Lercanidipine-Induced Cloudy Effluent in a Patient with Peritoneal Dialysis
Eray Eroglu1,2*, Aysenur Cirak2, Ismail Kocyigit1, Aydin Unal1, Murat Hayri Sipahioglu1, Bulent Tokgoz1 and Oktay Oymak1
1Division of Nephrology, Erciyes University School of Medicine, Kayseri, Turkey
2Department of Internal Medicine, Erciyes University School of Medicine, Kayseri, Turkey
- *Corresponding Author:
- Eray Eroglu
M.D, Medical Faculty, Divison of Nephrology
Department of Internal Medicine, Erciyes University School of Medicine
38039 Kayseri, Turkey
Tel: +905309220517
E-mail: drerayeroglu@hotmail.com
Received Date: July 19, 2017; Accepted Date: August 11, 2017; Published Date: August 14, 2017
Citation: Eroglu E, Cirak A, Kocyigit I, et al. (2017) Lercanidipine Induced Cloudy Effluent in a Patient with Peritoneal Dialysis. Jour Ren Med. Vol. 1 No.2: 10.
Abstract
Cloudy effluent is an important clinical finding in patients with continuous ambulatory peritoneal dialysis which is commonly related with peritonitis. The cloudy effluent is related with increased leukocytes which pass into the peritoneal fluid due to peritoneal infection or inflammation. However, there are several causes of cloudy effluents including eosinophil and/or erythrocyte involvement in the peritoneal fluid, malignant cells, trauma, increased fibrin structure and also drugs. Herein, we presented a case of continuous ambulatory peritoneal dialysis patient admitted to hospital with abdominal pain and cloudy effluent after the use of lercanidipine for hypertension. In the absence of clinical course of peritonitis patients’ turbidity of the peritoneal effluent has been cleared after the cessation of the drug.
Keywords
Lercanidipine; Cloudy effluent; Peritonitis
Introduction
Continuous ambulatory peritoneal dialysis (CAPD) is one of the renal replacement therapy modality for patients with end stage renal disease (ESRD). Infectious or non-infectious etiologies underlie the cloudy effluent in CAPD patients. Peritonitis is a serious complication of CAPD that is usually presented with cloudy effluent [1]. Polymorphonuclear leukocytes (PMNL) that passed through the peritoneal fluid are the main reason of the cloudy effluent in cases with peritonitis. Clinical course of peritonitis consists of nausea, vomiting, abdominal pain, fever and presence of microorganism which was generally obtained in culture of peritoneal fluid [2].
There are also non-infectious causes of cloudy effluent in patients with CAPD. In the absence of peritonitis noninfectious causes divided into cellular and non-cellular subgroups. Cellular reasons include eosinophilia (allergic reactions to PD solutions or Tenckhoff catheter, intraperitoneal malignancy, rupture of hydatid cyst, vasculitis, polyserositis), PMNL migration without infection, leakage of malignant cells or leakage of erythrocytes into the peritoneal cavity [1,3,4]. Culture negative cloudy effluent may also have related with acute abdomen such as cholecystitis, incarcerated hernia, pancreatitis, acute appendicitis and sterile organ rupture which was caused to PMNL migration into the peritoneal cavity in CAPD patients [5]. Non-cellular causes include drugs, trauma, fibrin and increased peritoneal triglyceride levels [1].
Among the drugs, cefalotin, cefazolin, gentamycin, chloramphenicol, amphotericin B, thrombolytic agents, vancomycin and dihydropyridine group of calcium channel blockers (CCBs) are associated with turbidity of the effluent [6,7]. Manidipine, benidipine, nifedipine and nizoldipine have also been reported that induce cloudy effluent among CCBs [8,9].
Herein, we report of a patient with CAPD and cloudy effluent occurrence after using lercanidipine 10 mg once daily within one day.
Case Report
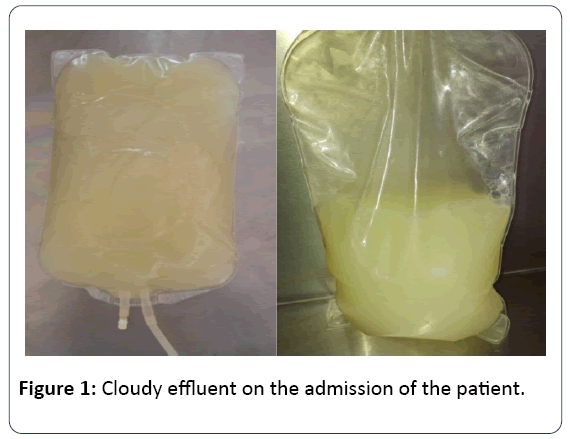
A 64-year-old female patient who had been treated with CAPD for one year was admitted our hospital due to turbid peritoneal effluent accompanied by constant abdominal pain that began one day before her admission (Figure 1). The underlying reason of ESRD is hypertension. She had no history of peritonitis. Her medical history includes hypertension for ten years. She had no smoking or alcohol habits. Her medication includes olmesartan 20 mg once daily, calcium acetate 700 mg three times daily, calcitriol 0.50 mcg once daily and darbepoetin alfa 20 mcg weekly injections. Patient had approximately 1500 mL urine; CAPD prescription consisted of four cycles of 2 L exchanges with 1.36% glucose solution per day. She had no fever, nausea and vomiting. On physical examination, she was fully oriented and cooperated.
Blood pressure was 160/100 mmHg, heart rate was 80 beats per minute and rhythmic, body temperature was 36.8°C and breathing rate was 18 per minute. There was no tenderness and rebound in the abdomen and bowel sounds were normal. Catheter exit site was clean. Samples taken from the effluent for the leukocyte count, gram staining and culture at the end of the 6-hour dwell period. There were no fibrin, blood particles and leucocytes in the specimen. Gram staining revealed no properties and there were no any bacterial or fungal pathogen in the culture.
Laboratory results revealed that, WBC (white blood cell) : 5,60 ×103/μL (4.8-10.7 × 103/μL), hemoglobin: 12.8 g/dL (14-18 g/dL), platelets: 274 × 103/μL (130-400 × 103/μL), BUN: 46.1 mg/dL (6-20 mg/dL), creatinine: 7.9 mg/dL (0.7-1.2 mg/ dL), sodium:140 mmol/L (136-145 mmol/L), potassium: 3.54 mmol/L (3.5-5.1 mmol/L), calcium: 9.2 mg/dL (8.6-10.2 mg/ dL), phosphorus: 4.48 mg/dL (2.5-4.5 mg/dL), albumin: 3.8 g/dL (3.5-5.2 g/dL), AST: 20 u/L (0-40 u/L), ALT:14 u/L (0-41 u/L), GGT: 59 u/L (10-71 u/L), LDH: 185 u/L(135-225 u/L), ALP: 90 u/L(40-130 u/L). Serum lipid levels were within normal range and iPTH level was 570 pg/mL (15-65 pg/mL). Ferritin levels were 132 ng/mL (15-150 ng/mL). C-reactive protein level was 5.4 mg/L (0-6 mg/L).
Urine and blood cultures were all negative in terms of any other infection. Analyses of the cloudy peritoneal effluent demonstrated triglyceride concentration 80 mg/dL. On reviewing her current medications, she had been prescribed lercanidipine 10 mg once daily a day ago. After exclusion of the acute abdomen and infectious peritonitis paracetamol 500 mg p.o. administered to the patient .6 hours later she was reported that the pain was completely regressed. After discontinuation of lercanidipine, the effluent was normalized within 24 hours (Figure 2).
Discussion
Calcium channel blockers have been used in the treatment of hypertension since the 1980s. They divided into different groups according to the functional molecular structures which include phenylalkylamine, benzodiazepine or dihydropyridine [6]. Possible causes of the formation of turbid effluent due to CCBs are thought to be lipophilic structures, gastroenterological system specific effects on lymph ducts and blood vessels [10,11].
Lercanidipine a new liphophilic vasoselective dihydropyridine- type calcium channel blocker with a growing use in recent years. It leads to systemic vasodilatation by blocking the influx of calcium ions through L-type calcium channels in cell membranes. It has a higher vascular selectivity, highly lipophilic feature, better tolerability and lower incidence of adverse effects, especially ankle edema. It has been rarely shown that lercanidipine have caused chyloperitoneum via increasing the trygliceride levels of peritoneal fluid, however the underlying mechanism of increasing tryglseride level has not been completely clarified [11].
A study that has been conducted in CAPD patients, turbid effluent has found to be 100% in patients with benidipine treatment, 42% in manidipine, 9% in nizoldipine and 0.6% in nifedipine [8]. Several studies have been reported the ratio of turbid effluent between 13% and 57% in patients with peritoneal dialysis under lercanidipine treatment [10-12]. In addition, Hsiao et al. have been demonstrated that 9 of 40 CAPD patients had cloudy effluent within two days of lercanidipine initiation. That 9 patients had higher mean triglyceride levels compared with the other 31 patients without cloudy effluent (19.3 ± 6.3 mg/dl vs. 0 mg/dl) [6]. Supportively, all triglyceride concentration in the cloudy effluent did not meet the criteria of chyloperitoneum (triglyceride levels over 110 mg/dl in the dialysate) in other reports. It has been reported that higher levels of triglyceride might be related with the large peritoneal dialysis volume due to increased peritoneal transport [13,14]. In addition, patients with lercanidipine-induced cloudy effluent tend to have a higher peritoneal membrane transport, which may lead to more lercanidipine accumulated in the peritoneal cavity through diffusion, and resulted in decreased lymphatic absorption [15,16]. Similarly, triglyceride levels of effluent in our case were mildly elevated and did not meet the criteria for chyloperitoneum, since she admitted earlier to hospital after taking the first dose of the lercanidipine.
Conclusion
In conclusion, physicians should be aware of drug induced cloudy effluent in CAPD patients in the absence of peritonitis. It is important to diagnose non-infectious causes of turbid effluent to avoid unnecessary antibiotics therapy and their costs. Drugs, trauma and other possible causes should be questioned carefully.
Disclosure Statement
The authors report no conflicts of interest.
References
- Rocklin MA, Teitelbaum I (2001) Non-infectious causes of cloudy peritoneal dialysate. Semin Dial 14:37-40
- Vas SI (1989) Peritonitis. In: Peritoneal Dialysis, (3rd edn), Nolph, KD (Eds), Kluwer Academic Publishers, Dordrecht 261.
- Rolleston HD (1914) A case of eosinophil ascites with remarks. Br Med J. 1:238-239.
- Harkavy J (1941) Vascular allergy: Pathogenesis of bronchial asthma with recurrent pulmonary infiltrations and eosinophilicpolyserositis. Arch Intern Med 67:709–734
- Steiner RW, Halasz NA(1990) Abdominal catastrophes and other unusual events in continuous ambulatory peritoneal dialysis patients. Am J Kidney Dis 15:1–7.
- Hsiao PJ, Lin HW, Sung CC, Wang CW(2010) Incidence and clinical course of lercanidipine-associated cloudy effluent in continuous ambulatory peritoneal dialysis. ClinNephrol 74:217-222
- Piraino BM, Silver MR, Dominguez JH, Puschett JB (1984) Peritoneal eosinophils during intermittent peritoneal dialysis. Am J Nephrol 4:152–157.
- YoshimotoK,SaimaS,NakamuraY,Nakayama M (1998) Dihydropyridine type calcium channel blocker-induced turbid dialysate in patients undergoing peritoneal dialysis. ClinNephrol 50: 90-93.
- Freiman JP, Graham DJ, Reed TG, Mc-Goodwin EB (1992)Chemical peritonitis following the intraperitoneal administration of vancomycin. Perit Dial Int 12: 57-60.
- Topal C, Sayarlioglu H, Dogan E, Erkoc R, Soyoral Y (2006)Cloudy dialysate due to lercanidipine. Nephrol Dial Transplant 21: 2997-2998.
- Yang WS, Huang JW, Chen HW, Tsai TJ, Wu KD (2008)Lercanidipine-induced chyloperitoneum in patients on peritoneal dialysis. Perit Dial Int 28: 632-636.
- Tsao YT, Chen WL (2009) Calcium channel blocker-induced chylous ascites in peritoneal dialysis. Kidney Int75:868.
- Levy RI, Wenk RE (2001)Chyloperitoneum in a peritoneal dialysis patient. Am J Kidney Dis 38: 12.
- Cardenas A, Chopra S (2002) Chylous ascites. Am J Gastroenterol 97: 1896-1900.
- Suzuki H, Inoue T, Kobayashi K, Shoda J, Nakamoto H (2006) The newly developed calcium antagonist, azelnidipine, increases drain volume in continuous ambulatory peritoneal dialysis patients. AdvPerit Dial 22:18-23.
- Rampino T, Dal-Canton A (2003)Peritoneal dialysis and epithelial-to-mesenchymal transition. N Engl J Med348:2037-2039.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences