ISSN : 2634-7164
Journal of Medical Microbiology and Immunology Research
Citokines Study in Lungs Infected with Pasteurellaceae and Mycoplasma spp.from Fattening Lambs
Sara Fernández1*, Javier Galapero1, Joaquín Rey2, Carlos J Pérez3, Alfonso Ramos3 and Luis Gómez4
1Department of Animal Medicine, Faculty of Veterinary Medicine, Histology and Pathological Anatomy Unit, University of Extremadura, Avda de la Universidad s/n, 10003, 9 Cáceres, Spain
2Department of Animal Health, Faculty of Veterinary Medicine, Infectious diseases Unit, University of Extremadura, Avda de la Universidad s/n, 10003, Cáceres, Spain
3Department of Mathematics, Faculty of Veterinary Medicine, Biostatistics Unit, University of Extremadura, Avda de la Universidad s/n, 10003, Cáceres, Spain
4Biotechnology Research Institute in Livestock & Cynegetic, Avda de la Universidad s/n, Cáceres, Spain
- *Corresponding Author:
- Sara Fernández
Department of Animal Medicine
Faculty of Veterinary Medicine
Histology and Pathological Anatomy Unit
University of Extremadura, Avda de la Universidad s/n
10003, 9 Cáceres, Spain
Tel: +34927257106
E-mail: safernandezd@unex.es
Received Date: Mar 12, 2018; Accepted Date: Apr 02, 2018; Published Date: Apr 11, 2018
Citation: Fernández S, Galapero J, Rey J, Pérez CJ, Ramos A, et al. (2018) Citokines Study in Lungs Infected with Pasteurellaceae and Mycoplasma spp. from Fattening Lambs. J Med Microbiol Immunol Res 2:1.
Copyright: © 2018 Fernández S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Cytokines have an important role in the inflammatory reaction and development of infection by respiratory microorganisms. In order to identify the density and distribution of different inflammatory cells in lamb lungs affected with Pasteurella multocida, Mannheimia haemolytica, Mycoplasma ovipneumoniae and Mycoplasma arginini under natural conditions, microbiological and immunohistochemical techniques were used. The experiment was conducted with forty fattening lambs from five feedlots in Extremadura (Southwestern Spain). The results showed an elevated count of labeled cells with a proinflammatory cytokine (TNF-α) in bronchial area, whereas, in alveolar area the anti-inflammatory cytokine, IL-10, presented the highest level of labelled cells. These observations suggested the chronologic order of these respiratory microorganisms in lungs according the different localization in lung tissues of the labelled cells. Different inflammatory response and possibly pathogenicity is also suggested, being M. haemolytica, which produced the most response of cytokines and M. arginini the lowest one.
Keywords
Respiratory syndrome; Immunohistochemistry
Introduction
Respiratory diseases are complex pathologies, occurring as a consequence of host interaction with various infectious agents under the influence of environmental factors [1]. These diseases cause great economic losses in Spanish sheep industry. This situation is especially important in Extremadura (Southwest, Spain), where sheep herds are present in 94% of municipalities, being the highest producer of lambs in Spain [2]. This problem is intensified by the environmental conditions in this region such as high temperatures and low relative humidity in warm months [3].
Pasteurellaceae (Pasteurella multocida and Mannheimia haemolytica) is highly prevalent among animal populations, where they are often found as part of the normal microbiota of the oral, nasopharyngeal, and upper respiratory tracts. P. multocida is a bacterial pathogen associated with a variety of diseases in animals, causing pneumonic and septicemic pasteurellosis in sheep’s [4]. Besides, M. haemolytica is one of the most important respiratory pathogens of domestic ruminants that cause serious outbreaks of acute pneumonia in neonatal, weaned and growing lambs [5].
Species of Mycoplasma spp. are also involved in respiratory pathologies. Mycoplasma ovipnuemoniae is one of the major pathogens that causes pneumonia in sheeps [6,7], and is highly infectious and prevalent. Mycoplasma arginini is a ubiquitous microorganism of many animal species, frequently isolated from the respiratory tracts of ill lambs, and sometimes it is present in mixed culture with M. ovipneumoniae [8]. Although M. arginini is not thought to be a major respiratory pathogen, it could increase the pathological damage that other microorganisms produce in the respiratory tract [9].
The innate immune system has the ability to eradicate pathogens by igniting a wide spectrum of biological responses involving numerous cell types and cell-signalling molecules [10]. The production of cytokines plays an important role in inflammatory reaction and development of infection. The proinflammatory cytokines, including interleukin 1 (IL-1), tumour necrosis factor-α (TNF-α) and interleukin 6 (IL-6), are of great importance during the innate immune response [11], IL-6 plays an important role in the regulation of the specific immune response during the final stage of inflammatory disease, albeit secondary to TNF-α and IL-1, which are considered to be the major inflammatory cytokines [12]. In contrast, interleukin 10 (IL-10) is considered an immunoregulatory cytokine [11,13] and, its main biological function seems to be the limitation and termination of inflammatory response, regulation of differentiation and proliferation of several immune cells [14]. In spite of the previous fact, IL-10 is a pleiotropic cytokine that can exert either immuno-stimulatory or immuno-suppressive effects on many cell types [13].
Several studies have examined the role of cytokines in the pathogenesis of ovine respiratory syndrome [5,15,16]; however, it is not clear how cytokines participate. Accordingly, the main aim of this study was to describe density and distribution of different cytokines in lamb lungs affected with P. multocida, M. haemolytica, M. ovipneumoniae or M. arginini under natural conditions, using histological and immunohistochemical techniques, in order to a best knowledge of the action of the implied agents.
Materials and Methods
Experimental design
A sample of 40 both sexes fattening lambs from five feedlots in Extremadura (Southwestern Spain) was considered in this study, specifically, 10 animals naturally infected with each studied microorganism (P. multocida, M. haemolytica, M. ovipneumoniae and M. arginini). The animals’ ages ranged from 70 to 75 days approximately at the beginning of the feedlot, and around 90-100 days at the end of the fattening period. This period was around from 15 to 21 days. Feeding (pellet concentrate and straw) and water were administered ad libitum. Production management were the same in all animals. Lungs were collected at abattoir and analyzed in the Infectious Diseases Laboratory of the University of Extremadura were samples collection, microbiology and immunohistochemical analyses were carried out. The experiment was approved by the Animal Experimentation Ethics Committee of the University of Extremadura (approval No. 19/2014, date 13/02/2014).
Microbiology
The microbiology study was carried out from samples of lungs that were inoculated in selective media for each studied microorganism.
Mycoplasma spp.: A 5 cm piece of lung was sliced from the cranial lobe for each examined animal. To examine the lung tissue, a 0.3 cm long section with a visible airway (i.e. bronchi or bronchiole) was inoculated in 2.5 ml of Eaton’s broth [17] from 7 to 14 days and checked daily for the detection of growth compatible with Mycoplasma spp. (e.g. slight turbidity, formation of typical colonies with “fried egg” morphology or it absence for M. ovipneumoniae) [18]. From 1.5 ml of broth cultures the genomic DNA was extracted and purified using a silica-membrane-based spin kit (GenEluteTM Bacterial Genomic DNA Kit, Sigma-Aldrich, Vienna, Austria) according to the manufacturer’s instructions. PCR of the 16S–23S Intergenic spacer sequence differentiated the cultured Mycoplasma species by their amplicon size as described by Tang et al. [19].
Pasteurellaceae: All samples were inoculated onto 10% Sheep Blood Agar and incubated at 37°C overnight. Colonies were identified by automatic system identification Phoenix (Becton Dickinson Diagnostics, Franklin Lakes, NJ USA), according to the manufacturer’s instructions. Molecular identification of P. multocida was carried out using the PCR technique to amplify the specific fragment of gene kmt1 (PMPCR) using the primers KMT1SP6 and KMT1T7 described by Townsend et al. [20]. The resulting PCR products were electrophoresed in 2% agarose gel, stained with ethidium bromide and photographed. Distilled water without any DNA was used as negative control. Electrophoresis conditions were 90 V for 60 min. NCTC 10322 strain was used as positive control of P. multocida. M. haemolytica identification was carried out with the same process as P. multocida. Besides, isolated were further characterised by indirect hemagglutination technique. Antisera for each serotype were produced in rabbits using the reference strains and NTCC provided by Robert Davies (Scotland).
Histopathology
Lung samples of 2-mm thick including lesioned tissues and undamaged adjacent area were taken. In those lungs without consolidation areas, 2-mm thick randomly selected samples of the cranioventral regions were taken. Samples were fixed in neutral buffered formalin ‘3.5%, 0.1 M and pH 7.2’, and routinely processed and embedded in paraffin wax. 5 μm sections were cut with a microtome (Leica RM2255®, Leica microsistemas, Barcelona, Spain), stained with Hematoxylin & Eosin and, after their histopathological study, classified in four pathological groups (Diffuse alveolar damaged (AD), interstitial pneumonia (PI), purulent bronchopneumonia (PB) and bronchointerstitial pneumonia (BI) group) according Caswell and Williams (2007). Once established the histopathological group to which each sample belongs, a total of fifteen samples were selected from each group in order to apply immunohistochemical technique.
Immunohistochemistry
Sections for immunohistochemical examination were rinsed and fixed in Bouin’s solution (formalin 10%, picric acid saturated solution in distilled water, and glacial acetic acid, 15:5:1) for 48 hours at room temperature, dehydrated in ascending scale of alcohols and embedded in paraffin. The avidin-biotin-peroxidase complex (ABC) method was used. All samples were dewaxing oven, rehydrated and then treated in hydrogen peroxide 3% in methanol for 15 min to eliminate the peroxidase activity. Samples were washed with TBS 0.01 M and pH 7.2. Antigen retrieval were subjected by using Tween 20 (Merck, München, Germany) 0.01% in 0.01 M TBS, pH 7.2, for 10 min at room temperature. After pretreatment, sections were given three 10-min rinses in TBS. Samples then were mounted in Sequenza Immunostaining Centre (Shandon Scientific, Runcorn, UK). Primary antibody cross-reactivity with tissue constituents was prevented using 1.5% normal serum block that matched the host species in which the link antibody was applied to the sections for 20 min. before incubation with the primary antibody at 4°C overnight. Details of primary antibodies used, specificity, concentration, and incubation time are summarized in Table 1. As the development and production of new monoclonal antibodies is both timeconsuming and expensive, it seems reasonable to investigate whether existing anti-cytokine antibodies can be used successfully in heterologous species of interest [15,21]. Accordingly, same studies have demonstrated that monoclonal antibodies specific for human, bovine and ovine cytokines cross-react with cytokines produced by peripheral blood mononuclear cells from sheep, [22] mouse, [23] swine, [24,25] dogs, [26] and horses [21,27].
Table 1: Details of primary antibodies, antigen retrieval, concentration, source and commercial origin for the immunohistochemical technique.
| Specificity | Type | Source | Comercial origin | Fixative | Dilution | Antigen retrieval |
|---|---|---|---|---|---|---|
| Human IL-1R | PAb | Rabbit | Cultek, SLU | Bouin | 1/400 | Tween 20® |
| Human IL-6 | PAb | Rabbit | Cultek, SLU | Bouin | 1/400 | Tween 20® |
| Human TNF-alpha | PAb | Rabbit | Cultek, SLU | Bouin | 1/1000 | Tween 20® |
| Human IL-10 | PAb | Rabbit | Cultek, SLU | Bouin | 1/500 | Tween 20® |
Biotinylated goat anti-rabbit antibody (Vector Laboratories, Burlingame, CA, USA) diluted 1 in 1000 in TBS containing normal goat serum 1.5% was used. The sections were then incubated with the ABC (Vectastain ABC Kit Elitew; Vector Laboratories), before applying for 1 min the chromogen 3,3′-diaminobenzidine-tetrahydrochloride-dihydrate (DAB; Sigma- Aldrich Chemie) in a solution (0.35 g/L) in 0.05 M Tris buffer (pH 7.6) containing hydrogen peroxide 0.1%. Slides were counterstained with Mayer’s haematoxylin.
Appropriate controls were included in each immunohistochemical run. These included sequential sections with an isotype control for each primary antibody or the omission of the primary antibody (positive or negative respectively).
The cell counting was determined with the number of immunolabelled cells/mm2 for each antibody using a method described by Redondo et al. [28]. Cells labelled by each antibody were counted in selected areas (septal zone, alveolar zone, bronchial luminal zone, and BALT zone) in 10 fields consecutives of 1 mm2 per block for each antibody.
Statistical analysis
Two-way analysis of variance (ANOVA) was used for statistical analysis. Each observation was categorized on the basis of two criteria - the isolated microorganism (P. multocida, M. haemolytica, M. ovipneumoniae and M. arginini) as well as the cytokine marker (IL-1, IL-6, TNFα and IL-10). These two factors were considered to address the cell counts in four lung zones (septal zone, alveolar zone, and bronchial luminal zone and BALT zone). The possible interaction between the cytokine markers and microorganisms was studied in each one of the four lung zones. Shapiro-Wilk’s test of normality and Levene’s test of homogeneity of variance were considered to analyze the ANOVA’s applicability conditions. The Bonferroni adjustment was considered to determine if there are statistically significant differences between the mean cell count with the considered antibody markers in a concrete microorganism (multiple comparisons).
Two-side P values less than 0.05 were considered statistically significant. Statistical analyses were performed by using IBM-SPSS software 19.0®.
Results
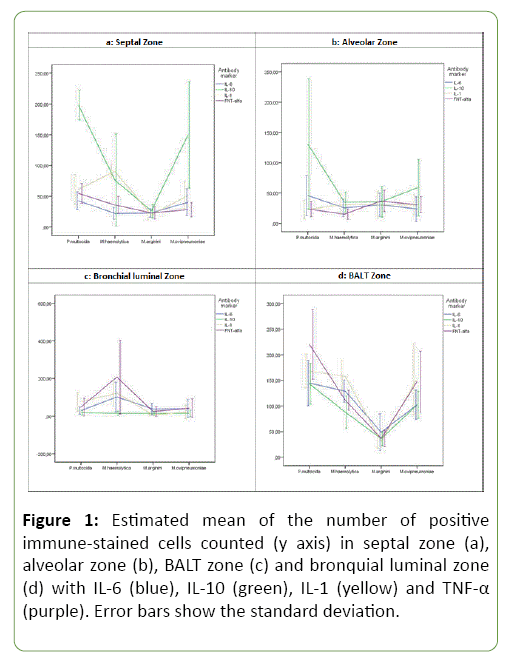
A significant interaction between the microorganisms and cytokine markers was observed in septal zone (P<0.001). Figure 1a shows the effect of markers and microorganisms studied for the count of cells in this zone. In general, IL-10 showed the highest mean count of immune-labelled cells. This cytokine presented statistically significant differences with the rest of cytokines markers in the mean cell counts within the groups of P. multocida (P<0.05) and M. ovipneumoniae (P<0.05). In animals with M. arginini, although IL-10 showed the highest mean count of immunolabelled cells, a statistically significant difference between markers was not revealed. In M. haemolytica, IL-10 and IL-1 provide the highest mean count of inmunolabeled cells (with no statistically significant differences between them), whereas the lowest ones were obtained with IL-6 and TNFα (also with no statistically significant differences between them). Mean and standard deviation of count cells under different studied cytokines with studied microorganisms are provided in Table 2.
Figure 1: Estimated mean of the number of positive immune-stained cells counted (y axis) in septal zone (a), alveolar zone (b), BALT zone (c) and bronquial luminal zone (d) with IL-6 (blue), IL-10 (green), IL-1 (yellow) and TNF-α (purple). Error bars show the standard deviation.
Table 2: Mean and standard deviation of cell counts under different cytokines with the studied microorganisms.
| Pasteurella multocida | Mannheimia haemolytica | Mycoplasma ovipneumoniae | Mycoplasma arginini | ||
|---|---|---|---|---|---|
| Cytokine | Localization | (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | (Mean ± SD) |
| IL-6 | Septal zone | 42.76 ± 20.25 | 22.12 ± 13.22 | 40.33 ± 30.33 | 22.86 ± 9.30 |
| Alveolar zone | 45.76 ± 46.33 | 25.42 ± 20.17 | 23.40 ± 28.33 | 30.47 ± 26.86 | |
| Bronchial luminal zone | 30.17 ± 26.24 | 101.70 ± 110.29 | 40.04 ± 66.57 | 38.30 ± 42.75 | |
| BALT zone | 144.61 ± 61.84 | 128.88 ± 29.57 | 102.37 ± 39.92 | 48.77 ± 49.94 | |
| IL-10 | Septal zone | 198.35 ± 33.96 | 76.32 ± 104.70 | 149.63 ± 120.71 | 26.25 ± 15.51 |
| Alveolar zone | 130.83 ± 150.50 | 35.08 ± 23.08 | 58.47 ± 64.99 | 35.51 ± 35.11 | |
| Bronchial luminal zone | 19.57 ± 17.36 | 15.55 ± 16.35 | 15.10 ± 24.33 | 15.02 ± 13.38 | |
| BALT zone | 143.06 ± 55.70 | 87.94 ± 44.04 | 101.92 ± 36.99 | 35.98 ± 18.95 | |
| IL-1 | Septal zone | 61.03 ± 33.87 | 90.52 ± 14.71 | 51.40 ± 30.62 | 19.58 ± 11.47 |
| Alveolar zone | 22.39 ± 21.53 | 31.24 ± 22.25 | 30.55 ± 23.59 | 30.44 ± 30.84 | |
| Bronchial luminal zone | 75.69 ± 70.63 | 122.53 ± 96.82 | 22.50 ± 49.42 | 7.53 ± 7.92 | |
| BALT zone | 167.92 ± 45.99 | 157.37 ± 45.28 | 152.64 ± 97.51 | 30.77 ± 18.33 | |
| TNF-α | Septal zone | 54.79 ± 22.49 | 35.94 ± 19.23 | 28.29 ± 15.92 | 22.86 ± 13.66 |
| Alveolar zone | 23.58 ± 17.53 | 14.98 ± 11.86 | 30.78 ± 18.46 | 36.76 ± 24.43 | |
| Bronchial luminal zone | 48.31 ± 65.23 | 207.91 ± 273.27 | 42.55 ± 68.12 | 25.22 ± 37.34 | |
| BALT zone | 220.18 ± 95.50 | 111.13 ± 27.13 | 147.48 ± 82.77 | 36.51 ± 22.06 | |
A significant interaction between the microorganism and cytokine markers was also observed in alveolar zone (P<0.001). Figure 1b shows the mean plot. In P. multocida, IL-10 marker showed the highest mean count of immunolabelled cells and revealed statistically significant differences with IL-1 (P<0.001), IL-6 (P<0.05) and TNFα (P<0.001). In the rest of microorganisms, IL-10 presented a high mean value of immunolabelled cells, although, no statistically significant differences were observed among the mean cell counts for the four cytokines markers.
Figure 1c presents the means and their corresponding 95% confidence intervals for the cell count in bronchial luminal zone. As in the previous zones, a significant interaction between microorganisms and cytokines markers has been found (P<0.001). In M. haemolytica, TNFα presented the highest mean count of immunolabelled cells with statistically significant differences in IL-6 (P<0.05) and IL-10 (P<0.001). In P. multocida, M. ovipneumoniae and M. arginini no statistically significant differences have been found. In contrast with previous zones, low mean numbers of immunolabelled cells with IL-10 marker were observed.
In BALT zone, there is another significant interaction between the microorganism and the cytokine marker (P<0.001). The mean plot is presented in Figure 1d. In P. multocida, TNFα presented the highest mean of immunolabelled cells, showing statistically significant differences in IL-6 (P<0.05) and IL-10 (P<0.05) On the other hand, in M. haemolytica the IL-1 marker presented the highest mean value, presenting statistically significant differences when compared with IL-10 (P<0.05). In the rest of microorganisms (M. arginini and M. ovipneumoniae) no statistically significant differences were observed among the mean cell counts for the four cytokines markers in this zone.
In general, high mean count of immunolabelled cells with IL-10 marker was observed in zones of the alveoli (septal zone and alveolar zone). In contrast, in areas of the bronchi (bronchial luminal zone and BALT zone) this marker presented low values and TNFα marked the highest mean counts.
Microscopic and statistical studies revealed different pathological groups and a significant interaction between them and the microorganism (Pasteurellaceae or Mycoplasmataceae) (P<0.001). Lesions in AD group, ciliar necrosis and compaction and alveolar denudation, and loss of type I pneumocytes and epithelial basement membrane. In PI group, the found lesions were characterized by septal damage, loss of type I pneumocytes and proliferation of type II pneumocytes. Septa were thickened, with mononuclear cell inflammatory infiltrates, marked congestion and oedema. Hyaline membranes were observed in junction interstitium and alveoli, composed of the presence of fibrin, eosinophilic proteinaceous material and cell debris. In PB group, the predominant lesional pattern was of exudative type. Neutrophils, macrophages and cells debris were observed within bronchial, bronchiolar and alveolar luminal. BALT was enlarged, as a multinodular structure. Interstitial pneumonia together with suppurative bronchopneumonia was also described. Inflammatory reaction within the interalveolar septa and exudative processes were concomitant in all the samples included in this group. This group was defined as bronchointerstitial pneumonia (BI).
In our study, 65% of animals with Pasteurellaceae presented BI and 20% presented PB. The relationship between this family and lung lesions indicated by a higher percentage of purulent and fibrinopurulent bronchopneumonia than diffuse alveolar damaged (0%) or interstitial pneumonia (15%). On the other hand, these values was higher in Mycoplasmataceae, 25% of animals presented AD and 16.3% PI. The value of BP was the same (20%) and of the percentage of BI was decreased (38.8%) with respect Pasteurellaceae.
Discussion
This study was proposed to analyse the role of cytokines in lungs of fattening lambs naturally infected with different microorganisms. Although respiratory microorganisms are a real problem in ovine production since they are responsible for important economical losses in the sheep industry in Spain [2], there are few studies that describe this cytokinemicroorganism association. For this reason, it is very important to know the immune response associated to these situations.
The production of cytokines plays an important role in inflammatory reaction and development of infection. Cytokines may also be synthesized by several other immune or non-immune cells including lymphocytes, neutrophils and fibroblasts [29]. Some cytokines may also act as regulators of macrophages activation. For example, TNF-α acts as potent activator of macrophages, whereas IL-10 inhibits activation of these cells [30]. In contrast, IL-10 is considered to be an immunosuppressive cytokine as it down-regulates the expression of several other cytokines including IL-1a, TNF-α, IL-6 and IL-10 itself [11,13].
In our study, an increase in labelled cells with TNF-α in bronchial luminal zone has been observed in infected lambs with M. haemolytica with respect to other markers. Lungs with this microorganism show the higher expression of proinflammatory cytokines indicating in this way a highest inflammatory reaction. This cytokine also presented elevated levels in the rest of microorganisms although were lower values than in M. haemolytica. In BALT area, labelled cells with TNF-α also were increased overall in P. multocida. In M. haemolytica an elevated level of IL-1 was also observed. Both cytokines are proinflammatory and are secreted by monocytemacrophages [12]. TNF-α and IL-1 promote neutrophilmediated tissue injury by stimulating neutrophil degranulation and the extracellular release of arachidonic acid metabolites, toxic oxygen radicals, and proteolytic enzymes [31,32] These cytokines play an important role in the inflammatory response. In these bronchial zones (bronchial luminal zone and BALT zone) a decreased number of labelled cells marked with IL-10 were observed. In BALT zone, was in Pasteurellaceae where pro-inflammatory cytokines were more elevated. Although there weren’t statistically significant differences in lungs with Mycoplasm spp., the expression of inflammatory reaction of implicated structure was higher with M. ovipneumoniae, and it could explain their more pathogenic character.
On the other hand, contrary values were obtained in the respiratory portion. Labelled cells marked with IL-10 were increased in septal zone, mainly in P. multocida and M. ovipneumoniae. In M. haemolytica an elevated level of IL-1 was also observed, in spite of the fact the IL-10 marker was high too. This fact was also observed in alveolar zone. IL-10 is a regulatory cytokine that, among other functions, inhibits the synthesis of pro-inflammatory cytokines [13], is a pleiotropic cytokine that can exert either immuno-stimulatory or immunosuppressive effect on many cell types [13], and can inhibit antigen-presenting cell and macrophage functions and can affect immune responses by inhibiting [33]. P. multocida and M. ovipneumoniae produce an increase of IL-10 that, in general, inhibits all activities that favour the inflammatory or specific cellular immune response [14]. In the case of M. haemolytica, IL-1, a pro-inflammatory cytokine, is also elevated, which indicates that this microorganism is who produces major reaction in this area. In the alveolar zone occurred the same process although in less expression.
In the present study, the presence of high levels of labelled cells marked with TNF-α in bronchial luminal zone and BALT zone indicated that this area was the first localization of these microorganisms and for this reason exist an inflammatory response to avoid the development of the disease acting as activators of defence cells as macrophages; besides, is in these areas where IL-10 presented the lowest value, incrementing the effect of proinflammatory cytokines. An elevated level of IL-10, a decreased one of TNF-α, and a lesser expression in the rest of cytokines markers were observed in respiratory portions (alveolar zone and septal zone). It was in these areas where an anti-inflammatory cytokine regulated the inflammatory response, helping to the establishment of these microorganisms in the lung tissues. This fact could help to understand the chronologic order of the impact of microorganisms and according to other authors; the alveolar inflammatory response was secondary to the bronchial or bronchiolar inflammatory response, as has previously been reported [34].
Conclusion
In conclusion, the presence of these respiratory microorganisms contributed to the inflammatory response and it was possible to immunohistochemically detect the expression of pro-inflammatory and anti-inflammatory cytokines by ovine pulmonary macrophages. In general, highest mean counts of labelled cells marked with IL-10 were observed in alveolar areas, whereas, TNF-α was the cytokine which presented the highest values in bronchial areas. In the main, it can be observed that all the studied microorganisms cause inflammatory response in lungs (although with different magnitude), being M. haemolytica as the most pathogenic microorganism according to.1 It is also important the reaction of P. multocida and M. ovipneumoniae. M. arginine was the microorganism that produces less inflammatory reaction, confirming its ubiquitous character, although it produces pathological damage in the respiratory tract [3].
Conflict of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgements
This research was partially supported by the Extremadura Regional Government under the Project PCE1007 from the PRI IV+DT+I program for strategic sector cooperation between research groups and companies, provided by the research group GRU10142. The Extremadura Regional Government and the European Social Fund also partially supported this research under the projects PD12131 and GR15106.
References
- Lacasta D, Ferrer LM, Ramos JJ, Gonzalez JM, De las Heras (2008) Influence of climatic factors on the development of pneumonia in lambs. Small Ruminant Res 80: 28-32.
- López F (2002) The sheep sector in Extremadura: Production and marketing. In: Agriculture and livestock in Extremadura. Caja Badajoz, Spain, pp: 145-173.
- Fernández S, Galapero J, Rey J, Perez CJ, Ramos A, et al. (2016) Investigations into the seasonal presence of Mycoplasma species in fattening lambs. Vet J 212: 80-82.
- Soriano-Vargas E, Vega-Sanchez V, Zamora-Espinosa JL, Acosta-Dibarrat J, Aguilar-Romero F, et al. (2012) Identification of Pasteurella multocida capsular types isolated from rabbits and other domestic animals in Mexico with respiratory diseases. Trop Anim Health Prod 44: 935-937.
- Ackermann MR, Brogden KA (2000) Response of the ruminant respiratory tract to Mannheimia (Pasteurella) haemolytica. Microbes Infect 2: 1079-1088.
- McAuliffe L, Hatchell FM, Ayling RD, King AIM, Nicholas RAJ (2003) Detection of Mycoplasma ovipneumoniae in Pasteurella-vaccinated sheep flocks with respiratory disease in England. Vet Rec 153: 687-688.
- Cheng C, Jun Q, Qingling M, Zhengxiang H, Yu M, et al. (2015) Serological and molecular survey of sheep infected with Mycoplasma ovipneumoniae in Xinjiang, China. Trop Anim Health Prod 47: 1641-1647.
- Azizi S, Tajbakhsh E, Rezail A, Nekouei SH, Namjoo AR (2011) The role of Mycoplasma ovipneumoniae and Mycoplasma arginini in pneumonic lungs of slaughtered sheep. Rev Med Vet-Toulouse 6: 310-315.
- Nicholas RAJ, Ayling RD, Loria GR (2008) Ovine mycoplasmal infections. Small Ruminant Res 76: 98.
- Parker TA, Cheng H, Willeford KO, Wu S (2001) Interleukin-6 expression in response to innate immune regulatory factor stimulation. Biomed Pharmacother 65: 90-94.
- Biron CA (2001) Interferons and other cytokines. In: Knipe D, Howley P, Griffin D, Lamb R, Martin M (Eds.) Fields of Virology. Lippincott Williams & Wilkins, Philadelphia, pp: 321-349.
- Murtaugh MP, Baarsch MJ, Yaling Z, Scamurra RW, Gaofeng L (1996) Inflammatory cytokines in animal health and disease. Vet Immunol Immunop 54: 45-55.
- Moore KW, Malefyt RW, Coffman RL, O’Garra A (2001) Interleukin-10 and the interleukin-10 receptor. Annual Rev Immunol 19: 683-765.
- Asadullah K, Sterry W, Volk HD (2003) Interleukin-10 Therapy- Review of a New Approach. Pharmacol Rev 55: 241-269.
- Carrasco L, Nunez A, Sanchez-Cordon PJ, Pedrera M, Farnandez de Marco M, et al. (2004) Immunohistochemical detection of the expression of pro-inflammatory cytokines by ovine pulmonary macrophages. J Comp Path 131: 285-293.
- Scheerlinck JP, Chaplin PJ, Wood PR (1998) Ovine cytokines and their role in the immune response. Vet Res 29: 369-383.
- Nicholas R, Baker S (1998) Recovery of mycoplasmas from animals. Methods Mol Biol 104: 37-43.
- Alley MR, Lonas G, Clarke JK (1999) Chronic non-progressive pneumonia of sheep in New Zealand-a review of the role of Mycoplasma ovipneumoniae. New Zeal Vet J 47: 155-160.
- Tang J, Hu M, Lee S, Roblin R (2000) A polymerase chain reaction based method for detecting Mycoplasma/Acholeplasma contaminants in cell culture. J Microbiol Meth 39: 121-126.
- Towsend KM, Frost AJ, Lee CW, Papadimitriou JM, Dawkins HJS (1998) Development of PCR assays for species- and type- specific identification of Pasteurella multocida isolates. J Clin Microbiol 36: 1096-1100.
- Pedersen LG, Castelruiz Y, Jacobsen S, Aasted B (2002) Identification of monoclonal antibodies that cross- react with cytokines from different animal species. Vet Immunol Immunop 88: 111-122.
- Rahman AN, Snibson KJ, Lee CS, Meeusen EN (2004) Effects of implantation and early pregnancy on the expression of cytokines and vascular surfaces molecules in the sheep endometrium. J Reprod Immunology 64: 45-58.
- Buendia AJ, Martinez CM, Ortega N, Rio LD, Caro MR, et al. (2004) Natural killer (NK) cells play a critical role in the early innate immune response to Chlamydophila abortus infection in mice. J Comp Path 130: 48-57.
- Salguero FJ, Ruiz-Villamor E, Bautista MJ, Sanchez-Cordon PJ, Carrasco L, et al. (2002) Changes in macrophages in spleen and lymphnodes during acute African swine fever: expression of cytokines. Vet Immunol Immunop 90: 11-22.
- Sánchez-Cordón PJ, Nunez A, Salguero FJ, Pedrera M, Fernandez de Macro M, et al. (2005) Lymphocyte apoptosis and thrombocytopenia in spleen during classical swine fever: role of macrophages and cytokines. Vet Pathol 42: 477-488.
- Catchpole B, Gould SM, Kellett-Gregory LM, Dobson JM (2002) Immunosuppressive cytokines in the regional lymphnodes of a dog suffering from an oral malignant melanoma. J Small Anim Pract 43: 464-467.
- Jaber JR, Fernandez A, Herraez P, Espinosa de los Monteros A, Ramirez GA, et al. (2003) Cross-reactivity of human and bovine antibodies in striped dolphin paraffin wax-embedded tissues. Vet Immunol Immunop 96: 65-72.
- Redondo E, Regodon S, Franco A, Masot J, Gazquez A, et al. (2003) Day-night changes in plasma melatonin levels and synaptophysin expression and ultrastructural properties of pinealocytes in developing female sheep under natural long and short photoperiods. Histol Histopathol 18: 333-342.
- Gómez-Laguna J, Salguero FJ, Barranco I, Pallares FJ, Rodriguez-Gomez IM, et al. (2010) Cytokine expression by macrophages in the lungs of pigs infected with the porcine reproductive and respiratory syndrome virus. J Comp Path 142: 51-60.
- Mitchell RN (2004) Immune diseases. In: V Kumar, R Cotran, S.L Robbins (Eds.) Basic Pathology. Elsevier Science, Philadelphia, pp: 103-164.
- Caswell JL, Middleton DM, Gordon JR (2001) The importance of interleukin 8 as a neutrophil chemoattractant in the lungs of cattle with pneumonic pasteurellosis. Can J Vet Res 65: 229-232.
- Sample AK, Czuprynski CJ (1994) Bovine neutrophil chemiluminescence is preferentially stimulated by homologous IL-1, but inhibited by the human IL-1 receptor antagonist. Vet Immunol Immunop 41: 165-172.
- Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, et al. (1993) Human IL-10 is produced by both type-1 helper (Th1) and type-2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immuno 150: 353-360.
- Kwon D, Chol C, Chae C (2002) Chronologic localization of Mycoplasma hyopneumoniae in experimentally infected pigs. Vet Pathol 39: 584-587.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences