ISSN : 2348-9502
American Journal of Ethnomedicine
Anti-oxidant, Anti-microbial Evaluation and GC-MS Analysis of Cyperus rotundus L. Rhizomes Chloroform Fraction
Department of Chemistry, Government Arts College (Autonomous), Kumbakonam, Tamilnadu, India-612 001
Abstract
Introduction: Plant has been used for the treatment of diseases all over the world and is known to contain substances that can be used for therapeutic purposes or as precursors for the synthesis of useful drugs.
Objective: To analyses chloroform extract of Cyperus rotundus rhizomes using GC-MS and to evaluate anti-oxidant, anti-microbial activities.
Method: The dried rhizomes of C. rotundus extracted with 90% methanol (MeOH) (4 X 500 ml) under reflux and has subjected to column chromatography. The anti-oxidant activity of C. rotundus rhizomes extracts in solvents of varying polarity has measured radical scavenging ability, using the stable radical, DPPH. The different extracts of C. rotundus rhizomes has evaluated for anti-microbial activity against clinical isolates of Escherichia coli, Staphylococcus aureus, Klebsiella pneumonia, Aspergillus niger, Aspergillus flavus and Candida albicans has selected based on anti-microbial activity of available drugs. Result: GC-MS analysis led to identification of five compounds namely (1) Trans-(2-chlorovinyl) dimethylethoxysilane, (2) 5-hydroxymethyl furfural, (3) Vanillin lactoside, (4) 2-propenoic acid, 3-(4-hydroxy-3-methoxy phenyl)-methyl ester, (5) 9, 12, 15-octadeca trienoic acid, 2, 3-bis [(trimethyl) oxy] propyl ester is the first report in the chloroform extracts of Cyperus rotundus rhizome.
Conclusion: The results showed the CHCl3 fraction of C. rotundus rhizomes can be considered as good sources of natural antioxidants and antimicrobial compounds present in it and has incorporated into the drug theories.
Keywords
Cyperus rotundus, Cyperaceae, GC-MS, Anti-oxidant, Anti-microbial activity.
INTRODUCTION
Plants and plant based medicaments are the basis of many of the modern pharmaceuticals we use today for our various ailments. It is clear that the plant kingdom harbors an inexhaustible source of active ingredients invaluable in the management of much intractable disease [1]. Plant have been used for the treatment of diseases all over the world before the advent of modern clinical drugs and are known to contain substances that can be used for therapeutic purposes or as precursors for the synthesis of useful drugs [2]. Cyperus rotundus L., (family Cyperaceae) also known as purple nut sedge or nut grass, is a common enduring world worst weed native to India. It grows in small clump up to 100 cm high with slender, scaly creeping rhizomes, bulbous at the base and arising singly from the tubers which are about 1-3 cm long. It therefore grows in various different habitats and environments. The tubers are externally blackish color and reddish white inside, with a characteristic odor. The stems grow to about 25 cm tall and the leaves are linear, dark green and grooved on the upper surface. Inflorescences are small, with 2-4 bracts, consisting of tiny flowers with a redbrown husk. The nut has three-angled, oblong-ovate, yellow and black when ripe. C. rotundus is indigenous to India, but has found in tropical, subtropical and temperate regions [3]. The tuber part of C. rotundus is one of the oldest known medicinal plants used to treat dysmenorrheal and menstrual irregularities [4]. It is a multipurpose plant, widely used in traditional medicine around the world to treat stomach ailments, wounds, boils and improves milk secretion in lactating woman and is an excellent immune modulator [5,6]. Ethyl acetate extract and two crude fractions, solvent ether and ethyl acetate of C. rotundus (Cyperaceae) has evaluated for several pharmacological and biological activities including antiinflammatory, anti-diabetic, anti-diarrheal, cytoprotective, anti-mutagenic, antimicrobial, anti-bacterial, anti-oxidant, cytotoxic and apoptotic, anti-pyretic and analgesic activities have reported for this plant [7-11].
The major compounds isolated from extracts of C. rotundus rhizome are α and β- cyperone, α and β-rotunol, β-pinene, β- selinene, camphene, copaene, cyperene, cyperenone, cyperol, cyperolone, cyperotundone D- copadiene, Depoxyguaiene, gamma-cymene, isocyperol, isokobusone, kobusone, limonene, linolenic-acid, myristic-acid, oleic-acid, pcymol, patchoulenone, pectin, polyphenols, rotundene, rotundenol, rotundone, selinatriene, sugeonol, and triterpenes including oleanolic acid and sitosterol, as well as flavonoids, sugars and minerals has reported previously [12-14]. Based on the literatures, we have decided the present work to analyses chloroform extract of Cyperus rotundus rhizomes using GC-MS and to evaluate anti-oxidant, anti-microbial activities.
MATERIAL AND METHODS
Plant material
Fresh rhizomes of C. rotundus growing wild has randomly collected in December, 2013 from the river basin of Cauvery in Thanjavur District, Tamilnadu (India) and authenticated by Prof. N. Ramakrishnan, (Department of Botany) and voucher specimens (GACBOT-202) has deposited at the Herbarium of the Department of Botany, Government Arts College (Autonomous), Kumbakonam, Bharathidasan University, India.
Extraction and fractionation
The dried rhizomes of C. rotundus extracted with 90% methanol (MeOH) (4 X 500 ml) under reflux. The methanol extract (76 g) has subjected to column chromatography with silica gel (60-120 mesh) as the stationary phase. The charged column has then eluted with different solvents of chloroform (4 x 250 ml) and ethyl acetate (4 x 250 ml) to yield several sub fractions. The fractions have collected and the solvent recovered by simple distillation and has concentrated in vacuo and left in an ice-chest for a week. The residue from chloroform fraction (37 g) has taken up in Me2CO and left in an ice - chest for two days when a brown solid separated and recrystallized from hot methanol.
GC-MS analysis
GC-MS analysis of CHCl3 fraction has performed on a Hewlett Packard HP 6890 Gas Chromatography with Hewlett Packard 5973 mass spectrometer system equipped with a DB-5 capillary column (30 m x 0.25 mm id, film thickness 0.25 μm). The oven temperature has programmed from 70-240 °C at the rate of 5°C/min. The ion source has set at 240 °C and electron ionization at 70 eV. Helium used as the carrier gas at a flow rate of 1 mL/min. Scanning range was 35 to 425 amu. Interpretation on mass spectrum of the unknown part has compared with the spectrum of the known components stored in the NIST library. The name, molecular weight and structure of the components of the test materials have ascertained.
DPPH free radical scavenging assay
The DPPH free radical is a stable free radical, which has widely accepted as a tool for estimating free radical-scavenging activities of antioxidants [15]. Hydrogen or electron donation abilities of the compounds have measured from the bleaching of the purple-colored methanol solution of 1, 1- diphenyl-2-picrylhydrazyl (DPPH). This spectrophotometer test uses the stable radical DPPH as a reagent. The sample solution of material (50 μL) at four concentrations (1.0, 0.5, 0.25 and 0.125 mg / mL) has mixed with freshly prepared methanolic solution of DPPH (634 μM) and allowed to stand for 30 min at room temperature. The absorbance has measured at 515 nm using a spectrophotometer and the inhibition of free radical DPPH in percent (%) has calculated using the formula below:
The percent of inhibition of DPPH reduction (decolourization),
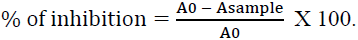
Where (A0) is absorbance of control (blank) and (Asample) is absorbance of test compound. The compound concentration demonstrating 50% inhibition (IC50) has calculated from the plot of inhibition percentage against sample concentration. Tests have carried out in triplicate. Samples and DPPH dissolved in methanol. Lascorbic acid has used as positive control.
Anti-microbial activity
Anti-microbial activity test has carried out in the following variation of the method originally described by Bauer et al. [16]. Muller Hinton ager has prepared and autoclaved at 15 lbs pressure for 20 minutes and cooled to 45˚C.The cooled media has poured on to sterile petriplates and allowed for solidification. The plates with media have seeded with the respective microbial suspension using sterile swab. The plant extracts has prepared at different dose individually placed on the each petriplates discs and placed control and standard (Streptomycin and Amphotericin) discs. The plates have incubated at 37˚C for 24 hrs. After incubation period, the zone of inhibition surrounding the discs has measured using a transparent ruler and the diameter recorded in mm.
Statistical analysis
The experimental results has expressed as statistical comparisons of Mean ± SEM carried out by one way analysis of variance (ANOVA) followed by Dunnet Multiple Comparisons Test. P values less than 0.05 has considered as statistically significant.
RESULTS AND DISCUSSION
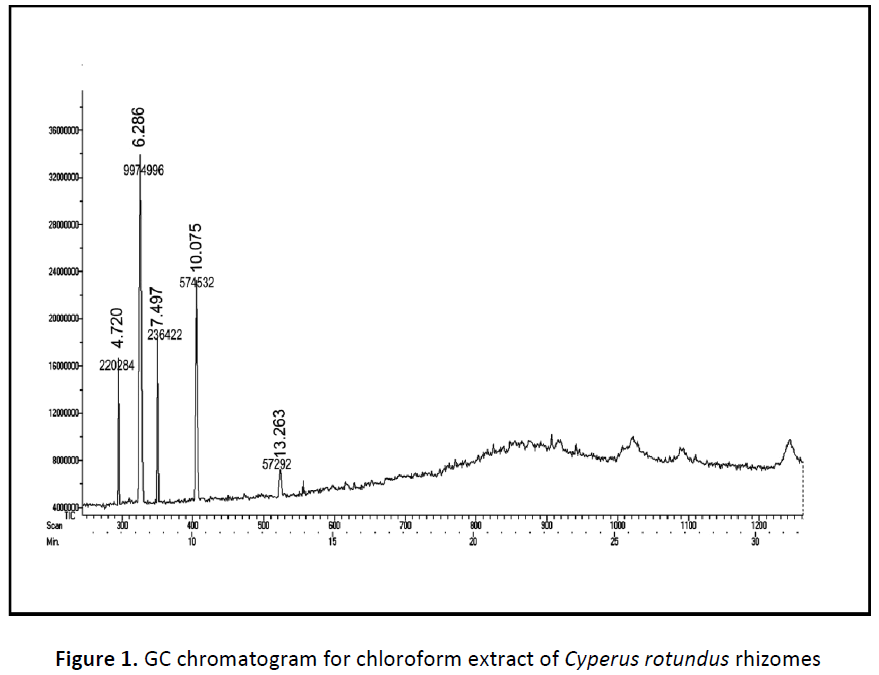
The components present in the chloroform fraction of Cyperus rotundus rhizomes have identified by GC-MS analysis (Fig 1). The active principles with their retention time (RT), molecular formula, molecular weight (MW), peak area has presented in the Table 1. The GC-MS spectrums showed a molecular ion peak at m/e 164.042, 126.031, 476.15, 208.073 and 496.340 follow a molecular formulas of C6H13ClOSi (MW 164), C6H6O3 (MW 126), C20H28O3 (MW 476), C11H12O4 (MW 208) and C27H52O4Si2 (MW 496). Based on GCMS analysis the following compounds have identified namely (1) Trans-(2-chlorovinyl) dimethylethoxysilane, (2) 5-hydroxymethyl furfural, (3) Vanillin lactoside, (4) 2- propenoic acid, 3-(4-hydroxy-3-methoxy phenyl)-methyl ester, (5) 9, 12, 15-octadeca trienoic acid, 2, 3-bis [(trimethyl) oxy] propyl ester.
Table 1: GC-MS data for chloroform extract of Cyperus rotundus rhizomes
| S. No. | Compound name | Molecular formula | Molecular weight | Retention time | Peak area | % of peak area |
|---|---|---|---|---|---|---|
| 1 | Trans-(2-chlorovinyl) dimethylethoxysilane | C6H13ClOSi | 164 | 4.720 | 220284 | 1.99 |
| 2 | 5-hydroxymethyl furfural | C6H6O3 | 126 | 6.286 | 9974996 | 90.16 |
| 3 | Vanillin lactoside | C20H28O3 | 476 | 7.497 | 236422 | 2.137 |
| 4 | 2-propenoic acid, 3-(4- hydroxy-3-methoxy phenyl)-methyl ester | C11H12O4 | 208 | 10.075 | 574532 | 5.19 |
| 5 | 9, 12, 15-octadeca trienoic acid, 2, 3-bis [(trimethyl)oxy]propyl ester | C27H52O4Si2 | 496 | 13.263 | 57292 | 0.518 |
Anti-oxidant activity
The anti-oxidant activity of C. rotundus rhizomes extracts in solvents of varying polarity has measured with hydrogen granting or radical scavenging ability, using the stable radical, DPPH. The method has based on reduce alcoholic DPPH solutions in the presence of a hydrogen granting anti-oxidant. The DPPH free radical scavenging activity of the Chloroform extract of C. rotundus rhizomes showed the highest scavenging activity (% inhibition 87.01, 84.92, 78.47 and 74.19 at 1.0, 0.5, 0.25 and 0.125 mg/ml respectively), followed by ethyl acetate extract. Methanol extract showed least DPPH radical scavenging ability with % inhibition 81.62, 74.80, 71.92 and 50.76 at 1.0, 0.5, 0.25 and 0.125 mg/ml respectively. The results of the free radical scavenging activity of C. rotundus assessed by DPPH test and amount of the sample needed for 50% inhibition of free radical activity, IC50 values has summarized in Table 2. Lower IC50 value suggests higher anti-oxidant activity. Based on the results found the anti-oxidant activity of C. rotundus rhizomes methanol extract (IC50: 91.85 μg / ml) was quite comparable with standard anti-oxidant of L-ascorbic acid.
Table 2: DPPH free radical scavenging activity of the rhizomes extracts of Cyperus rotundus
| Concentration (mg/ml) | IC50 (μg/ml) | ||||
|---|---|---|---|---|---|
| Samples | 1.0 | 0.5 | 0.25 | 0.125 | IC50 |
| Radical scavenging effect (%) | |||||
| C. rotundus chloroform extract | 87.01 ± 0.05 | 84.92 ± 0.20 | 78.47 ± 0.01 | 74.19 ± 0.70 | 131.0 ± 0.04 |
| C. rotundus ethyl acetate extract | 86.08 ± 0.05 | 80.71 ± 0.21 | 74.98 ± 0.60 | 64.83 ± 0.15 | 105.21 ± 0.01 |
| C. rotundus methanol extract | 81.62 ± 0.02 | 74.80 ± 0.15 | 71.92 ± 0.70 | 50.76 ± 0.01 | 91.85 ± 0.06 |
| L-ascorbic acid | 97.06 ± 0.05 | 94.11 ± 0.20 | 92.32 ± 0.02 | 87.88 ± 0.15 | 70.93 ± 0.01 |
Values are expressed in Mean ± Standard Deviation (M±SD).
Anti-microbial activity
The methanol, chloroform and ethyl acetate extracts of C. rotundus rhizomes has evaluated for anti-microbial activity against clinical isolates of Escherichia coli, Staphylococcus aureus, Klebsiella pneumonia, Aspergillus niger, Aspergillus flavus and Candida albicans has selected based on anti-microbial activity of available drugs. The small amount of the extract has carried over with these inoculums has easily removed by diffusion into the agar and the effect denied by spreading the inoculums over a large area. Chloroform extract showed better inhibitory activity against most of the pathogens. The maximum zone of inhibition against S. aureus (31 ± 0.04 mm) followed by K. pneumonia (28 ± 0.02 mm) and E. coli (18 ± 0.01 mm). If ethyl acetate and methanol extract showed high anti-microbial activity against S. aureus with 26 ± 0.07 and 22 ± 0.12 mm and lesser active against E. coli with 15 ± 0.60 and 14 ± 0.04 mm (Table 3). Also both extracts showed extended antifungal activity against C. albicans followed by A. flavus and A. niger. The inhibition zone has viewed against C. albicans with chloroform extract 26 ± 0.67 mm, ethyl acetate 22 ± 0.02 mm and with methanol extract 15 ± 0.20 mm. Thus the results held C. rotundus rhizomes extracts revealed better control of the pathogens than the commercially available antibiotics.
Table 3: Inhibition zones formed by Cyperus rotundus rhizomes extracts and standard antibiotics
| S. No. | Microorganisms | Diameter of inhibition zones (mm/50 µL) (M±SD) | |||
|---|---|---|---|---|---|
| Chloroform extract | Ethyl acetate extract | Methanol extract | Standard (10 µg) | ||
| 1 | Staphylococcus aureus | 31 ± 0.04 | 26 ± 0.07 | 22 ± 0.12 | 30 ± 0.65* |
| 2 | Escherichia coli | 18 ± 0.01 | 15 ± 0.60 | 14 ± 0.04 | 26 ± 0.02* |
| 3 | Klebsiella pneumonia | 28 ± 0.02 | 20 ± 0.05 | 17 ± 0.12 | 32 ± 0.01* |
| 4 | Candida albicans | 26 ± 0.67 | 22 ± 0.02 | 15 ± 0.20 | 29 ± 0.05** |
| 5 | Aspergillus niger | 19 ± 0.05 | 14 ± 0.60 | 11 ± 0.01 | 24 ± 0.04** |
| 6 | Aspergillus flavus | 21 ± 0.01 | 16 ± 0.70 | 12 ± 0.04 | 27 ± 0.20** |
Used concentrations: 50 μL of 10 mg / mL of Cyperus rotundus extracts.
Bacteria Standard* - Ciprofloxacin (10 µg); Fungal Standard** - Amphotericin - B (10 µg). Values are expressed in Mean ± Standard Deviation (M±SD).
CONCLUSION
In the present study, the rhizomes of C. rotundus has subjected to GC-MS analysis. The GC-MS pattern showed five different phytochemical constituents found in chloroform fraction of C. rotundus rhizomes. This fraction shows excellent antioxidant and antimicrobial activities. It has widely observed and accepted the medicinal value of a plant lies in the bioactive compounds present in it.
REFERENCES
- Shariff, Z.U. (2001): Modern Herbal Therapy for Common Ailments. Nature Pharmacy Series (Volume 1). Spectrum Books Limited, Ibadan, Nigeria. In Association with Safari Books (Export) Limited, United Kingdom. pp.9-84.
- Sofowora, A. (1982): Medicinal Plants and Traditional Medicine in Africa. 3rd edn, John Willey and Sons Ltd, Ibadan. pp.8-14.
- Uddin, S.J, Mondal, K, Shilpi, J.A, Rahnan, M.T. (2006): “Antidiarrhoeal activity of Cyperus rotundus”. Fitoterapia. 77 (2), 134- 136.
- Yu, J, Lei, G, Cai, L, Zou, Y. (2004): “Chemical composition of C. rotundus extract’’. J. Phytochemistry. 65, 881-889.
- Puratuchikody, A, Nithya, D.C, Nagalakshmi, G. (2006): “Wound Healing Activity of Cyperus rotundus Linn.” Indian J. Pharm. Sci. 68, 97-101.
- El-Kamali, H.H and El-Khalifa, K.F. (1999): “Folk medicinal plants of riverside forests of the Southern Blue Nile district, Sudan”. Fitoterapia. 70, 493-497.
- Sundaram, M.S, Sivakumar, T, Balamurugan, G. (2008): “Anti- inflammatory effect of Cyperus rotundus Linn Leaves on acute and subacute inflammation in experimental rat models”. Biomedicine. 28, 302-304.
- Raut, N.A and Gaikwad, N.J. (2006): “Anti- diabetic activity of hydro-ethanolic extract of Cyperus rotundus in alloxan induced diabetes in rats”. Fitoterapia. 77, 585-588.
- Neffatti, A, Ben Ammar, R, Dijoux-Franca, M.G, Ghedira, K, Chekir-Ghedira, L. (2008): “In vitro evaluation of anti-bacterial, anti-oxidant, cytotoxic and apoptotic activities of the tubers infusion and extracts of Cyperus rotundus”. Bioresour. Technol. 99, 9004-9008.
- Sundaram, M.S, Sivakumar, T, Balamurugan, G. (2008): “Anti- inflammatory effect of Cyperus rotundus Linn Leaves on acute and sub-acute inflammation in experimental rat models”. Biomedicine. 28, 302-304.
- Gupta, M.B, Palit, T.K, Singh, N, Bhargava, K.P. (1971): “Pharmacological studies to isolate the active constituents from Cyperus rotundus possessing anti-inflammatory, anti- pyretic and analgesic activities”. Indian Journal of Medical Research. 59:76-82.
- Thebtaranonth, C, Thebtaranonth, Y, Wanauppathamkul, S, Yuthavong, Y. (1995): “Anti-malarial sesquiterpenes from tubers of Cyperus rotundus: structure of 10, 12-peroxyca-lamenene, a sesquiterpene endoperoxide”. Phytochemistry. 40, 125-128
- Sonwa, M.M and König, W.A. (2001): “Chemical study of the essential oil of Cyperus rotundus”. Phytochemistry. 58(5): 799-810.
- Jeong, S.J, Miyamoto, T, Inagaki, M, Kim, Y.C, Higuchi, R. (2000): “Rotundines A-C, three novel sesquiterpene alkaloids from Cyperus rotundus”. J. Nat. Prod. 63, 673- 675.
- Hu, F, Lu, R, Huang, B, Liang, M. (2004): “Free radical scavenging activity of extracts prepared from fresh leaves of selected Chinese medicinal plants”. Fitoterapia. 75: 14-23.
- Bauer, A.W, Kirby, W.M, Sherries, M, Durk, M. (1966): “Antibiotic susceptibility testing by a standard single disc method”. Amer J Clin Pathol. 36:493-496.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences