ISSN : ISSN: 2576-1412
Journal of Applied Microbiology and Biochemistry
Antifungal Activity of Artemisia Nilagirica Essential Oil from the Western Ghats Nilgiris against Food Borne Fungi
Kishmu Lingan*
Department of Biotechnology, Karpagam University, Coimbatore, Tamil Nadu, India
- *Corresponding Author:
- Kishmu Lingan
Department of Biotechnology
Karpagam University, Coimbatore
Tamil Nadu 641021, India
Tel: +91 91764 24990
E-mail: kishmubiotech@gmail.com
Received Date: April 27, 2018; Accepted Date: May 10, 2018; Published Date: May 18, 2018
Citation: Lingan K (2018) Antifungal Activity of Artemisia Nilagirica Essential Oil from the Western Ghats Nilgiris against Food Borne Fungi. J Appl Microbiol Biochem. Vol.2 No.2:6
DOI: 10.21767/2576-1412.100022
Abstract
Commercial antifungal drugs lead to develop fungal resistance and cause their own side effects to humans. Alternative use of plant essential oil as antifungal agent was attempted. The present study revealed that the extracted essential oil from Artemisia nilagirica was assessed for its chemical constituents and antifungal activity against four food borne fungi. Forty compounds were present in the essential oil out of which thujone was the major compound followed by γ- curcimene, α- caryophyllene, lavundulol and germacrene. Minimum inhibitory concentration for Aspergillus flavus, Aspergillus niger, Fusarium oxysporum and Penicillium notatum was 57.33, 32.66, 87.66 and 13.66 μl/L air respectively. Results obtained showed that the Essential oil has more potency against P. notatum followed by other fungal species at a slightly higher concentration.
Keywords
Artemisia nilagirica; Food borne fungi; Essential oil; Antifungal activity
Introduction
Fungal infections are spread all over the environment, especially on the fruits, vegetables and other food products stored for longer period. These food borne fungi not only spoil the stored food products, but they are also responsible for various deadly diseases. In this respect, essential oil from plant was effective against the food borne fungi and act as an alternative to synthetic antifungal products [1,2] and used as herbal medicine against the disease caused by fungi [3]. They have wide application against variety of fungal infections, treatment and prevention [4] and also helpful in effective management of fungi in buildings and indoor environment [5]. These fungi are not only responsible for the spoilage of food, they also produce certain toxins such as alfatoxins ochratoxin-A, which is the secondary metabolites produced by the Aspergillus species [6]. Synthetic fungicides have been used for the control of pathogenic fungi, however, their potential toxicological problems and considerable deterioration of the environmental quality and human health have generated considerable interest in preservation of grains by using naturally occurring compounds [7]. In connection in our study we focused on finding novel antifungal agent from natural source (plant essential oil) and its food preservative potential. Therefore A. nilagirica essential oil can be used as biocontrol agent against stored-product insects. Even though essential oil from Artemisia species have been reported for its insecticidal activity, yield of plant essential oil and its chemical composition may vary based on the location, season, plant part and method of extraction. Therefore our plant may be a novel source for its unique chemical composition, yield and insecticidal activity. Favorable eco-toxicological properties of essential oil make them potentially suitable for control of food borne fungi.
Materials and Methods
Plant collection
Artemisia nilagirica was collected from Kagguchi village (latitude- 11.33691°N, longitude- 76.81061°E), Western Ghats, The Nilgiris, based on the information provided by the local tribes. The collected plant was identified and authenticated by Botanical Survey of India, Coimbatore (authentication number – BSI/ SRC/5/23/2012-13/Tech.1741).
Extraction of essential oil
Essential oil was extracted from dried leaf powder (20 g) by hydro-distillation at 96°C for 4 hours. Extracted essential oil was stored at 4°C until use.
Analysis of essential oil components
Qualitative analysis of A. nilagirica essential oil was performed using Gas Chromatography and Mass Spectroscopy (Shimadzu, GC-MS QP 2010+). Various constituents of essential oil were identified by comparing their mass spectra with those stored in NIST MS search 2.0 (MS library).
Antifungal activity
Well diffusion method: Aspergillus flavus (KUMBF07), Aspergillus niger (KUMBF13), Fusarium oxysporum (KUMBF56) and Penicillium notatum (KUMBF57) were obtained from Karpagam Microbial Culture Collection Center (KMCC), Karpagam University, Coimbatore. Fungal spore suspensions (1.5 x 105 CFU ml-1) were spread separately on sterile potato dextrose agar medium. Three wells were cut in each plate. First well is loaded with 50 μl of essential oil (sample), second well with 50 μl of ketaconazole (50 μg-standard drug), and third well with 50 μl of methanol (negative control). Zone of inhibition was observed after 60 h of incubation.
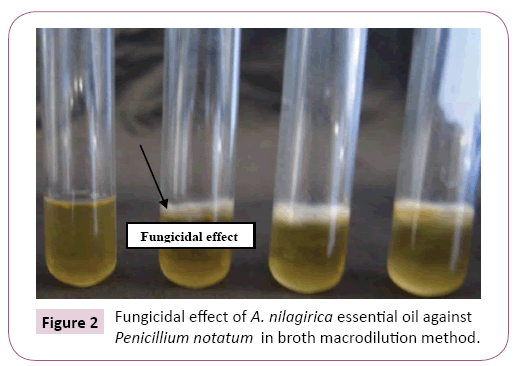
Broth macro dilution method: Sterile potato dextrose broth containing tween-20 at a concentration of 4% was aliquated into five tubes with 900 μl each. To the first tube 100 μl of Essential oil was added, mixed well and 100 μl was taken and added to second tube, thus all tubs were serially diluted, similar steps were followed for the four fungal species with replicates. Fungal spore suspension- 10 μl (1.5 x 105 CFU ml-1) was added to all the test tubes and incubated at room temperature (27°C). Minimum inhibitory and fungicidal concentrations of essential oil were observed after 60 h.
Results and Discussion
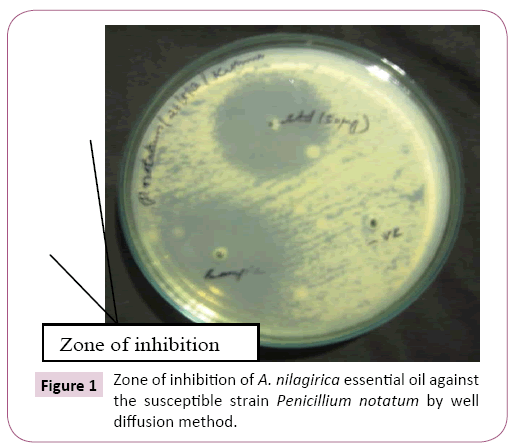
GC-MS studies enabled the detection of forty compounds in A. nilagirica essential oil (Table 1) out of which, α-thujone (34.24%) is the major compound followed by γ- curcimene (11.40%), α- caryophyllene (9.97%), lavundulol (4.03%) and germacrene (3.81%). Essential oil of A. nilagirica from northern hilly areas of India which contained forty three compounds, out of which, α-thujone (36.35%) is the major compound followed by β-thujone (9.37%), germacrene (6.32%), 4-terpineol (6.31%), camphene (5.47%), β-caryophyllene (5.43%) and borneol (4.12%) to those obtained by Sati et al. [8]. In comparison, the analyzed A. nilagirica essential oil from two extreme origin of India has nearly same number of compounds and their major compound percentage have slightly varied. From this study we concluded that the bioavailability of A. nilagirica was widely present in the hilly regions of India with similar chemical constituents. A. nilagirica essential oil was required 5.7% v/v for inhibition of A. niger, but against P. notatum it required only 1.3% v/v (Table 2). Maximum zone of inhibition was found to be against P. notatum 27.66 ± 0.43 (Figure 1). The minimum inhibitory concentration of essential oil required is varied based on the fungal species and also the type of oil (Figure 2). Similarly, the isolated essential oil from Indian medicinal plants showed their minimum inhibitory concentration between 0.5 and >4% v/v against A. niger [2].
Figure 1: Zone of inhibition of A. nilagirica essential oil against the susceptible strain Penicillium notatum by well diffusion method.
Table 1 Chemical composition of Artemisia nilagirica essential oil.
| Compound | R Time | Area % | Compound | R Time | Area % |
|---|---|---|---|---|---|
| Sabinene | 6.596 | 0.94 | Elemene | 15.367 | 0.56 |
| 1-octen-3-ol | 6.667 | 1.22 | α-Caryophyllene | 15.773 | 9.97 |
| Cymene | 7.679 | 0.37 | Germacrene | 16.115 | 3.81 |
| Limonene | 7.778 | 0.52 | β-Sesquiphellandrene | 16.320 | 2.23 |
| 1,8- Cineol | 7.848 | 0.69 | β-Humulene | 16.614 | 1.35 |
| γ-Terpinene | 8.428 | 0.60 | Neoallocimene | 16.726 | 0.45 |
| α- Thujone | 9.625 | 34.24 | γ-Curcumene | 17.231 | 11.40 |
| Thujol | 10.366 | 1.45 | Lepidozene | 17.471 | 2.22 |
| Cis verbenol | 10.465 | 0.85 | Cedrene | 17.533 | 0.52 |
| Furandione | 10.525 | 1.75 | γ-Cadinene | 17.793 | 0.68 |
| Geraniol | 10.876 | 2.96 | 4-Octylphenol | 18.338 | 0.52 |
| Terpinol | 11.132 | 1.29 | Palustrol | 18.608 | 0.39 |
| β- Terpinol | 11.369 | 0.90 | Ledol | 19.240 | 0.42 |
| Cis-Carveol | 11.907 | 0.43 | Cadinol | 10.043 | 1.80 |
| Perilaladehyde | 13.106 | 1.77 | Eudesmol | 20.158 | 0.74 |
| Lavundulol | 13.342 | 4.03 | Iodine | 20.302 | 0.73 |
| Perillol | 13.616 | 1.88 | Bisabolol | 20.462 | 1.37 |
| Peugenol | 14.668 | 0.84 | Triene | 23.135 | 0.66 |
| Copaene | 15.098 | 1.03 | Rimuen | 24.765 | 0.45 |
| Zingiberene | 15.278 | 0.60 | Phytol | 26.477 | 0.74 |
R Time-Retention Time. Indicated in bold font- Major compounds
Table 2 Antifungal activity of Artemisia nilagirica essential oil against food borne fungi.
| Test fungi | Zone of inhibition (mm) | MIC | MFC | |
|---|---|---|---|---|
| Ketaconazole (50 µg) | Essential oil (50 µl) | |||
| A. flavus | 11.83 ± 0.54 | 10.33 ± 0.43 | 57.33 ± 0.43 | 93.33 ± 0.39 |
| A. nigerr | 11.83 ± 0.43 | 12.66 ± 0.43 | 32.66 ± 0.62 | 56.33 ± 0.43 |
| F. oxysporum | 13.33 ± 0.43 | 10.66 ± 0.43 | 87.66 ± 0.43 | 142.33 ± 0.43 |
| P. notatum | 10.33 ± 0.43 | 27.66 ± 0.43 | 13.66 ± 0.62 | 24.66 ± 0.43 |
MIC: Minimum Inhibitory Concentration, MFC: Minimum Fungicidal Concentration, Values are based on six determinations and expressed as mean ± standard error
Conclusion
The present study revealed that Artemisia nilagirica essential oil showed effective antifungal activity against all the food borne fungi tested at tested concentrations. This plant is a weed and abundantly present all around the hilly regions of India, therefore it can be used as economically and environmentally safe alternative to the synthetic fungicides. Further bioactive compound present in the essential oil responsible for the antifungal activity will be predicted, which may lead to development of commercial biofungicide formulation against food borne fungi. The bioactive compounds present in this essential oil can be used as fumigants in the warehouses were rice, wheat, cereals and pulses are stored for longer duration.
References
- Ferdes M, Ungureanu C (2012) Antimicrobial activity of essential oils against four food-borne fungal strains. Sci Bull 74: 87-98.
- Bansod S, Rai M (2008) Antifungal activity of essential oils from indian medicinal plants against human pathogenic Aspergillus fumigatus and Aspergillus niger. World J Med Sci 3: 81-88.
- Omran SM, Esmailzadeh S (2009) Comparison of anti-Candida activity of thyme, pennyroyal, and lemon essential oils versus antifungal drugs against Candida species. Jundishapur J Microbiol 2: 53-60.
- Uniyal V, Bhatt RP, Saxena S, Talwar A (2012) Antifungal activity of essential oils and their volatile constituents against respiratory track pathogens causing Aspergilloma and Aspergillosis by gaseous contact. J Appl Nat Sci 4: 65-70.
- Verma RK, Chaurasia L, Kumar M (2011) Antifungal activity of essential oils against selected building fungi. Indian J Nat Prod Res 2: 448-451.
- Fente CA, Jaimez OJ, Vizquez BI, Franco CM, Cepeda A (2001) A new additive for culture media for rapid determination of alfatoxin-produsing Aspergilus strain. Appl Environ Microbiol 67: 4858-4862.
- Dambolena JS, López AG, Meriles JM, Rubinstein HR, Zygaldo JA (2012) Inhibitory effect of 10 natural phenolic compounds on Fusarium verticillioides. A structure-property-activity relationship study. Food Control 28: 163-170.
- Sati SC, Sati N, Ahluwalia V, Walia S, Sati OP (2013) Chemical composition and antifungal activity of Artemisa nilagirica essential oil growing in northern hilly areas of India. Nat Prod Res 27: 45-48.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences