ISSN : 2348-1927
Annals of Biological Sciences
Effect of the Environmental Factors on Diketopiperazine Cyclo (Pro-Phe) Production and antifungal Activity of Bacillus amyloliquefaciens Q-426
Jian-Hua Wang1, Yu-Xuan Zhang1, Cui Zhang1, Jian Lu1, Chun-Shan Quan2*
1Key Laboratory of Coastal Environmental Processes and Ecological Remediation, Yantai Institute of Coastal Zone Research, PR China
2Life Science College, Dalian Nationalities University, Dalian, PR China
Abstract
Bacillus amyloliquefaciens Q-426 with strong antifungal activity was isolated from compost samples in Dalian of China. Four kinds of diketopiperazines were extracted from the strain including cyclo (Pro-Phe) (cPP) which had both D- and L-isomers. Studies in this paper found that cPP production and antifungal activity were affected by environmental factors including incubation time, temperature, pH and carbon source in the media. The results indicated that cPP had a significant effect on the antifungal activity of B. amyloliquefaciens Q-426, and the previous studies found that cPP showed a positive response to biosensors which were used to detect signal molecules. Based on these studies, diketopiperazines were suspected as the signal molecule of B. amyloliquefaciens Q-426.
Keywords
diketopiperazines, cPP, Antifungal activity, Signal molecule
Introduction.
Many bacteria isolated from nature, have been proven to be a rich source of diverse arrays of bioactive metabolites with great potential for pharmaceutical and medical applications. Bacillus amyloliquefaciens was discovered in soil 1943 by Fukumoto [1], who gave the bacterium its name because it produced a liquifying amylase. In 1987 a group of scientists including Priest et al. [2] established it as a separate species. B. amyloliquefaciens is a species of Grampositive bacteria which can produce many structurally diverse antimicrobial compounds, such as iturins, surfactins, fengycins.
Cyclo (Pro-Phe) (cPP) is a kind of diketopiperazines which formed from two amino acids by cyclodehydration, and diketopiperazines have been found in various microorganisms including Bacillus sp. [3,4]. diketopiperazines including cPP were found to have a wide range of biological functions, such as anti-tumour [5], antiviral [6], antibacterial [7-9]. With a growing awareness of the diversity and biological roles played by many of the diketopiperazines found in nature, more and more attention have been paid to these compounds. diketopiperazines were firstly suspected to be signal molecule of Pseudomonas aeruginosa by Holden et al. [10] and then Degrassi et al. [11] found diketopiperazines in Pseudomonas putida WCS358. They both observed that diketopiperazines showed positive response to signal molecule biosensor Agrobacterium tumefaciens NT1 (pDCI41E33) or Escherichia coli JM109 (pSB401).
B. amyloliquefaciens Q-426 was isolated from compost samples collected in Dalian of China based on its antifungal activities and identified according to morphological and biochemical characteristics and 16S rDNA sequence (deposited in NCBI with a GenBank accession NO. HM130462). In previous studies [12,13], diketopiperazines and antifungal compounds like bacillomycin D, fengycin A and B were found produced in B. amyloliquefaciens Q-426. Here we found that environmental factors played a great influence on cPP production and antifungal activity. There were some relative connections between cPP yield and antifungal activity and diketopiperazines were suspected as the signal molecule of B. amyloliquefaciens Q-426.
Materials and Methods
Microorganisms and culture conditions
B. amyloliquefaciens Q-426 was preserved in the China Center for Type Culture Collection (CCTCC NO. M2010237). The strain was grown at 37°C with 200 rpm in a growth medium CA, which contains (g/L): K2HPO4, 1.5; KH2PO4, 0.6; NH4Cl, 2.0; MgSO4.7H2O, 0.5; Na-Citrate, 7.5, yeast extract, 8.0 and the initial pH was 6.5-7.0.
Candida albicans CGMCC 2.538 was purchased from the China General Microbiological Culture Collection Center and the growth medium YPD have been described earlier [14].
Extraction and quantitative analysis of diketopiperazine cPP
The method of extraction of cPP from the culture supernatants of B. amyloliquefaciens Q-426 has been described previously [15]. Briefly, supernatants (20 ml) from stationary-phase cultures of sample bacteria extracted with equal volume of acidified ethyl acetate (0.5% acetic acid) three times. The extract was removed by rotary evaporation (40~45°C) and the residue re-suspended in 1 ml acetonitrile. The crude extracts which contained cPP were stored at -20°C prior to analysis.
Quantitative analysis of cPP in the extract was performed by gas chromatography coupled with electron-impact mass spectrometry on GCMS-QP2010 Plus (Shimadzu Corporation, Kyoto, Japan) and the detailed parameters set were described previously [15]. The cPP produced by B. amyloliquefaciens Q-426 under different conditions were quantified basing on the peak area in total ion chromatogram (TIC) of GC-MS.
Extraction of antifungal substance and antifungal assays
The extraction of antifungal substance from B. amyloliquefaciens Q-426 was based on the method described elsewhere [15]. After concentrating the culture supernatant, the antifungal substance was co-precipitated by addition of ammonium sulfate and the resulting pellet was redissolved in phosphate buffer. About 1ml antifungal substance was obtained from 20 ml culture supernatant.
The antifungal activity of the samples was measured by the rate of inhibition on C. albicans and 1 ml antifungal substance was added to 5 ml C. albicans culture medium (inoculum 0.1%), at the same time a negative control without antifungal substance was prepared. The inhibition rate of the antifungal substance was calculated by the following formula [15]:
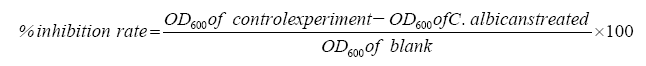
Where OD600 is the optical density at 600 nm of the sample examined. All of the results were quantified at least 5 times.
The environmental factors investigated
Diketopiperazine cPP yields were compared under different conditions, including variation of incubation time, temperature, pH level and carbon source in the media. The incubation time was varied between 0~72 h (0, 6, 12, 24, 48 and 72 h). The temperatures investigated were 25, 31, 37, 43 and 49°C. The pH levels studied were 3, 4, 5, 7, 8, 9 and 10. The carbon sources used here were sodium citrate (C), sodium acetate (A), sodium format (F), glucose (G), lactose (L) and maltose (M).
Results
Characterization and quantification of cPP
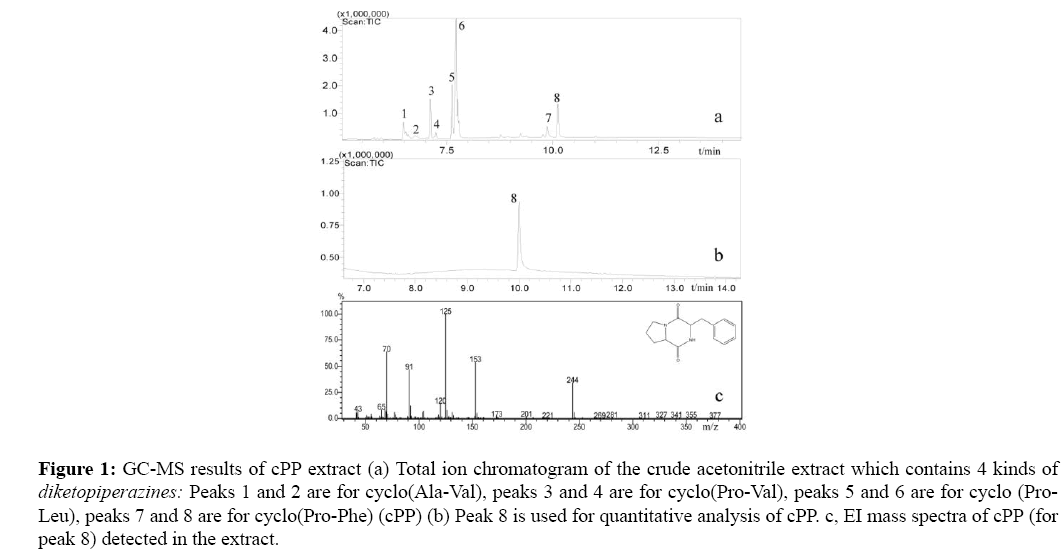
As already mentioned, the crude acetonitrile extract was detected by GC-MS and mass analysis was performed in full-scan mode (m/z 40-400). The GC-MS result of is shown in Figure 1. In TIC shown in Figure 1a, the peaks 1-8 are all for diketopiperazines [12,15] and peaks 7 and 8 correspond to cPP by comparision the EI-MS (Figure 1c) with mass spectral library. The standard compound L-cyclo(Pro-Phe) gave the same retention time as peak 8, so peak 8 is for L-cyclo(Pro-Phe) and peak 7 is for D-cyclo(Pro-Phe). In this study, cPP was quantified on the basis the area of peak 8.
Figure 1: GC-MS results of cPP extract (a) Total ion chromatogram of the crude acetonitrile extract which contains 4 kinds of diketopiperazines: Peaks 1 and 2 are for cyclo(Ala-Val), peaks 3 and 4 are for cyclo(Pro-Val), peaks 5 and 6 are for cyclo (Pro- Leu), peaks 7 and 8 are for cyclo(Pro-Phe) (cPP) (b) Peak 8 is used for quantitative analysis of cPP. c, EI mass spectra of cPP (for peak 8) detected in the extract.
cPP synthesis in B. amyloliquefaciens Q-426 is incubation time dependent
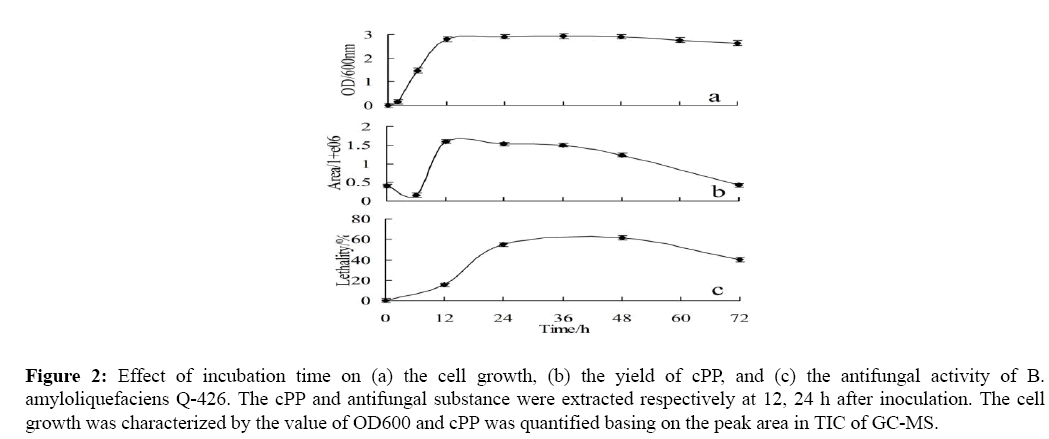
To determine the pattern of cPP production in B. amyloliquefaciens Q-426, DKPs were extracted from the culture at different growth stages and analyzed by GC-MS. The cPP production and antifungal activity variation pattern during growth is shown in Figure 2. Figure 2a and Figure 2b show that the yield of cPP increased with bacteria growth and reached the maximum in late exponential phase (about 12 h after inoculation), which in line with the growth pattern of general signal molecule. As the complex medium used to culture B. amyloliquefaciens Q-426 includes yeast extract, which can produce cyclic peptides by heat sterilization [16]. A little of cPP was produced by the culture medium, however it is certain that the cPP formed by the medium is much less than the cPP produced by the bacteria. After the late exponential phase, the concentration of bacteria maintained and declined slowly (shown in Figure 2a) and the cPP concentration reduced gradually until almost to zero.
Figure 2: Effect of incubation time on (a) the cell growth, (b) the yield of cPP, and (c) the antifungal activity of B. amyloliquefaciens Q-426. The cPP and antifungal substance were extracted respectively at 12, 24 h after inoculation. The cell growth was characterized by the value of OD600 and cPP was quantified basing on the peak area in TIC of GC-MS.
The antifungal activity gradually improved to the maximum at 24 h and last for 24 h, then decreased with the reduction of cell biomass (Figure 2c). It was indicated that antifungal substance are produced only after completion of the bacteria growth phase. The highest antifungal activity came after the yield maximum of cPP, which suggested that the antifungal activity was under the regulation of diketopiperazines.
cPP maintains a stable production in a wide range of temperature
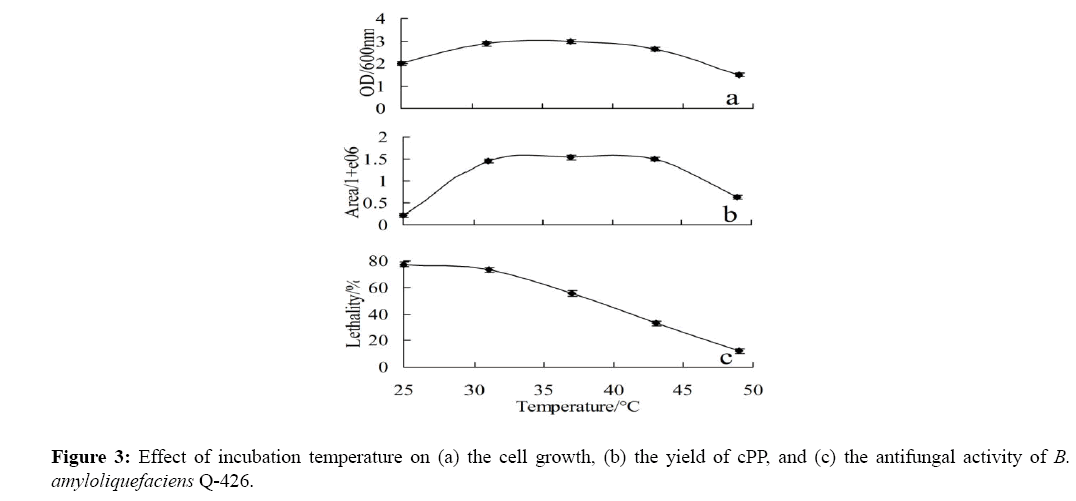
In a wide temperature range of 31-42°C, B. amyloliquefaciens Q-426 has a stable yield of cPP, which is consistent with the biomass of the strain (Figure 3a and Figure 3b). This result confirmed that the amount of cPP is in proportion with the bacterial biomass, and the cPP yield was not dependent upon temperature changes in a specific range. Amazingly, the antifungal activity of the culture extracts reduced with the temperature increased, which is quietly different from the trends of the biomass. Two hypotheses may explain the decrease of antifungal activity in high temperature: (1) It may because that low temperature is conducive to the synthesis of antifungal substances. (2) In the high-temperature environment, B. amyloliquefaciens Q-426 enters into stationary phase in advance, and then become to wither up. The bacteria cannot maintain a good growth until 48 h and the synthesis of secondary metabolites stopped, so the antifungal activity detected at 48 h reduced.
Influence of the carbon source on cPP production
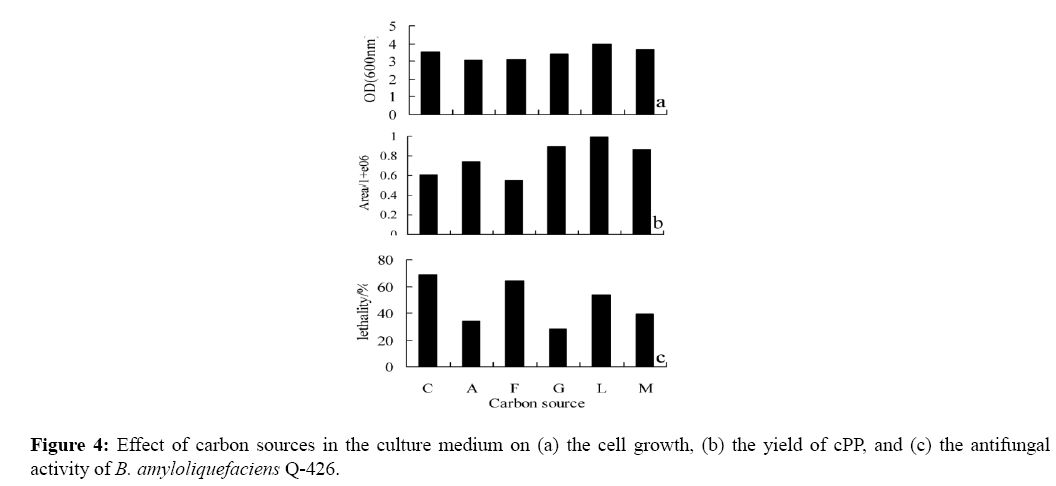
To investigate the carbon source influence on diketopiperazines synthesis and antifungal activity, we changed the sodium citrate in CA medium with sodium acetate, sodium formate, glucose, lactose and maltose. Figure 4 shows that supplied with these carbon sources, B. amyloliquefaciens Q-426 can grow well, which was reflected in the values of OD600 (Figure 4a). However, the yields of cPP were quite different; cultured with glucose, lactose or maltose (sample G, L, M), B. amyloliquefaciens Q-426 produced more cPP than the others (sample C, A, F) at 12 h (shown in Figure 4b). It might be because that the growth speed of the strain was different in mediums with different carbon sources, and the time went to late logarithmic phase and the bacteria biomass reached at stationary phase were quite different. B. amyloliquefaciens Q-426 could produce antifungal substance at different culture medium, just with the different antifungal activity. Moreover, the antifungal activity was related to bacteria density and cPP production (shown in Figure 4c).
Cultured the strain with glucose, lactose or maltose (sample G, L, M), B. amyloliquefaciens Q-426 grows better and produced more cPP, but the antifungal activity was lower. Compared with the sodium citrate and lactose mediums (sample C, L), we found that the same thing happen. It was indicated that the antifungal activity of the strain was not only related to the density of the bacteria, but also be inhibited by the diketopiperazines synthesis.
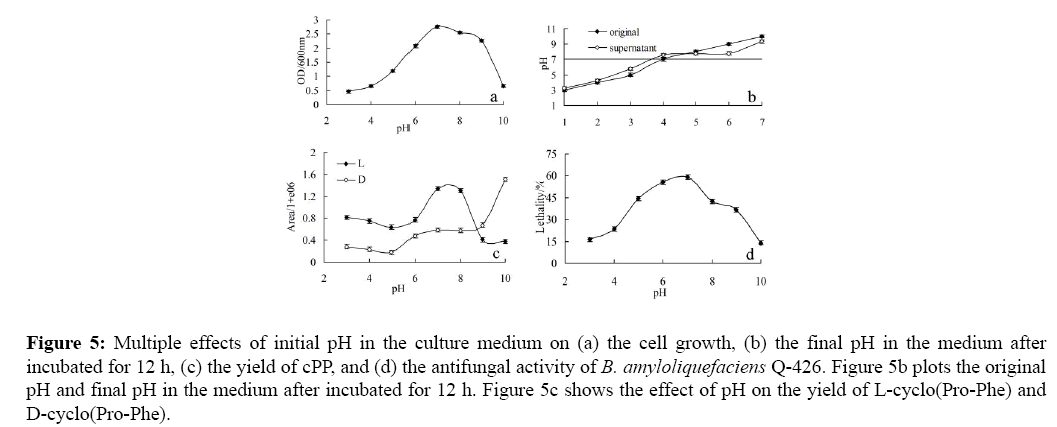
Multiple effect of the medium pH on cPP production
To investigate the pH influence on cPP synthesis, the yield of cPP was plotted as the function of pH levels (Figure 5). Like most bacteria, B. amyloliquefaciens Q-426 grows well in the approximate neutral environment (pH 6-9), other than strong acid or alkaline environment (shown in Figure 5a). The strain could make a slight modification to the pH level of the initial culture medium (Figure 5b) and the pH level of the supernatant would be raised when the initial culture was neutral or acid and be reduced when the initial culture was alkaline. As shown in Figure 5c, the cPP produced by B. amyloliquefaciens Q-426 was given priority to L-isomer in acidic and slight alkaline environment (pH ≤ 8) and D-isomer in alkaline conditions (pH ≥ 9).
Figure 5: Multiple effects of initial pH in the culture medium on (a) the cell growth, (b) the final pH in the medium after incubated for 12 h, (c) the yield of cPP, and (d) the antifungal activity of B. amyloliquefaciens Q-426. Figure 5b plots the original pH and final pH in the medium after incubated for 12 h. Figure 5c shows the effect of pH on the yield of L-cyclo(Pro-Phe) and D-cyclo(Pro-Phe).
Coincidentally, this phenomenon exists in other diketopiperazines and it was indicated that the chirality of the diketopiperazines was affected by the acid-base property of the culture medium. In terms of total cPP, L- and Disomers, cultured in neutral medium, the strain could produce the most cPP and then more in alkaline than acid medium. The Figure 5d showed that neutral environment was the most suitable for antifungal substances synthesis and with the pH increase or declining, antifungal activity were both decreased. Contrast the strain biomass, cPP yield, antifungal activity produced in weakly acidic (pH=6) and alkaline (pH=8) environment, we found that the weakly alkaline environment was more conducive to bacteria growth and cPP synthesis, while antifungal activity dropped compared with weakly acidic environment. It suggested that diketopiperazines might play a negative regulation role in antifungal substances synthesis.
Discussion
Four kinds of diketopiperazines were found in the supernatant of B. amyloliquefaciens Q-426 [12] and as we discussed previously [15], diketopiperazines showed positive response to biosensors which used to detect AHLs. Previous studies on this compound showed that diketopiperazines have been suspected to be the signal molecule of P. aeruginosa [10] and P. putida [11]. Studies in this paper suggested that diketopiperazine cPP might have some influence on the antifungal activity of the strain and cPP were suspected to be the signal molecule of B. amyloliquefaciens Q-426.
As other organism behaviors, cPP production and antifungal activity are processes apparently dependent on many environmental factors, such as incubation time, temperature, carbon source and pH. Research on the relationship of the cPP production and the antifungal activity of B. amyloliquefaciens Q-426 cultured in different environment will help us further understand the mechanism of action of diketopiperazine in the strain.
When the cells concentration reached the threshold, cPP is secreted to the medium gradually, and climb to the maximum until the late exponential phase or the early stationary phase (Figure 2a and Figure 2b). In other words, the concentration of cells and cPP climb to the maximum at the same stage, which proved that the production of cPP is a quorum sensing behavior. The hypothesis was supported by Figure 3a and Figure 3b, which indicated that cPP yield and cell biomass maintains high in a wide temperature range of 31-42°C. The result shown in Figure 5a and Figure 5c also indicated that the yield of cPP was directly related to the concentration of bacteria. The bacteria could consume cPP as carbon and nitrogen sources because of lacking of nutrition, so cPP almost depleted at 72 h (Figure 2b).
Variations in the fermentation environment factors often result in an alteration in antibiotics production. The alternation involves changes in both yield and composition of the compound [17]. Roitman et al. [18] reported that by varying the conditions under which B. cepacia is grown, the yield and composition of the phenylpyrrole metabolites could be changed. Antibiotic produced by B. cepacia NB-1 also was greatly influenced by nutritional and environmental factors [19]. Studies in this work indicated that the environmental factors might influence the yield of cPP and the antifungal activity of B. amyloliquefaciens Q-426. The Figure 2 showed that the antifungal activity increased rapidly after the yield of DKPs reached the maximum, which indicated that antifungal substance production was under the regulation of diketopiperazine.
The results consisted with previous studies that the concentration of signal molecule reached a threshold can cause the expression of the gene which functioned the antibiotic production [20,21]. Figure 4b and Figure 4c showed that cPP played a negative effect on the synthesis of antifungal substance when the bacteria maintained a good growth, and it was supported by the results shown in Figure 5b and Figure 5c. The present data clearly established that the antifungal activity of B. amyloliquefaciens Q-426 is not only influenced by environmental factors, but also is under the signaling regulation and the diketopiperazine cPP may act as signal molecule.
Acknowledgement
This work was supported by the grant from Science and Technology Project of Yantai (2016ZH073), One Hundred- Talent Plan of Chinese Academy of Sciences and the Two-Hundred Talents Plan of Yantai.
References
- Fukumoto, J., J Agr Chem Soc, 1943. 19: pp. 487-503.
- Priest, F.G., et al., Bacteriol, 1987. 37: 69-71.
- Trischman, J.A., et al., Mar Biotechnol, 2004, 6: pp. 215-220.
- Lu, X., et al., Chem Nat Compd, 2009. 45: pp. 244-246.
- Nicholson, B., et al., Anti-Cancer Drugs, 2006. 17: pp. 25-31.
- Sinha, S., et al., Nucleot Nucl, 2004. 23: pp. 1815-1824.
- De Kievit, T.R. and Iglewski, B.H., Infect Immun, 2000. 68: pp. 4839-4849.
- Fdhila, F., et al., J Nat Prod, 2003. 66: pp. 1299-1301.
- Kozlovsky, A.G., et al., Appl Biochem Microbiol, 2003. 39: pp. 393-397.
- Holden, M.T.G., et al., Mol Microbiol, 1999. 33: pp. 1254-1266.
- Degrassi, G., et al., Curr Microbiol, 2002. 45: pp. 250-254.
- Wang, J.H., et al., World J Microbiol Biotechnol, 2016. 32: p. 143.
- Zhao, P.C., et al., J Basic Microbiol, 2014. 54: pp. 448-456.
- Li, X., et al., FEMS Microbiol Lett, 2008. 285: pp. 250-256.
- Wang, J.H., et al., Fan Anal Bioanal Chem, 2010. 396: pp. 1773-1779.
- Skwierczynski, R.D. and Connors, K.A., Pharma Res, 1993. 10: pp. 1174-1180.
- Upadhyay, R., Visintin, L. and Jayaswal, R., Can J Microbiol, 1991. 37: pp. 880-884.
- Roitman, J.N., Mahoney, N.E. and Janisiewicz, W.J., Appl Microbiol Biotech, 1990. 34: pp. 381-386.
- El-Banna, N. and Winkelmann, G., J Appl Microbiol, 1998. 85: pp. 69-78.
- Marahiel, M.A., Nakano, M.M. and Zuber. P., Mol Microbiol, 1993. 7: pp. 631-636.
- Stein, T., et al., Mol Microbiol, 2002. 44: pp. 403-416.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences