered gluteraldehyde for 4 h, and then fixed in 1% osmium tetroxide in 0.1 mol/L for 2 h. The specimens were dehydrated in ascending grades of alcohol, semithin sections were stained with toluidine blue to select the proper sites for ultrathin sections (50-60 nm thick), then were cut by using an ultramicrotome, then sections were double stained with 2% uranyl acetate and lead citrate, finally examined and photographed using a JEM-1200EXII, Transmission Electron Microscope UK transmission electron microscope at the Faculty of Medicine, Benha University [29].
Immunohistochemical study: Immunohistochemical study was done by using anti-CD44 antibodies for staining of the CD44 antigen to detect MSCs markers by using the avidin–biotin peroxidase complex technique [30]. Paraffin sections were deparaffinized and hydrated. After blocking the endogenous activity of peroxidase using 10% hydrogen peroxide, the sections were incubated with primary antibodies. CD44 antibody is a mouse monoclonal antibody clone 2A4 (Lab Vision Corporation, Neomarkers Laboratories, Westing House, and Thurmont, California, USA). Then, the secondary antibody was applied (biotinylated goat antirabbit) after washing with phosphate buffer.
Then sections were incubated with labeled avidin–biotin peroxidase, which binds to the biotin on the secondary antibody. The site of antibody binding was visualized as a brown precipitate by peroxidase. CD44-positive cells showed brown cytoplasmic deposits. Sections were counterstained with Meyer’s hematoxylin. The mean number of CD44 immunopositive cells from each section was estimated.
Morphometric study: Image analysis was used to measure the mean epithelial height of cornea, stromal corneal thickness and mean number of CD44 immuno positive cells. In each specimen measurements were performed within 10 random nonover lapping fields at × 400 magnification. These measurements were done at the Histology Department, Faculty of Medicine, Cairo University (‘Olympus BX40, Ships to USA).
Real-time PCR and Enzyme-linked Immunosorbent Assay (ELISA)
For analysis of mRNA expression: The mRNA expression levels of macrophage inflammatory protein-1 alpha (MIP-1a), tumor necrosis factor-alpha (TNF-a) and vascular endothelial growth factor (VEGF) in the corneas were evaluated using polymerase chain reaction (real-time PCR). At the end of experiment the corneas from each group were dissected and each one cut in half; one half was analyzed using real-time PCR, and the other half was analyzed using an enzyme-linked immunosorbent assay (ELISA). TRIzol (Invitrogen, Carlsbad, CA) was used to harvested corneas for RNA extraction and detection of its concentration. DNase I digestion (Ambion, Austin, TX) was used to remove Genomic DNA. PrimeScript RT reagent kit (Takara, Dalian, China) was used to reverse transcribed of 1 mg of RNA into cDNA, then the cDNA was putted in 100-well plates of Real-time PCR to perform and analysis with an ABI PRISM 7000 sequence detection system (Applied Biosystems Inc., Foster City, CA). The relative gene expression levels were calculated according to [31]. VEGF protein levels in the corneas were determined using an ELISA kit (R&D Systems, Minneapolis, MN). Mortar was used to homogenize the cornel tissue, then lysed in 500 mL buffer and centrifuged at 10,000 rpm for 10 min. The supernatants were used for ELISA.
Statistical analysis: Quantitative data were calculated as means and SD and compared using one-way analysis of variance. P-values of less than 0.005 were considered statistically significant. Statistical analyses were done by SPSS IBM, United States. (Version 16 windows) software.
Results
Characteristics of expanded undifferentiated bone marrow - mesenchymal stem cells
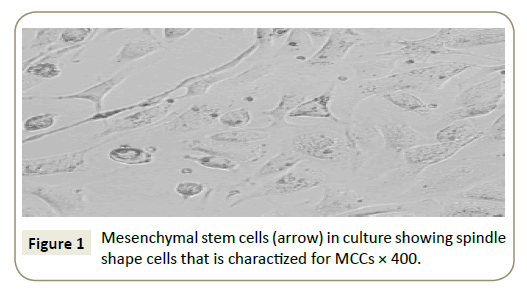
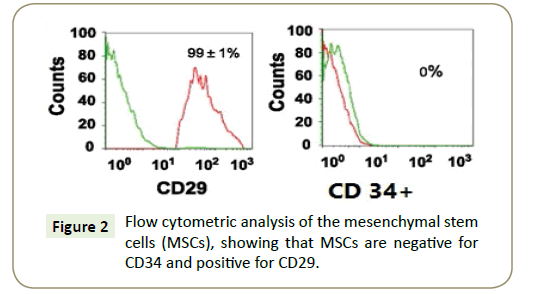
Identification of cells in culture as being MSCs by their spindleshaped appearance (Figure 1) and by detection of CD29, the flow cytometric analysis of the surface markers of the MSCs were negative for CD34 and positive for CD29 (Figure 2).
Figure 2: Flow cytometric analysis of the mesenchymal stem cells (MSCs), showing that MSCs are negative for CD34 and positive for CD29.
From above results we indicated that the isolated MSCs relatively purified bone marrow-derived mesenchymal stem cells.
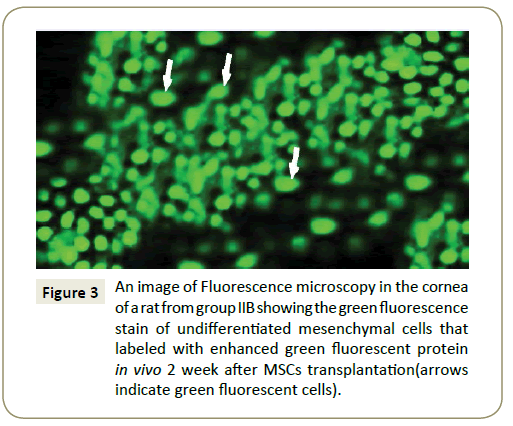
Fluorescent microscopic result: Fluorescence microscopy images showing in the cornea of group IIb (Figure 3) and group III B the green fluorescence of MSCs labeled with enhanced green fluorescent protein in vivo 2 week after MSCs injection.
Figure 3: An image of Fluorescence microscopy in the cornea of a rat from group IIB showing the green fluorescence stain of undifferentiated mesenchymal cells that labeled with enhanced green fluorescent protein in vivo 2 week after MSCs transplantation(arrows indicate green fluorescent cells).
Histological result
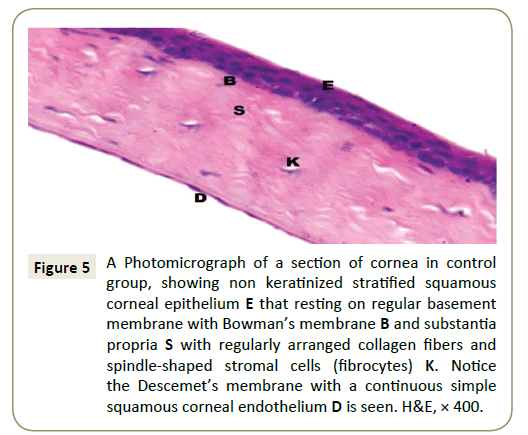
Light microscopic results (H&E): In the control group (Group I) cornea revealed smooth covering with a non-keratinized stratified squamous epithelium which formed of basal columnar cells with oval nuclei resting on a clear basement membrane, intermediate polyhedral cells with rounded nuclei and superficial flattened squamous cells with flattened nuclei and under the epithelium there was a thin acidophilic membrane revealed Bowman’s membrane (Figure 4). Regularly arranged bundles of collagen fibers with spindle-shaped corneal fibroblasts (keratocytes) between them are seen in the avascular stroma, also Descemet’s membrane was appeared clearly as a continuous single layer of flat endothelial cells (Figure 5).
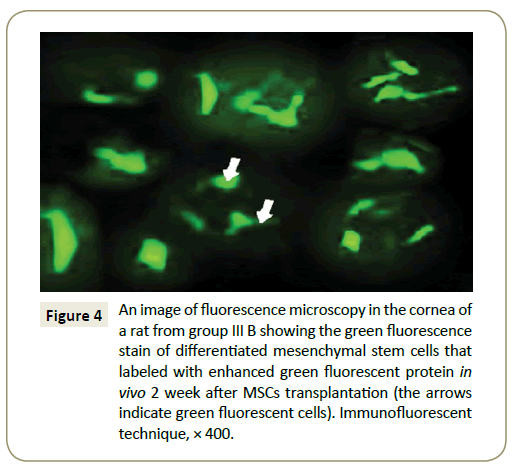
Figure 4: An image of fluorescence microscopy in the cornea of a rat from group fibers B showing the green fluorescence stain of differentiated mesenchymal stem cells that labeled with enhanced green fluorescent protein in vivo 2 week after MSCs transplantation (the arrows indicate green fluorescent cells). Immunofluorescent technique, × 400.
Figure 5: A Photomicrograph of a section of cornea in control group, showing non keratinized stratified squamous corneal epithelium E that resting on regular basement membrane with Bowman’s membrane B and substantia propria S with regularly arranged collagen fibers and spindle-shaped stromal cells (fibrocytes) K. Notice the Descemet’s membrane with a continuous simple squamous corneal endothelium D is seen. H&E, × 400.
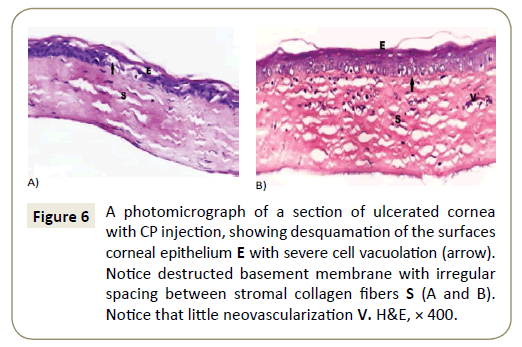
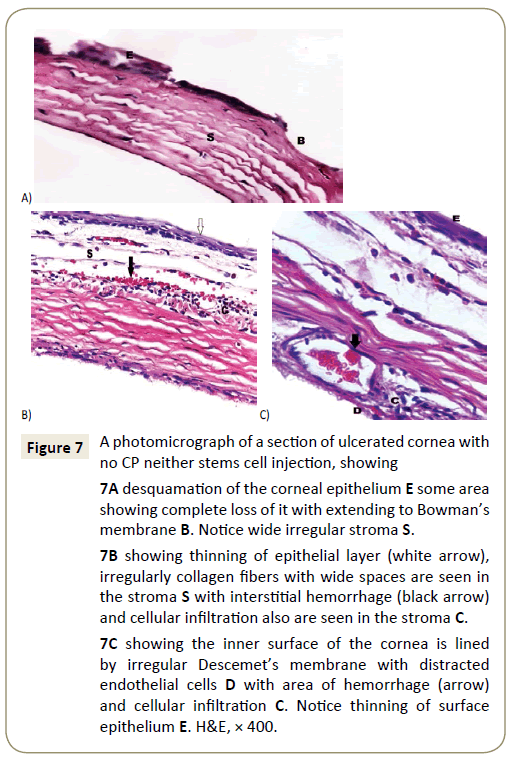
In corneas of subgroup IIa showed desquamation and degeneration of superficial cells, with regularly arranged bundles of collagen fibers in some parts of the stroma but other showed separated and disturbed collagen fibers (Figure 6A) with neovascularization between these bundles (Figure 6B). While Corneas of subgroup IIIa showed different degrees of affection, some corneas showed epithelial thinning with desquamation, degeneration and vacuolation with irregulary arranged epithelial cells (Figure 7A).
Figure 6: A photomicrograph of a section of ulcerated cornea with CP injection, showing desquamation of the surfaces corneal epithelium E with severe cell vacuolation (arrow). Notice destructed basement membrane with irregular spacing between stromal collagen fibers S (A and B). Notice that little neovascularization V. H&E, × 400.
Figure 7: A photomicrograph of a section of ulcerated cornea with no CP neither stems cell injection, showing
7A desquamation of the corneal epithelium E some area showing complete loss of it with extending to Bowman’s membrane B. Notice wide irregular stroma S.
7B showing thinning of epithelial layer (white arrow), irregularly collagen fibers with wide spaces are seen in the stroma S with interstitial hemorrhage (black arrow) and cellular infiltration also are seen in the stroma C.
7C showing the inner surface of the cornea is lined by irregular Descemet’s membrane with distracted endothelial cells D with area of hemorrhage (arrow) and cellular infiltration C. Notice thinning of surface epithelium E. H&E, × 400.
Neovascularization, interstitial hemorrhage with inflammatory cellular infiltration and degenerated keratocytes were seen between wide space irregularly arranged collagen fibers of the stroma with degeneration and even losting of Bowman’s membrane are also seen (Figure 7B). Also Descemet’s membrane showed separation from stroma in some parts leaving wide spaces in between with neovascularization in stroma (Figure 7C).
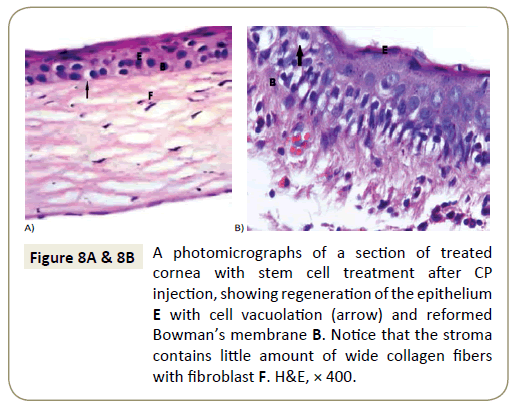
Corneal sections of rats left to recover with stem cells treatment for two weeks after CP injection (Group II b) showed desquamated and degenerated of the superficial cells of their epithelium ,other showed vacuolation of epithelial cells with wide separation of fibers of stroma with interstitial hemorrhage between them (Figure 8A and 8B).
Figure 8A & 8B: A photomicrographs of a section of treated cornea with stem cell treatment after CP injection, showing regeneration of the epithelium E with cell vacuolation (arrow) and reformed Bowman’s membrane B. Notice that the stroma contains little amount of wide collagen fibers with fibroblast F. H&E, × 400.
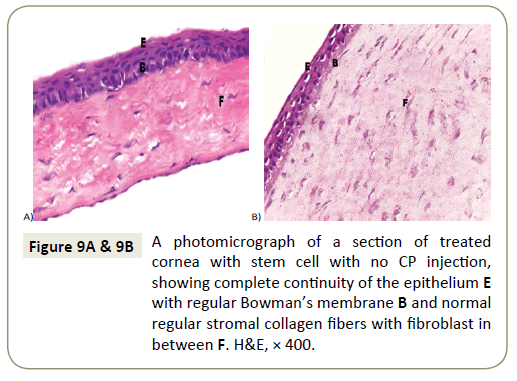
While corneas of group III b (rats treated with stem cells without CP injection) showed nearly normal corneal epithelium with intact Bowman’s membrane. Stroma was nearly normal with wide separation of its fibers.
Endothelial cells in the Descemet’s membrane were more or less normal (Figure 9A and 9B).
Immunohistochemical results
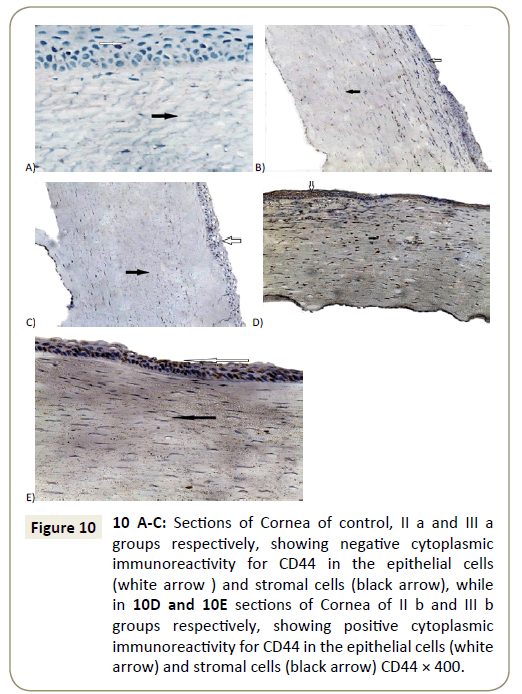
Negative immunoreactivity for anti-CD44 was detected in corneal sections of control group, II a and III a respectively (Figure 10A-10C), while examination of sections in both groups (group II b and III b) was showed positive immunoreactivity for anti-CD44 within the epithelial cells as well as within the stroma of corneal sections (Figure 10D and 10E).
Figure 10: 10 A-C: Sections of Cornea of control, II a and fibers a groups respectively, showing negative cytoplasmic immunoreactivity for CD44 in the epithelial cells (white arrow ) and stromal cells (black arrow), while in 10D and 10E sections of Cornea of II b and fibers b groups respectively, showing positive cytoplasmic immunoreactivity for CD44 in the epithelial cells (white arrow) and stromal cells (black arrow) CD44 × 400.
Electron microscopic results
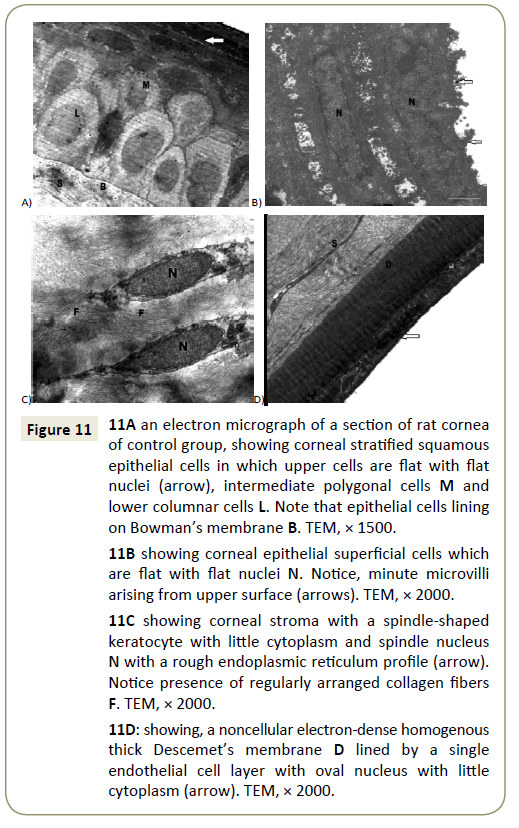
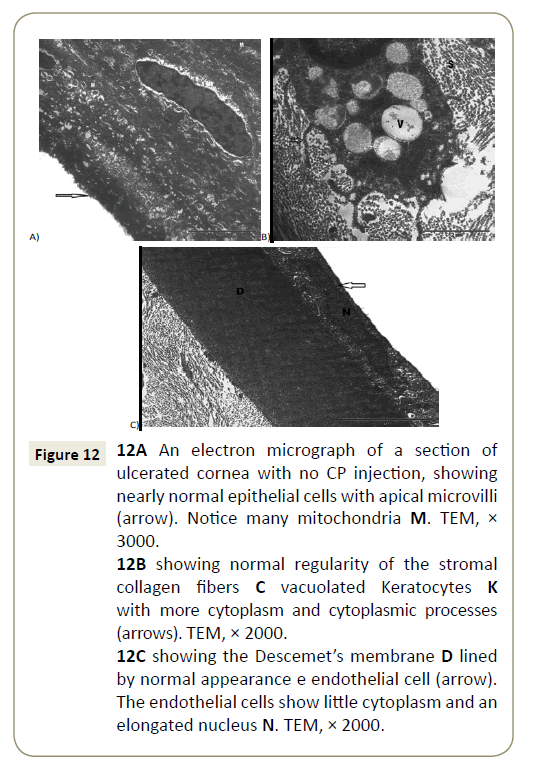
Electron microscopic sections of the cornea of the control group showed covering epithelium which formed of cuboidal basal cells, polygonal intermediate cells and top squamous cells, these cells showed few cytoplasmic organelles (Figure 11A). The superficial flat cells have flattened euchromatic nuclei and minute microvilli arising from their superficial surface (Figure 11B). The basal cells were cubical and were attached together by multiple junctions and they were lined on a basement membrane (Bowman’s membrane) (Figure 11A). The corneal stroma was thick, avascular and formed of many regularly layers of collagen fibers with spindle shaped keratocytes cells that had elongated nuclei with little cytoplasm (Figure 11C). Also Descemet’s membrane was appeared as a cellular homogenous layer with flat endothelial cells at the posterior surface of the cornea (Figure 11D). However, EM sections of ulcerated cornea with CP showed that cells of the superficial epithelial layer revealed an apparently normal appearance with apical microvilli (Figure 12A), also normal and regularly appearance of stromal collagen fibers but with vacuolated keratocytes (Figure 12B) and normal Descemet’s membrane (Figure 12C).
Figure 11: 11A an electron micrograph of a section of rat cornea of control group, showing corneal stratified squamous epithelial cells in which upper cells are flat with flat nuclei (arrow), intermediate polygonal cells M and lower columnar cells L. Note that epithelial cells lining on Bowman’s membrane B. TEM, × 1500.
11B showing corneal epithelial superficial cells which are flat with flat nuclei N. Notice, minute microvilli arising from upper surface (arrows). TEM, × 2000.
11C showing corneal stroma with a spindle-shaped keratocyte with little cytoplasm and spindle nucleus N with a rough endoplasmic reticulum profile (arrow). Notice presence of regularly arranged collagen fibers F. TEM, × 2000.
11D: showing, a noncellular electron-dense homogenous thick Descemet’s membrane D lined by a single endothelial cell layer with oval nucleus with little cytoplasm (arrow). TEM, × 2000.
Figure 12: 12A An electron micrograph of a section of ulcerated cornea with no CP injection, showing nearly normal epithelial cells with apical microvilli (arrow). Notice many mitochondria M. TEM, × 3000.
12B showing normal regularity of the stromal collagen fibers C vacuolated Keratocytes K with more cytoplasm and cytoplasmic processes (arrows). TEM, × 2000.
12C showing the Descemet’s membrane D lined by normal appearance e endothelial cell (arrow). The endothelial cells show little cytoplasm and an elongated nucleus N. TEM, × 2000.
While sections of ulcerated cornea with no CP injection showed marked ultrastructural changes in the form of separation of the superficial cells from underlying cells and showed multiples cytoplasmic vacuoles with wide intercellular spaces, these cells showed disfigurement in their shapes with degenerated nuclei (Figure 13A), while stroma showed an irregularly arranged collagen fibers with degenerated kerratocytes with cytoplasmic vacuoles, lysosomes and degenerated mitochondria (Figure 13B). Neovascularization was also observed in the stroma with irregularity of its fibers (Figure 13C). The Descemet’s membrane was lined by destructed endothelial cell with cytoplasmic vacuoles (Figure 13D).
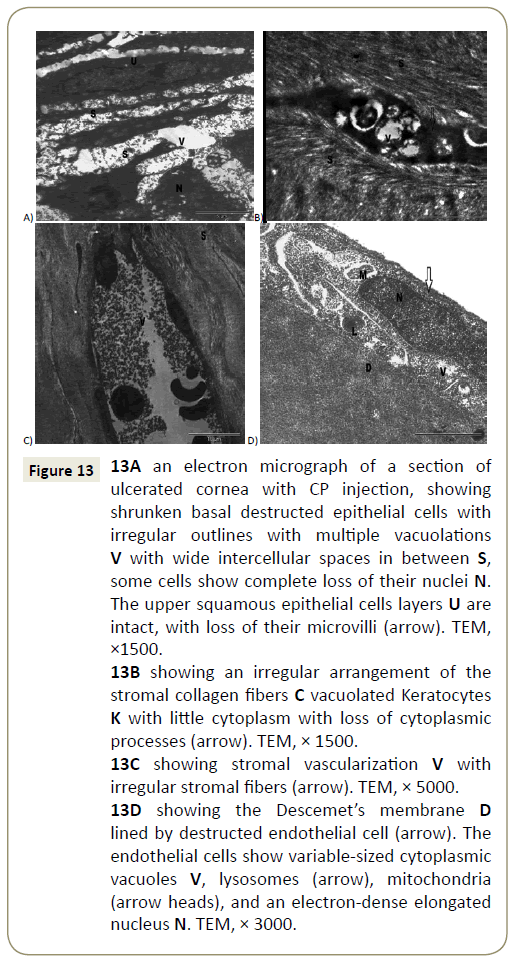
Figure 13: 13A an electron micrograph of a section of ulcerated cornea with CP injection, showing shrunken basal destructed epithelial cells with irregular outlines with multiple vacuolations V with wide intercellular spaces in between S, some cells show complete loss of their nuclei N. The upper squamous epithelial cells layers U are intact, with loss of their microvilli (arrow). TEM, ×1500.
13B showing an irregular arrangement of the stromal collagen fibers C vacuolated Keratocytes K with little cytoplasm with loss of cytoplasmic processes (arrow). TEM, × 1500.
13C showing stromal vascularization V with irregular stromal fibers (arrow). TEM, × 5000.
13D showing the Descemet’s membrane D lined by destructed endothelial cell (arrow). The endothelial cells show variable-sized cytoplasmic vacuoles V, lysosomes (arrow), mitochondria (arrow heads), and an electron-dense elongated nucleus N. TEM, × 3000.
Ulcerated cornea with CP injection and stem cell treatment, showed apparently normal superficial corneal epithelial (Figure 14A) with nearly normal regularity of the corneal stroma and stromal keratocytes showed euchromatic nuclei with scanty cytoplasm (Figure 14B). The Descemet’s membrane appeared normal with endothelial cells with elongated nuclei (Figure 14C).At end ulcerated cornea with stem cell treatment with no CP injection, showed apparently normal superficial corneal epithelial cell with apical microvilli (Figure 15A). Normal appearance of corneal stroma with normal keratocytes (Figure 15B). Descemet’s membrane with elongated endothelial cell which is similar to those of the control group (Figure 15C).
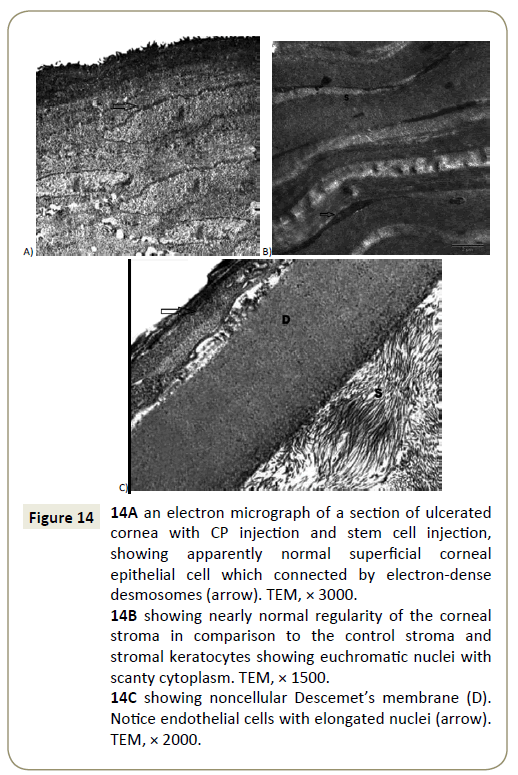
Figure 14: 14A an electron micrograph of a section of ulcerated cornea with CP injection and stem cell injection, showing apparently normal superficial corneal epithelial cell which connected by electron-dense desmosomes (arrow). TEM, × 3000.
14B showing nearly normal regularity of the corneal stroma in comparison to the control stroma and stromal keratocytes showing euchromatic nuclei with scanty cytoplasm. TEM, × 1500.
14C showing noncellular Descemet’s membrane (D). Notice endothelial cells with elongated nuclei (arrow). TEM, × 2000.
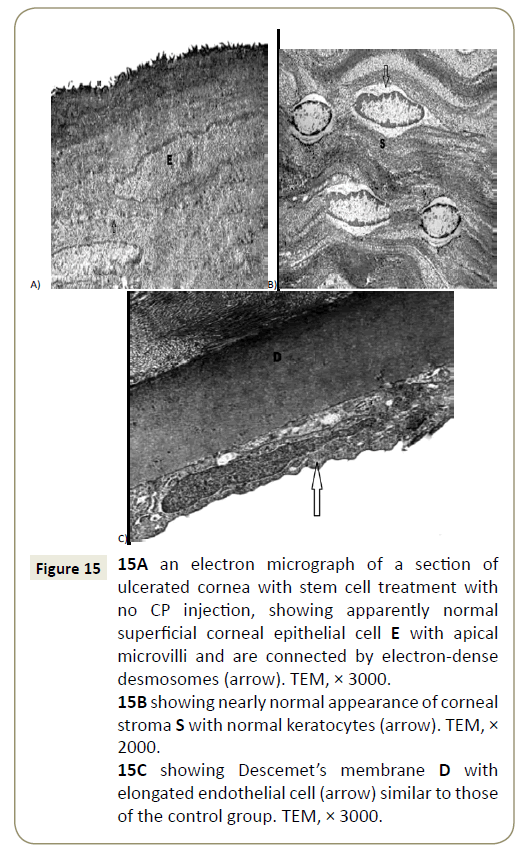
Figure 15: 15A an electron micrograph of a section of ulcerated cornea with stem cell treatment with no CP injection, showing apparently normal superficial corneal epithelial cell E with apical microvilli and are connected by electron-dense desmosomes (arrow). TEM, × 3000.
15B showing nearly normal appearance of corneal stroma S with normal keratocytes (arrow). TEM, × 2000.
15C showing Descemet’s membrane D with elongated endothelial cell (arrow) similar to those of the control group. TEM, × 3000.
Morphometric results
These results confirm the histological results and showed highly significant decrease in the corneal epithelial thickness and corneal stromal thickness in ulcerated corneas those not treated with stem cells especially in rats injected with CP when compared with the control corneas. While, there was a nonsignificant difference in corneal epithelial thickness and corneal stromal thickness in stem cells treated rats with no CP injection and in rats treated with stem cells with CP injection when they compared with the control. While these treated animals with stem cells showed a highly significant increase in all previous measures when compared to the non-treated corneas (Table 1). Also, Mean number of corneal CD44 immunopositive cells ± SD in the studied groups (Table 2).
Table 1: Mean ± SD corneal epithelial and stromal thickness in the studied groups.
| Group | Mean ± SD of corneal epithelial thickness | Mean ± SD of corneal stromal thickness |
|---|---|---|
| Group I | 42.02 ± 354 | 44.41 ± 312 |
| Subgroup IIa | 29.89 ± 659* | 28.98 ± 659* |
| Subgroup IIb | 40.31 ± 194 | 42.14 ± 365 |
| Subgroup III a | 37.41 ± 143 | 40.31 ± 367 |
| Subgroup III b | 41.02 ± 354** | 44.01 ± 152** |
*Significantly different from the value of the control group at P<0.05.
** Significantly different from the value of the ulcerated non treated group at P<0.05.
Table 2: Mean number of corneal CD44 immunopositive cells ± SD in the studied groups.
| Group | Mean ± SD |
|---|---|
| Group I | 0 |
| Subgroup IIa | 0.8 ± 0.6** |
| Subgroup IIb | 3.9 ± 1.0* |
| Subgroup III a | 1.7± 2.0 |
| Subgroup III b | 9.4 ± 2.3* |
*Significantly different from the value of the control group at P<0.05.
** Significantly different from the value of the stem cells treated group at P<0.05.
Anti-Inflammatory role of MSCs in the cornea
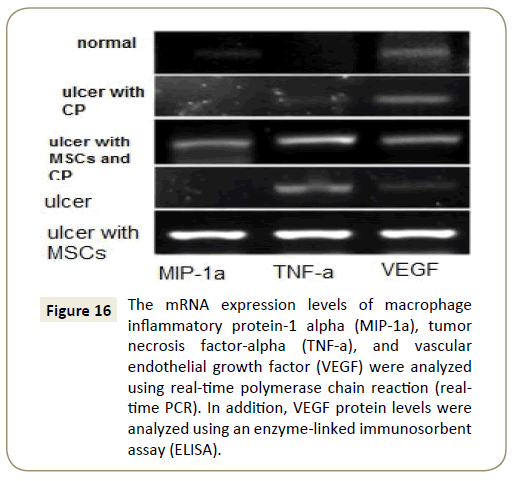
We used PCR and ELSA to detect the releasing of chemotactic factors by MSCs to assess their mechanism in inflammation. The result was found that the levels of MIP-1a were significantly higher in the MSC group than in the control group (p=0.005). However, the expression of MCP-1 and MIP-1 were not different between the two groups (p=0.016).TNF (an immunostimulatory cytokine) was higher in the MSC group than in the control group (p=0.005) (Figure 16).
Figure 16: The mRNA expression levels of macrophage inflammatory protein-1 alpha (MIP-1a), tumor necrosis factor-alpha (TNF-a), and vascular endothelial growth factor (VEGF) were analyzed using real-time polymerase chain reaction (realtime PCR). In addition, VEGF protein levels were analyzed using an enzyme-linked immunosorbent assay (ELISA).
Discussion
Common ocular injury is chemical corneal burn which leads to massive corneal damage with impairment of vision. Therefore, it is necessary to find accurate treatment. Recently, a promising approach for treatment of corneal ulcer is amultipotent cells known as MSCs [32,33].
Those multipotential stem cells can migrate and home to specific tissues, differentiate and multiple in response to the local microenvironment conditions, and stimulate a local repair response [34], so in this study we study the effect of systemically transplanted MSCs to chemically induced corneal injury. Local alkali corneal injury stimulates a systemic reaction to help corneal healing. The bone marrow is stimulated as result of injury that consider as main source of inflammatory cells and multipotential progenitor cells [35]. In our study, the ulcerate corneal rats that had bone marrow suppression by CP showed many histological changes in the form of epithelial thinning with desquamation, degeneration, vacuolation of cells. Loss of normal arrangement of stroma collagen fibers with neovascularization and inflammatory cellular infiltration between their fibers with degenerated Keratocytes and degenerated endothelial cells in the Descemet’s membrane. Ultrastructural and morphometrical study confirmed these results as Ultrastructural examination revealed degenerated superfacial epithelial cells with loss of microvilli with irregular basal cell and wide separation of corneal stroma. The present histological corneal alteration explain the changes in PCR and these findings agreed with other investigators who reported that in bone marrow suppressed rabbits, corneal alkali injury did not induce a rapid and massive release of inflammatory cells from bone marrow into bloodstream [36]. In stem cells – treated rats, light microscopic examination revealed that the corneal structure that appeared almost similar to that of the control rats. In addition, there was a significant increase (P<0.05) in the mean corneal thickness compared with ulcerated untreated group. Ultrastructural examination showed preserved corneal epithelial cells, except for a few small vacuoles in the cytoplasm of the basal and intermediate epithelial cell layers. Many reports shown that MSCs has immunoregulatory properties in various diseases as promote the wound healing [37] regression of experimental autoimmune encephalomyelitis [35] and reduce myocardial inflammation [38]. In our study, we found that the MSCs have anti-inflammatory property for restoration of corneal epithelium and this was detected by PCR that revealed that the levels of MIP-1a were significantly higher in the MSC group than in the control group (p=0.005). The mechanism by which MSC act in this study could be due to the ability of MSCs to help the remnant corneal epithelial cells to regaining the stem cell niche in the cornea or may be differentiate into corneal stromal cells (keratocytes). Chemocytokines are substances released as result of any stress as burn lead to migration of stem cells and localize them into specific tissue [39]. Also, stem cell potentiate by this substances in inducing the mobilization of endogenous MSCs into peripheral blood, [40] which is consistent with our results that indicate the coincidence of increased frequencies of circulating MSCs with presence of higher level of CD 29 which is marker of stem cell.
In conclusion, our study revealed that systemic injection of autologous MSCs lead to corneal epithelial regeneration and inhibits neovascularization in an induced chemical corneal burn. This result revealed that the MSCs have therapeutic effects by suppressing the early inflammatory reaction of local corneal cells, but further study need to detect methods to keep MSCs in place and how they differentiate.
References
- Sappino AP, Schurch W, Gabbiani G (1990) Differentiation repertoire of fibroblastic cells Expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest 63:144-161.
- Merle H, Gérard M, Schrage N (2008) Ocular burn. J Fr Ophtalmol 31:723-734.
- Kao WW, Zhu G, Benza R, Kao CW (1996) Appearance of immune cells and expression of MHC II DQ molecule by fibroblasts in alkali-burned corneas. Cornea 15: 397-408.
- Krause DS, Theise ND, Collector MI (2001) Multi-organ, multilineage engraftment by a single bone marrow-derived stem cell. Cell 105: 369-377.
- Mori L, Bellini A, Stacey MA (2005) Fibrocytes contribute to the myofibroblast population in wounded skin and originate fromthe bonemarrow. Exp Cell Res 304: 81-90.
- Asahara T, Murohara T, Sullivan A (2007) Isolation of putative stem endothelial cells for angiogenesis. Science 275: 964-967.
- Brazelton TR, Rossi FM, Keshet GI, Blau HM (2000) From marrow to brain: expression of neuronal phenotypes in adult mice. Science 290: 1775-1779.
- Hristov M, Weber C (2007) Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med 8: 498-508.
- Youn OJ, Mee Kum K, Mi Sun S (2008) The Anti-Inflammatory and Anti-Angiogenic Role of Mesenchymal Stem Cells in Corneal Wound Healing Following Chemical Injury. Stem Cells 26: 1047-1055.
- Yanling MA, Youngshing XU, Zhifeng X (2006) Reconstruction of Chemically Burned Rat Corneal Surface by Bone Marrow-Derived Human Mesenchymal Stem Cells. Stem Cells 24: 315-321.
- Chamberlain G, Fox J, Ashton B, Middleton J (2007) Concise review mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25: 2739-2749.
- Sato K, Ozaki K, Mori (2010) Mesenchymal stromal cells for graft-versus-host disease: basic aspects and clinical outcomes. J Clin Exp Hematop 50: 79-89.
- Larijani B, Esfahani EN, Amini P (2012)Stem cell therapy in treatment of different diseases. Acta Med Iran 50: 79-96.
- Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogenic immune cell responses. Blood 105: 1815-1818.
- Zappia E, Casazza S, Enrico P (2005) Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 106: 1755-1761.
- Kuroda Y, Kitada M, Wakao S, Dezawa M (2011) Bone marrow mesenchymal cells: How do they contribute to tissue repair and are they really stem cells? Arch Immunol Ther Exp (Warsz) 59: 369-378.
- Yang MCS, Chi NH, Chou NK (2010) The influence of rat mesenchymal stem cell CD44 surface markers on cell growth, fibronectin expression, and cardiomyogenic differentiation on silk fibroin- Hyaluronic acid cardiac patches. Biomaterials 31: 854-862.
- Brodsky RA, Chen AR, Dorr D (2010) High-dose cyclophosphamide for severe aplastic anemia:long-term follow-up. Blood 115: 2136-2141.
- Oh SY, Choi JS, Kim EJ (2010) The role of macrophage migration inhibitory factor in ocular surface disease pathogenesis after chemical burn in the murine eye. Mol Vis 16: 2402-2411.
- Zhou S, Xie Z, Xiao O (2010) Inhibition of mouse alkali burn induced-corneal neovascularization by recombinant adenovirus encoding human vasohibin-1. Mol Vis 16: 1389-1398.
- Lu P, Li L, Mukaida N, Zhang X (2007) Alkali-induced corneal neovascularization is independent of CXCR2-mediated neutrophil infiltration. Cornea 26: 199-206.
- Horn MM, Paz AH, Duarte ME (2008) Germinative testicular cells and bone marrow mononuclear cells transplanted to a rat model of testicular degeneration. Cloning Stem Cells 10: 543-546.
- Nakamura T, Torimura T, Sakamoto M (2007) Significance and therapeutic potential of endothelial progenitor cell transplantation in a cirrhotic liver rat model. Gastroenterology 133: 91-107.
- Shuai TJ, Li C, Wei-Ying J (2010) Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Molecular Vision 16: 1304-1316.
- Popp FC, Slowik P, Eggenhofer E (2007) No contribution of multipotent mesenchymal stromal cells to liver regeneration in a rat model of prolonged hepatic injury. Stem Cells 25:639-645.
- Haasters F, Prall WC, Anz D (2009) Morphological and immunocytochemical characteristics indicate the yield of early progenitors and represent a quality control for human mesenchymal stem cell culturing. J Anat214: 759-767.
- Niki H,Hosokawa S, Nagaike K, Tagawa T (2004) A new immunofluorostaining method using red fluorescence of PerCP on formalinï¬ÂÂxed parafï¬ÂÂn-embedded tissues. J Immunol Methods 293:143-151.
- Bancroft JD, Layton C (2013) The Hematoxylin & eosin and Carbohydrates. In:Theory & Practice ofhistological techniques, Suvarna SK, Layton C and Bancroft JD editors. Churchill Livingstone of El Sevier, Philadelphia.
- Wu F, Jin W, Feng J (2010) Propamidine decrease mitochondrial complex III activity of Botrytis cinerea. BMB Rep43: 614-621.
- Yang MC, Chi NH, Chou NK (2010) The influence of rat mesenchymal stem cell CD44 surface markers on cell growth, fibronectin expression, and cardiomyogenic differentiation on silk fibroin- Hyaluronic acid cardiac patches. Biomaterials 31: 854-862.
- Lee J, KimE, MiChoi S, Kim D K (2016) Microvesicles from brain-extract— treated mesenchymal stem cells improves neurological functions in a rat model of ischemic stroke. Scientific Reports 6: 33038.
- Oh JY, Kim MK, Shin MS (2008) The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cell 26:1047-1055.
- Jiang TS, Cai L, Ji WY (2010)Reconstruction of the corneal epithelium with induced marrow esenchymal stem cells in rats. Mol Vis 16:1304-1316.
- Yamada M, Kubo H, Kobayashi S (2004) Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide- induced lung injury. J Immunol 172: 1266-1272.
- Ye J, Yao K, Kim JC (2006) Mesenchymal stem cell transplantation in a rabbit corneal alkali burn model:engraftment and involvement in wound healing. Eye (Lond) 20:482-490.
- Ye J, Lee SY, KooK K H, Yao K (2008) Bone marrow-derived progenitor cells promote corneal wound healing following alkali injury Graefes Arch Clin Exp Ophthalmol246: 217-222.
- Lazarus HM, Koc ON, Devine SM (2005) Cotransplantation of HLAidentical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant 11: 389 -398.
- Guo J, Lin GS, Bao CY(2007)Anti-Inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation 30: 97-104.
- Reinshagen H, Auw-Haedrich C, Sorg RV (2011) Corneal surface reconstruction using adult mesenchymal stem cells in experimental limbal stem cell deficiency in rabbits. Acta Ophthalmol 89:741-748.
- Lan Y, Kodati S, Lee HS (2012) Kinetics and Function of Mesenchymal Stem Cells in Corneal Injury. IOVS 53: 7.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences