Antifungal Activities of Pseudomonas Strains against Indigenous Phytopathogenic Fungi of Phaseolus Vulgaris L. Isolated from a Greenhouse in Western Algeria
Slimane Mokrani1,2, Lakhder Belabid2 and Elhafid Nabti1*
1Laboratoire de Maitrise des Énergies Renouvelables, Faculté des Sciences de la Nature et de la Vie, Université de Bejaia, Algeria
2University of Mustapha Stumbouli, Department of Agronomy, Laboratory of Research on Biological Systems and Geomantic, Algeria
*Corresponding Author:
Elhafid Nabti
Laboratoire de Maitrise des Énergies Renouvelables
Faculté des Sciences de la Nature et de la Vie
Université de Bejaia, Algeria
E-mail: elhnabti1977@yahoo.fr
Received date: October 05, 2018; Accepted date: December 26, 2018; Published date: January 02, 2019
Citation: Mokrani S, Belabid L, Nabti E (2019) Antifungal Activities of Pseudomonas Strains Against Indigenous Phytopathogenic Fungi of Phaseolus Vulgaris L. Isolated from a Greenhouse in Western Algeria. Res J Plant Pathol Vol. 2 No.1: 07
Copyright: © 2019 Mokrani S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
In the present study, two phytopathogenic fungi were isolated from Phaseolus vulgaris L. Macroscopic and microscopic identification of the fungi attributed them to the genera Fusarium and Sclerotinia. Antifungal activities of three Pseudomonas strains; P7 Pseudomonas plantarii P30, P. fluorescens Biovar 5 and P36 P. fluorescens Biovar 5 revealed percent inhibition of the phytopthogenic fungi ranging from 47.78% to 100%. The determination of the antifungal mechanism of the strain P7 revealed a mycelium lysis of Sc-sc (Sclerotinia sclerotiorum) and deformation of Fop (Fusarium oxysporum f sp. phaseoli). The results lead us to think on the capability of utilization of the three strains as biocontrol agents against the phytopathogenic fungi.
Keywords
Antifungal activity; Phaseolus vulgaris L.; Pseudomonas; Phytopathogenic fungi
Introduction
Diseases cause 80-100% yield loss of common bean on agriculture. Among all the transmittable seed-borne diseases of common bean, fungi cause the most damage [1]. In addition, the increasing importance of dermatophytosis and emerging resistance of dermatophytes to current synthetics antifungal stimulated the search for safer and more effective alternative drugs from natural sources [2]. In other side, biological protection of plants includes different types of amensalism, especially antibiosis as well as a competition between protective microorganisms and pathogens for nutrients, energy and habitat [3]. Although different microorganisms can be used as biofertilizer [4] or biological control agents, important evidence exists regarding the role of antibiotic production by bacteria isolated from soil such as suppressors and inhibitors of pathogens development [5]. Particularly, the use of plant growth-promoting bacteria (PGPB) with antifungal properties is an attractive alternative of xenobiotic compounds application [6,7]. More particularly, different Pseudomonas species colonizing the rhizosphere possess several interesting characteristics, which make them attractive for utilization as biological control agents. Their ability to colonize roots and maintain a high population density is remarkable [8]. The over goal of this study is to isolate and identify the phytopathogenic fungi of Phaselus vulgaris L then, highlight the antifungal activities and mechanisms of the Pseudomonas strains against the isolated phytopathogenic fungi.
Material and Methods
Origin and traits of Pseudomonas strains
Three strains P7 P. plantarii, P30 P. fluorescens Biovar 5 and P36 P. fluorescens Biovar 5 were isolated, identified and characterized for their PGP traits from different plants in previous study which is shown in Table 1 [9].
| Strain | Species | SED | IAA | PS | HCN | PEC |
|---|---|---|---|---|---|---|
| P7 | P. plantarii | ++ | - | + | - | - |
| P30 | P. fluorescens Biovar 5 | + | - | + | - | + |
| P36 | P. fluorescens Biovar 5 | + | - | + | + | + |
+: low positive reaction; +: high positive reaction; -: negative reaction. SED: siderophores; IAA: indol-3- acetic acid, PS: phosphate solubilization; HCN: hydrogen cyanid; PEC: pectinase.
Table 1: Tall height of the tomato plant and the length of the root (During the ripening period 72nd day).
Characterization of fungal bean diseases
The isolation of the phytopathogenic fungi was carried out from bean plants of 3-4 months old cultivated in greenhouse at Tighanif Mascara (35°24’ N 0°19’E). Bean plants are none treated with chemical as pesticides, fungicides or fertilizers that weakened plants health, reduce their resistance to telluric diseases and especially promote the development of phytopathogenic microorganisms.
Isolation of phytopathogenic fungi
The isolation of the phytopathogenic fungi was achieved by inoculation of the infected plant parts (leaves or/and stems) catted in small slices sterilely, then deposited on PDA agar, followed by incubation at 25°C/5-7 days [10] The fungal isolates were purified by sub-culturing successively two to three times by re-inoculation a piece of agar containing mycelium on a new PDA medium, followed by incubation at 25°C/7days. Typical colonies were then conserved and characterized [11].
Identification of phytopathogenic fungi
Macroscopic identification: For macroscopic examination of the fungi, colonies obtained after culture on PDA medium were characterized for their filamentous aspects: appearance; relief; size and color. Presence of a scattering pigment in the agar as well as other parameters such as growth speed of the colonies or the temperature of development can be good indicators for fungal macroscopic identification [12].
Microscopic identification: Microscopic fungal identification was realized applying a mycelium fragment between glass blade and coverslip, then passed directly under microscopic examination (×10) and/or (×40) [13]. To observe the fungal reproductive forms, an activation of sporulation was carried out using the method described by [14] Sporulation of Fop isolate was activated by applying thermal shock (heat treatment of 140 °C for 30-60 s followed by freeze treatment at -20 °C/5 min). Whereas, Sc-sc isolate sporulation was activated by applying UV light at ambient temperature for 16 h followed by incubation at 25 °C/3-5 day(s) in the darkness. Phytopathogenic fungal identification was monitored according to [15]. Moreover, Fusarium and Sclerotinum species are identified according to [16,17] respectively.
Antifungal activity
Antagonist activity was and the inhibitory effect Pseudomonas strains are estimated by calculation of the percent inhibition of fungal mycelial growth according to the following formula:
Percent inhibition= (r control - r test)/(r test) ×100
Antifungal mechanism
The determination of the antifungal activities of strains P7 P. plantarii against the phytopathogenic fungal isolates for Fusarium oxysporum fs. phaseoli and Sc-sc Sclerotina sclerotiorum was determined by studying the contact zones using a modified method of [18].his technique consists to observe the contact zones between P7 and the phytopathogenic fungus obtained after dual culture on PDA medium. Coverslip was gently deposited at the contact zone. Then, Microscopic observation was performed (× 10 and/or × 40).
Statistical analysis
A completely randomized design was used for statistical analysis of percent inhibition. One-way analysis of variance with a significance level of p<0.05 was applied. Similarly, when significant differences were found, a comparison of means was performed using Dunnett multiple comparison tests (p<0.05). Means and standard deviation were also calculated.
Results
Characterization of fungal bean diseases
Characterization of fungal diseases exhibited on Phaseolus vulgaris L plants revealed two characteristic symptoms (Figure 1). The first disease was characterized by leaves representing brown spots, invasive white mycelium was clearly observed on stems (Shown in “a”). While, the second disease showed symptoms of bean wilting characterized by desiccated leaves (Shown in “b”).
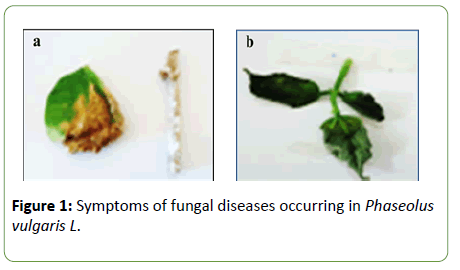
Figure 1: HPLC chromatogram of the nine reference compounds in 50% aqueous methanol, measured at 370nm. Retention times for rutin, sutherlandin A, sutherlandin B, kaempferol-3-O-rutinoside, sutherlandin C, sutherlandin D, quercitrin, quercetin and kaempferol were 11.9, 12.7, 13.8, 15.3, 16.2, 17.0, 18.0, 26.2 and 28.1 minutes, respectively.
Identification of phytopathogenic fungi
Macroscopic identification: Macroscopic observation of the two phytopathogenic fungi revealed two different aspects (Figure 2). After isolation and purification of the two fugal isolates on PDA agar at 25°C/7days, two isolates showed a cream-colored mycelial colonies. The isolate growth rapidly occupying the total Petri plate at the end of incubation. The diameter of colonies formed was 40 mm. Whereas, Sc-sc colonies were white, gray containing small black-stick sclerotia. Colonies were 40 mm in diameter characterized by high speed growth. Sc-sc colonies have a vegetative part and a reproductive part. The vegetative portion ensures the development of the thallus and the construction of the producing part. Whereas, the reproductive part results in globular black shape sclerotia. The isolate could produce 15 to 20 sclerotia on PDA per Petri plate.
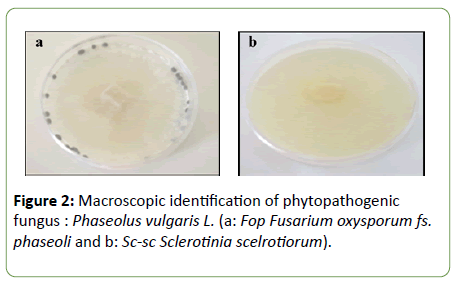
Figure 2: Macroscopic identification of phytopathogenic fungus : Phaseolus vulgaris L. (a: Fop Fusarium oxysporum fs. phaseoli and b: Sc-sc Sclerotinia scelrotiorum).
Microscopic identification: Microscopic observation of the two phytopathogenic fungi revealed distinct myceliums appearances (Figure 3). Microscopic observation of “Fop” showed a septal mycelium and conidia characteristic of the genus Fusarium. The microscopic observation was characterized by spindle-shaped mycelium. Formation of specifics chlamydospores and macroconidia were also observed. Where, the thallus of Sc-sc was coenocytic and branched hyphae, endowed with distinctive cylindrical, elongated ascospores of 8 to 16 cells.
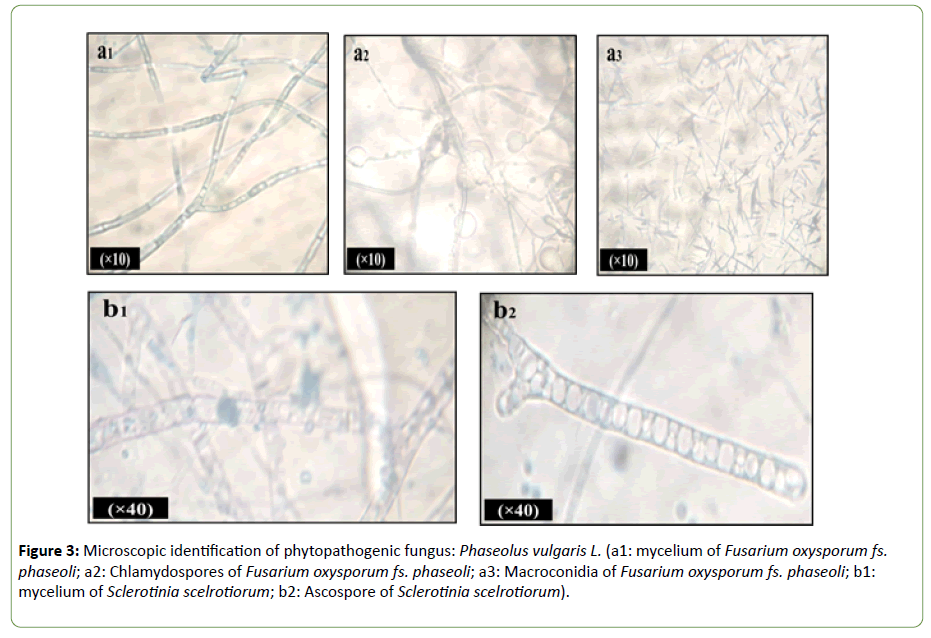
Figure 3: Microscopic identification of phytopathogenic fungus: Phaseolus vulgaris L. (a1: mycelium of Fusarium oxysporum fs. phaseoli; a2: Chlamydospores of Fusarium oxysporum fs. phaseoli; a3: Macroconidia of Fusarium oxysporum fs. phaseoli; b1: mycelium of Sclerotinia scelrotiorum; b2: Ascospore of Sclerotinia scelrotiorum).
Antifungal activity
Study of the antifungal activities of Pseudomonas strains against the phytopathogenic fungal isolates Sc-sc and Fop revealed variation of the inhibition zones formed (Figure 4).
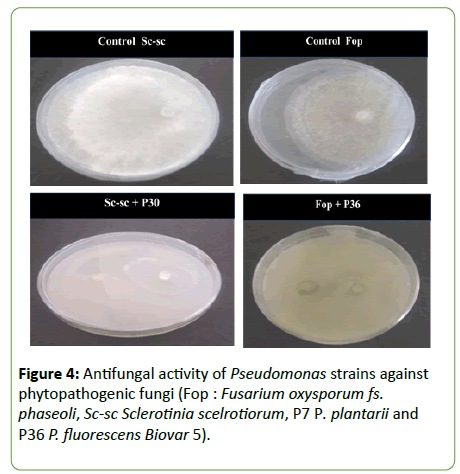
Figure 4: Antifungal activity of Pseudomonas strains against phytopathogenic fungi (Fop : Fusarium oxysporum fs. phaseoli, Sc-sc Sclerotinia scelrotiorum, P7 P. plantarii and P36 P. fluorescens Biovar 5).
The antifungal activities of the PGPR bacterial strains showed that percentages of mycelium growth inhibition of the fungal isolates Sc-sc and Fop varies from one isolate to another (Figure 5). Strains P7 and P30 wield percent inhibition of 100% of Sc-sc, P36 exhibited percent inhibition of 38.89%. However, the isolate Fop was inhibited 100% by strain P36; moderate percent inhibition of 47.78% and 48.89% were exerted by P7 and P30, respectively.
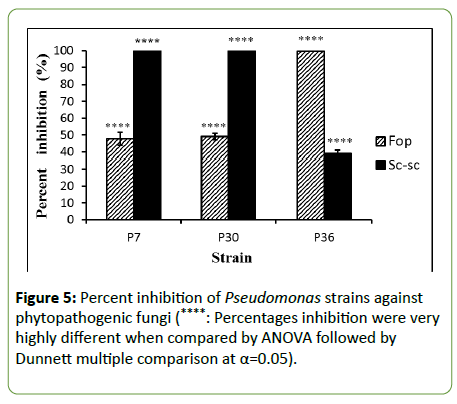
Figure 5: Percent inhibition of Pseudomonas strains against phytopathogenic fungi (****: Percentages inhibition were very highly different when compared by ANOVA followed by Dunnett multiple comparison at α=0.05).
Antifungal mechanism
Observation of the contact zones of the strain P7 Pseudomonas plantarii with fungal isolates Sc-sc Sclerotinia sclerotiorum and Fop Fusarium oxysporum fs. phaseoli revealed different effects in co cultures (Figure 6). Microscopic examination showed alterations of Fop mycelium characterized by cells bloating. While, the effects observed on Sc-sc were characterized by the lysis of the fungal mycelium.
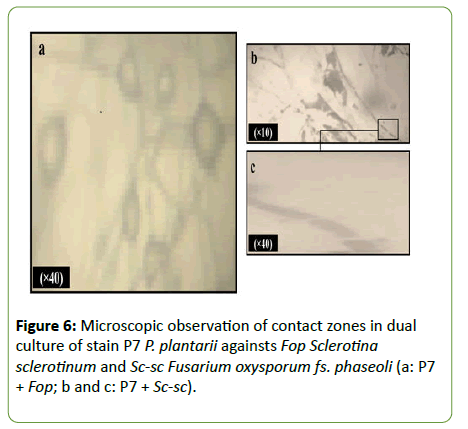
Figure 6: Microscopic observation of contact zones in dual culture of stain P7 P. plantarii againsts Fop Sclerotina sclerotinum and Sc-sc Fusarium oxysporum fs. phaseoli (a: P7 + Fop; b and c: P7 + Sc-sc).
Discussion
Pseudomonas species are ubiquitous microorganisms present in agricultural soils and well adapted to grow in the rhizosphere. This rhizobacterium possesses many traits to act as a biocontrol agent and to promote the plant’s growth ability. Also, PGPR traits make them interesting candidates for biological control of various fungal plant diseases.
In this work, two genera of fungus bean diseases are characterized; the brown spot and bean wilt in greenhouse experiments. Plant diseases are sometimes grouped by types of symptoms, type of organ, affecting them and type of plant affected, but the most useful criteria is classification according to the pathogen responsible for the plant disease [19]. The present study revealed also that the isolates Fop Fusarium oxysporum fs. phaseoli and Sc-sc Sclerotinia sclerotiorum were affected by the characterized bean diseases. Fungi belonging to the genera Fusarium and Sclerotinia are important fungi that contaminate bean crops [20]. Detection of plant pathogens is generally carried out by conventional methods. Isolation and study of cultural characteristics that are most often carried out on solids media [21]. Other liquid or solid culture media proved to be favorable for fungal sporulation, which remains an essential step for the identification of phytopathogenic fungi [22]. In addition, Identification of phytopathogenic fungi by cultural traits, microscopic observation of sporulation forms is an important step for genus discrimination. Identification by molecular biology techniques, including 16S rDNA sequencing, remains an essential step for confirmation of species identification.
Pseudomonas strains tested in this work for their antifungal activities showed high significant inhibition percentages against the two phytopathogenic fungal isolates Fop and Sc-sc [23] reported that the strain RhiNA Pseudomonas protegens exerted a potential inhibition of mycelial growth when confronted to the fungi: Botrytis cinerea, Aspergillus niger, Mucor sp. and Aspegillus flavus. The use of biological control in the management of agricultural pests and diseases is an effective alternative to the use of pesticides, which often accumulate in plants and are lethal to beneficial organisms present in the soil [24]. Since effective biocontrol agents often act through the combination of several different mechanisms, a selection process allowed us to find positive antagonistic strains with more than one target [25]. Pseudomonas antifungal activities observed could be attributed to different PGPR traits [26]. Fungal inhibition assay using mutants of different phenotypes classes suggested that all the four traits (siderophore, HCN, antibiotics and fluorescent pigments) might be involved in the biocontrol of the pathogen [27]. Phytohormones such as IAA (- auxin-indol acetic acid) produced by microbes are more effective in plant growth due to their continuous and slow release [28]. Also, hydrolytic enzymes can degrade the structural matrix of fungal cell walls and therefore can act as antifungal factors [29,30]. For example, Pseudomonas is capable to produce pectinase which is a group of enzyme known to catalyze the pectic substance through depolymerization and deexrification reaction. This enzyme has the role in preventing plant from infection caused by pathogens [31]. As well as several reports indicated that different bacterial species, particularly rhizosphere colonizing bacteria, have the ability to release organic phosphates or to solubilize insoluble inorganic phosphate compounds such as tricalcium phosphate, dicalcium phosphate, hydroxyapatite, and rock phosphate. These bacteria make available the soluble phosphates to the plants, and in return gain root borne carbon compounds, mainly sugars and organic acids, necessary for bacterial growth [32].
Finally, concerning the antifungal activities mechanisms, observation of the contact zones of strain P7 Pseudomonas plantarii against phytopathogenic fungi Fop and Sc-search exhibited a lysis and characteristic deformation of mycelia, respectively. It was published that two bacterial strains Bacillus amyloliquefaciens and Burkholderia cepacia caused various morphological changes in terms of vacuolization, enlargement and swelling of Foa mycelium. Such alterations have been associated with the weakening of mycelial cell walls and cytoplasm expulsion [33]. Lytic enzymes such as chitinase, b-1,3- glucanase have been found in several Bacillus extracts and Burkholderia species [34], these compounds may explain the lysis of Foa cell walls in co-culture with these antagonistic bacteria [35]. Rai et al. reported that Pseudomonas spp. DF41 revealed a highly effective inhibitory effect on S. sclerotiorum by action on mycelial growth and suppression of sclerotia and ascospore germination. Besides, intense researches have been devoted to study the beneficial effects of natural products on plants (marine algae, plant extracts etc.)[22,34]. Furthermore, PGPR bacteria are a promising candidate for the biological control of many fungal diseases causing very high deserter of crop cultures every year.
Conclusion
The present study showed the occurrence of some phytopathogenic fungi of Phaseolus vulgaris causing fungal diseases described in literature by simple isolation in the common PDA medium. Macroscopic and microscopic identification had also confirmed which kind of fungal isolates simultaneously to the attribution of the characteristic symptoms of the fungal diseases observed.
We can conclude that Pseudomonas strains are the effectiveness PGPB affecting fungal growth and acting by different mechanisms, including lysis or deformation of fungal mycelium. These results could be crucial for an eventual investigation of biologic control agents.
References
- Kator L, Ogo-Oluwa AT, Kemi AB (2016) Isolation and Identification of Seed Borne Fungi of Common Bean (Phaseolus vulgaris L.) from Selected Markets in Makurdi International. J Appl Agri Sci 2: 75-78.
- Ranjbariyan A, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M (2014) Antifungal activity of a soil isolate of Pseudomonas chlororaphis against medically important dermatophytes and identification of a phenazine-like compound as its bioactive metabolite. J Mycol Med 24: 57-64.
- Mokrani S, Belabid L, Bedjaoui B, Nabti E (2018) Growth Stimulation of Phaseolus Vulgaris L Plantules by Strain Bacillus Amyloliquefaciens Hla Producer of Beneficial Agricultural Enzymes. JOJ Hortic Arboric 2: 555581.
- Raaijmakers JM, Vlami M, de Souza JT (2002) Antibiotic production by bacterial biocontrol agents. Antonie Van Leeuwenhoek 81: 537–547.
- Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 7: 4951–4959.
- Bensidhoum L, Nabti E, Tabli N, Kupferschmied P, Weiss A, et al. (2016) Heavy metal tolerant Pseudomonas protegens isolates from agricultural well water in northeastern Algeria with plant growth promoting, insecticidal and antifungal activities. European J Soil Biol 75: 38-46.
- Haas D, Keel C (2003) Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant disease. Annu Rev Phytopathol 4: 117–153.
- Mokrani S, Rai A, Belabid L, Cherif A, Cherif H, et al. (2018) Pseudomonasdiversity in western Algeria: role in the stimulation of bean germination and common bean blight biocontrol. Eur J Plant Pathol 152: 1-19.
- Grewal RK, Jhooty JS (1984) Rating of gram Cicer arietinum blight in fungicidal trials. Crop Improv 11: 71-72.
- Terbeche F (2011) Etude de l'activité protéolytique et le profil protéique total chez Ascochyta rabiei. Mémoire de magister, université Ahmed Ben Bella, Es Senia, Oran, Algérie.
- Lecellier A (2013) Caractérisation et identification des champignons filamenteux par spectroscopie vibrationnelle. Thèse de doctorat. Université de Reims Champgne-Ardenne, France.
- Cahagnier B, Richard-Molard D (1998) Analyse mycologique in Moisissures des aliments peu hydratés. Tec and Doc, Lavoisier, Paris, France.
- Su YY, Qi YL, Cai L (2012) Induction of sporulation in plant pathogenic fungi. Mycology 3: 195-200.
- Kohn LM (1979) A monographic revision of the genus Sclerotinia. Mycotaxon 9: 365-444.
- Tivoli B (1988) Guide d'identification des différentes espèces ou variétés de Fusarium rencontrées en France sur la pomme de terre et dans son environnement. Agronomie 8: 211-222.
- Ekins MG, Aitken EAB, Goulter KC (2002) Carpogenic germination of Sclerotinia minor and potential distribution in Australia. Australian Plant Pathol 31: 259-265.
- Mitoi ME, Helepciuc FE, Brezeanu A, Cornea CP (2012) Characterization of the impact of Bacillus licheniformis and Pseudomonas aeruginosa against Alternaria alternata by phase contrast microscopy and transmission electron microscopy. Analele Stiintifice ale Universitatii" Al. I. Cuza" din Iasi 58: 5-18.
- Benjama A (2003) Méthode d‘évaluation rapide du degré d’attaque de lolivier par la tuberculose causée par Pseudomonas savastanoi pv savastanoi, en verger au Maroc. Fruits 58: 213-219.
- Ospina HF, Schwartz HF (1981) Bean diseases caused by fungi and their control. Ed. Centre International de Agricultura Tropical (CIAT), Cali, Colombia.
- Liu D (2010) Molecular Detection of Foodborne Pathogens. CRC Press Taylor and Francis group, USA.
- Tatiana TMSR, Luiz AM, Onkar DD, Eduardo SGM (2010) In vitro production of conidia of Alternaria solani. Trop Plant Pathol 35: 203-212.
- Nogórska K, Bikowski M, Obuchowski M (2007) Multicellular behavior and production of a wide variety of toxic substances support usage of Bacillus subtilis as a powerful biocontrol agent. Acta Biochim Pol 54: 495-508.
- Cain CC, Lee D, Waldo 3rd RH, Henry AT, Casida Jr EJ, et al. (2003) Synergistic antimicrobial activity of metabolites produced by a nonobligate bacterial predator Antimicrob Agents Chemother 47: 2113-2117.
- Tabli N, Rai A, Bensidhoum L, Palmieri G, Gogliettino M, et al. (2018) Plant growth promoting and inducible antifungal activities of irrigation well water-bacteria. Biological Control 117: 78-86.
- Pal KK, Tilak KVBR, Saxena AK, Dey R, Singh CS (2000) Antifungal characteristics of a fluorescent Pseudomonasstrain involved in the biological control of Rhizoctonia solani Microbiol Res 155: 233-242.
- Gupta G, Parihar SS, Ahirwar NK, Snehi SK, Singh V (2015) Plant growth promoting rhizobacteria (PGPR): current and future prospects for development of sustainable agriculture. J Microb Biochem Technol 7: 96-102.
- Cattelan AJ, Hartel PG, Fuhrmann JJ (1999) Screening for plant growth-promoting rhizobacteria to promote early soybean growth. SSSAJ 63: 1670-168.
- Hu C (2005) Induction of growth promotion and stress tolerance in Arabidopsis and tomato by plant growth promoting rhizobacteria. Dissertation, Auburn University, USA
- Reetha S, Selvakumar G, Bhuvaneswari G, Thamizhiniyan P, Ravimycin T (2014) Screening of cellulase and pectinase by using Pseudomonas fluorescens and Bacillus subtilis. International Letters Natural Sci 13:75-80.
- Khan MS, Zaidi A, Ahemad M, Oves M, Wani PA (2010) Plant growth promotion by phosphate solubilizing fungi-current perspective. Arch Agric Soil Sci 56: 73-98.
- Dihazi A, Jaiti F, Jaoua S, Driouich A, Baaziz M, et al. (2012) Use of two bacteria for biological control of bayoud disease caused by Fusarium oxysporum in date palm (Phoenix dactylifera L) seedlings. Plant Physiol Biochemi 55:7-15.
- Buensanteai N, Yuen GY, Prathuangwong S (2008) The biocontrol bacterium Bacillus amyloliquefaciens KPS46 produces Auxin, Surfactin and extracellular proteins for enhanced growth of Soybean plant. Thai J Agricul Sci 41: 101-116.
- Savchuk SC (2002) Evaluation of biological control control of Sclerotinia scleroiorum on Canola (Brassica napus) in the lab, in the greenhouse and in the field. M.Sc. Thése de doctorat, University of Manitoba, Canada.
- Nabti E, Bensidhoum L, Tabli N (2018) Effect of the marine algae cystoseira mediterranea on growth of hordeum vulgare (l.) and it chlorophyll content. Trends Horticulture 1: 1-8.
- Rai A, Bensidhoum L, Tabli N, Bouaoud Y, Naili F, et al. (2016) A Pseudomonas Protegenswith High Antifungal Activity Protects Apple Fruits Against Botrytis Cinerea Gray Mold. Int J Scientific Res Sci Technol 2: 227-237.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
