ISSN : 0976-8505
Der Chemica Sinica
A Practical Approach to the Effective Synthesis of 1,8-Dioxỏ̮̦̉ctahydroxanthene Derivatives By The Use of ICl3 /SiO2 and In(CF3 SO3)3 As Recyclable and Highly Efficient Catalysts
Bahador Karami1*, Khalil Eskandari2 and Goodarz Ansari3
1Department of Chemistry, Yasouj University, Yasouj, Iran
2Young Researchers and Elites Club, Shahrekord Branch, Islamic Azad University, Shahrekord, Iran
3Department of Chemistry, Gachsaran Branch, Islamic Azad University, Gachsaran, Iran
Abstract
Silica gel supported iodine trichloride and indium triflate were found as efficient and reusable catalysts to the synthesis of a series of xanthenes as potentially interesting biological active molecules in high rates and yields. A broad range of xanthene derivatives were efficiently prepared via a three component reaction between cyclic β-diketones and arylaldehydes (2:1 ratio) with high yield and purity by the use of a catalytic amount of ICl3 /SiO2 and In(CF3 SO3 )3 as Lewis acids under solvent-free and mild conditions. This newly achieved route has some advantage such as facile and simple handling, employing of neat and reusable catalysts, using readily available chemicals, short span of needed time, avoid of employing hazardous solvents, and including green chemistry aspects.
Keywords
Recyclable catalyst, Condensation reaction, Cyclic β-diketone, Solvent free, 1,8-dioxoöctahydroxanthene
Introduction
Nowadays, the role of solid acids in environmentally safe and economical technologies, mainly in chemical manufacturing processes and green chemistry is inevitable and undeniable [1-5]. Furthermore, the key role of solid acids as catalyst in the synthesis of heterocycles and other organic compounds is well-known and well-recognized [6-9], especially, after completion of reaction, they can easily separate from reaction mixture [10,11]. Furthermore, considering green chemistry aspects, due to reducing of environment pollutions, use of eco-friendly technologies such as running organic reactions under green conditions is one of the more important perspective that nowadays has received the interest of chemists [12-18]. The use of solvent-free conditions in chemical reactions is one of these technologies. To the point of green chemistry view, loading chemical reactions under solvent-less conditions has significant advantages such as avoiding use of harmful solvents, reducing environmental pollution, bringing down handling costs, predigestion of experimental and, work up procedures, and also frugality in labor [12-18].
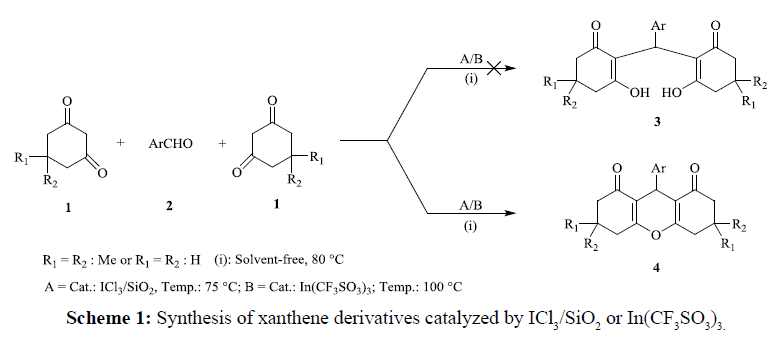
Among organic compounds, phrmacological and therapeutic virtues of xanthene derivatives make them more notable and important molecules. Therefore, they have recently received more attentions by chemists and pharmacologists [19,20]. In addition, the importance of xanthenes clearly is understood by their applications including, xanthene dyes [21], anti-cancer activity [22], applicable for the evaluation of biomolecules via its fluorescent activity [23], and also in laser and optic technologies [24]. As well as, some of the xanthene-based heterocyles have recognized as antagonists and in photodynamic therapy [25]. As a matter of fact cancer is the leading cause of death in the developed countries, and second leading cause of death in the developing countries, therefore improving and developing of new and more effective compounds to treat of cancer is inevitable [16]. Considering above, for instance, some derivatives of 1,8-dioxoöctahydroxanthene were synthesized and assessed for their anticancer activities in vitro [16]. The obtained results from this experiment, reveals this type of compounds have shown anti-proliferative properties against a number of cancer cell lines including, human neuroblastoma (IMR32), human colon carcinoma (Colo-205), and human chronic myeloid leukemia (K562) cells [16]. Despite, taking attempts to 1,8-dioxoöctahydroxanthene derivatives synthesis and evaluation of their properties toward tumor and normal cell lines [26], the leishmanicidal assay of these compounds were evaluated. The results of this experiment, revealed that some derivatives of 1,8-dioxoöctahydroxanthenes would show anti-leishmanial activity [27]. Also Up to date several natural products and herbal medicines are isolated and characterized from natural sources which have xanthene motifs in their chemical structures [28]. Nowadays, there are numerous reported methods in which lead to produce of xanthenes and their derivatives, including the reaction between aryloxymagnesium halides and triethylorthoformate [29], cyclodehydration [30], reaction of benzynes with phenols [31], intramolecular condensation reactions between benzaldehydes and acetophenones [32], and cyclocondensation of ortho-hydroxyarylaldehydes and β-tetralone [33]. Turning to the matter of xanthene derivatives, some other procedures are well-known that cause to produce of these heterocycles, such as catalytic condensation between 2-naphthol with aldehydes or acetals by the use of silica sulfuric acid (SSA), HCl/CH3COOH or H3PO4[34]. Bacause of some of these methods are possessed unfavourable effects such as having long times and harsh condition of reaction, and also low yields, modifying and improving of them have been endlessly sought. Therefore, following to our interest in highly efficient catalytic synthesis of a broad class of heterocycles and organic compounds via multicomponent and one pot reactions [35-38], in this work, an efficient, green adapted and new route to the synthesis of 1,8-dioxoöctahydroxanthenes (4) was reported via condensation reaction between 1,3-cyclohexanediones (1) with arylaldehydes (2) by employing ICl3/SiO2 (iodine trichloride supported on silicagel) and In(CF3SO3)3 as powerful, cheap and recyclable catalysts (Scheme 1).
Materials and Methods
Experimental
Materials and methods
An elecrtothermal KSB1N apparatus was applied to determine melting points (m.p.). JASCO FT-IR-680 plus spectrometer along with KBr as matrix was used to deduce IR spectra. FT-NMR Bruker Avance Ultra Shield Spectrometer at 400.13 and 100.62 MHz in CDCl3 as solvent was used to determine 1H NMR and 13C NMR spectra respectively. A Heraeus Rapid analyzer was got for the measurement of elemental analyses (C, H, N, S) and the results were in good agreement with the calculated values (± 0.3 %). TLC-Grade silica gel-G/UV 254 nm plates (eluents: n-hexane, and ethyl acetate 2:1) was applied to control of reaction progress. All chemicals were also purchased from Merck and Sigma-Aldrich chemical companies.
Preparation of ICl3/SiO2
Firstly, the 5 g of silica gel [60 Å, 35-75 μm particle size] for 4 h was heated in oven at 140°C. Then dried silica gel (1 g) was stirred with iodine trichloride (0.117 g, 0.5 mmol) in chloroform (10 mL), and then was heated under reflux conditions for appropriate time (4 h). In continuous, the mixture was filtered, washed thoroughly with chloroform (3 × 10 ml), and the obtained catalyst was dried at 70°C for 2 h.
General Procedure for the Synthesis of 9-Aryl-Substituted 1,8-Dioxoöctaoctahydro-xanthenes
Cyclic 1,3-diketone (2 mmol), was added to the mixture of arylaldehyde (1 mmol) and ICl3/SiO2 (0.117 g, 0.05 mmol) or In(CF3SO3)3 (0.011 g, 0.02 mmol), and then the mixture was heated at 70°C for the time demonstrated in Table 1. The progress of the reaction was controlled by TLC (eluents: n-hexane, and ethyl acetate 2:1). After confirming of reaction completion, CHCl3 (10 mL) was poured to the reaction mixture, Afterwards, to separate catalyst, the mixture was filtered. In continuous, solvent was evaporated from the filtrate in vacuum to remain the crude product. Crude product was recrystallized by boiling EtOH to obtain the crystalline pure product. At the end, the separated catalyst from reaction mixture was washed with boilng ethanol, then dried at 120°C for 1 h (ICl3/SiO2: dried at 70°C for 8 h), and reused four more times in other reactions.
| Compound 4 | ICl3/SiO2 | In(CF3SO3)3 | M.P. (°C)/[lit.] |
|---|---|---|---|
| Time (min)/Yield (%)a | Time (min)/Yield (%)a | ||
 |
60/90 | 60/95 | 202-204 (201-202) [45] |
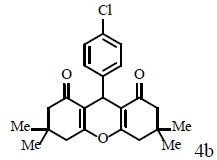 |
70/90 | 70/92 | 230-232 (230-232) [45] |
 |
70/87 | 70/90 | 215-217 (216-217) [46] |
 |
40/88 | 40/90 | 219-221 (221-223) [46] |
 |
80/89 | 70/88 | 226-227 (226-228) [47] |
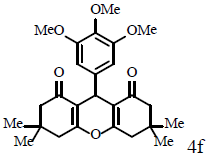 |
60/88 | 60/92 | 209-211 (210-212) [48] |
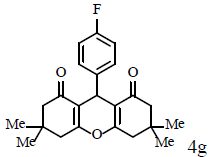 |
60/90 | 65/92 | 223-225 (224-226) [47] |
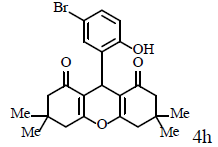 |
60/84 | 70/89 | 250-252 (249-252) [49] |
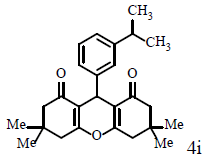 |
60/82 | 70/85 | 189-191 (190-191) [50] |
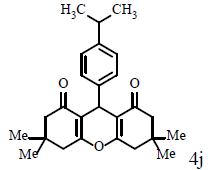 |
70/88 | 70/90 | 238-239 (236-239) [30] |
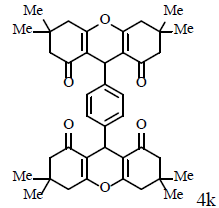 |
60/86 | 60/89 | 245-247 (>300) [51] |
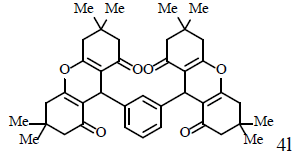 |
75/85 | 75/88 | 238-240 (236-238) [52] |
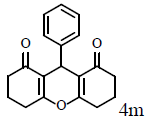 |
60/92 | 70/95 | 271-273 (272-273) [53] |
 |
65/88 | 65/90 | 260-262 (262-263) [45] |
 |
60/91 | 55/95 | 224-227 (224-226)[54] |
 |
70/88 | 70/89 | 250-252 (249-252)[29] |
 |
80/84 | 60/88 | 170-172 (169-171) [55] |
 |
60/87 | 60/90 | 282-285 (280-282) [56] |
 |
90/81 | 80/85 | 141-143 (139-141) [13] |
Table 1: Catalytic synthesis of 9-aryl-substituted-1,8-dioxoöctahydroxanthenes by the use of ICl3/SiO2 and In(CF3SO3)3 under solvent-free conditions.
Typical procedure to the synthesis of product 4a
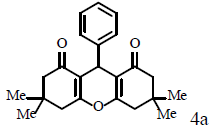
Compound 4a was synthesized based on the general procedure, by employing dimedone 1 (R1, R2=CH3) (0.280 g, 2 mmol), benzaldehyde 2a (0.106 g, 1 mmol) and ICl3/SiO2 (0.117 g, 0.05 mmol) or In(CF3SO3)3 (0.011 g, 0.02 mmol). The progress of the reaction was controlled by TLC. When the reaction progress was completed, CHCl3 (10 ml) was poured to the mixture, and then the mixture was filtered to separate the catalyst. Removing solvent from filtrate by vaccum, lead to obtain crude product which was recrystallized by boiling EtOH to afford white crystals as pure product.
Representative spectral data
Compound 4a: mp 202-204°C; IR (KBr) νmax: 699, 742, 1200, 1468, 1624, 1662, 2958, 3059 cm-1; 1H NMR (400 MHz, CDCl3) δ (J, Hz): 0.79 (s, 6H, 2CH3), 0.90 (s, 6H, 2CH3), 2.00 (dd, 1J=16.4, 4J=28.8, 4H, 2CH2), 2.27 (s, 4H, 2CH2), 4.55 (s, 1H, CH), 6.90-7.10 (m, 5H, CHAr) ppm; 13C NMR (100 MHz, CDCl3) δ 28.5, 30.4, 33.0, 33.4, 42.0, 51.9, 116.8, 127.5, 129.2, 129.5, 145.2, 163.4, 196.7 ppm; Anal. Calcd.: C, 78.83; H, 7.48, (C23H26O3); Found: C, 78.79; H, 7.51.
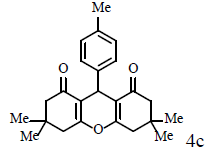
Compound 4c: mp 260-262°C; IR (KBr) νmax: 827, 1125, 1175, 1466, 1615, 1658, 2955, 3049 cm-1; 1H NMR (400 MHz, CDCl3); δ (J, Hz): 2.01 (m, 4H, 2CH2), 2.26 (s, 3H, CH3), 2.35 (m, 4H, 2CH2), 2.59 (m, 4H, 2CH2), 4.78 (s, 1H, CH), 7.03 (d, J=7.2, 2H, Ar-H), 7.19 (2H, d, J=7.2, CHAr); 13C NMR (100 MHz, CDCl3); δ: 20.3, 21.1, 27.1, 31.2, 37.0, 117.0, 128.2, 128.8, 135.8, 141.6, 163.8, 196.6 ppm; Anal. Calcd.: C, 79.09; H, 7.74, (C24H28O3); Found: C, 79.12; H, 7.69.
Compound 4f: mp 209-211°C; IR (KBr) νmax: 844, 1089, 1189, 1375, 1479, 1620, 1659, 2995, 3049 cm-1; 1H NMR (400 MHz, CDCl3) δ (J, Hz): 1.04 (s, 6H, 2CH3), 1.12 (s, 6H, 2CH3), 2.24 (s, 4H, 2CH2), 2.47 (s, 4H, 2CH2), 3.78 (s, 3H, OCH3), 3.81 (s, 6H, 2OCH3), 4.72 (s, 1H, CH), 6.52 (s, 2H, CHAr); 13C NMR (100 MHz, CDCl3) δ: 27.2, 29.4, 31.8, 32.2, 40.9, 50.7, 56.1, 60.7, 105.7, 115.6, 136.6, 139.7, 152.8, 162.3, 196.4; Anal. Calcd.: C, 70.89; H, 7.32, (C26H32O6); Found: C, 70.91; H, 7.28.
Compound 4g: mp 223-225°C; IR (KBr) νmax: 1179, 1160, 1199, 1359, 1500, 1619, 1659, 2970, 2989, 3039 cm-1; 1H NMR (400 MHz, CDCl3) δ (J, Hz): 1.00 (s, 6H, 2CH3), 1.11 (s, 6H, 2CH3), 2.21 (q, J=16.4, 4H, 2CH2), 2.47 (s, 4H, 2CH2), 4.73 (s, 1H, CH), 6.91 (m, 2H, CHAr), 7.27 (m, 2H, CHAr); 13C NMR (100 MHZ, CDCl3) δ 27.3, 29.3, 32.2, 40.8, 50.7, 114.7, 114.9, 115.5, 129.9, 140.0, 160.1, 162.6, 196.3; Anal. Calcd.: C, 74.98; H, 6.84, (C23H25FO3); Found: C, 74.91; H, 6.79.
Compound 4i: mp 189-191°C; IR (KBr) νmax: 1139, 1159, 1199, 1375, 1449, 1615, 1659, 2879, 2959, 3065 cm-1; 1H NMR (400 MHZ, CDCl3) δ (J, Hz): 1.01 (s, 6H, 2CH3), 1.10 (s, 6H, 2CH3), 1.18 (d, J=5.2, 6H, 2CH3), 2.21 (m, 4H, 2CH2), 2.46 (s, 4H, 2CH2), 2.79 (bb, 1H, CH), 4.73 (s, 1H, CH), 7.05 (d, J=6.8, 2H, CHAr), 7.19 (m, 2H, CHAr); 13C NMR (100 MHZ, CDCl3) δ: 23.9, 27.5, 29.2, 31.3, 32.2, 33.6, 40.9, 50.8, 126.1, 128.1, 141.4, 146.5, 162.1, 196.5; Anal. Calcd.: C, 79.56; H, 8.22, (C26H32O3); Found: C, 79.61; H, 8.25.
Compound 4k: mp 245-247°C; IR (KBr) νmax: 809, 1004, 1161, 1199, 1364, 1424, 1461, 1619, 1665, 2956, 3039 cm-1; 1H NMR (400 MHZ, CDCl3) δ (J, Hz): 0.97 (s, 12H, 4CH3), 1.07 (s, 12H, 4CH3), 2.18 (s, 8H, 4CH2), 2.44 (dd, 1J=36.4, 4J=17.6, 8H, 4CH2), 4.71 (s, 2H, 2CH), 7.08 (s, 2H, CHAr), 7.27 (s, 2H, CHAr); 13C NMR (100 MHZ, CDCl3) δ: 25.0, 27.7, 29.0, 30.7, 32.2, 40.8, 50.6, 115.7, 127.9, 141.7, 162.4, 196.4; Anal. Calcd.: C, 77.14; H, 7.45, (C40H46O6); Found: C, 77.09; H, 7.48.
Compound 4l: mp 238-240°C; IR (KBr) νmax: 769, 1158, 1203, 1462, 1629, 1660, 2958, 3094 cm-1; 1H NMR (400 MHZ, CDCl3) δ (J, Hz): 1.03 (s, 12H, 4CH3), 1.08 (s, 12H, 4CH3), 2.15 (dd, 2J=24, 4J=16, 8H, 4CH2), 2.48 (dd, 2J=45.2, 4J=17.6, 8H, 4CH2), 4.72 (s, 2H, 2CH), 7.07-7.09 (m, 3H, CHAr), 7.15 (s, 1H, CHAr); 13C NMR (100 MHZ, CDCl3) δ 28.0, 29.6, 31.8, 32.6, 41.3, 51.3, 116.0, 126.8, 128.2, 144.0, 162.7, 196.7; Anal. Calcd.: C, 77.14; H, 7.45, (C40H46O6); Found: C, 77.21; H, 7.39.
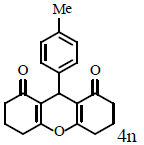
Compound 4n: mp 260-262°C; IR (KBr) νmax: 827, 1125, 1176, 1468, 1617, 1657, 2956, 3052 cm-1; 1H NMR (400 MHZ, CDCl3) δ (J, Hz): 2.01 (m, 4H, 2CH2), 2.26 (s, 3H, CH3), 2.35 (m, 4H, 2CH2) 2.59 (m, 4H, 2CH2) 4.78 (s, 1H, CH), 7.03 (d, J=7.2, 2H, CHAr), 7.19 (d, J = 7.2, 2H, CHAr); 13C NMR (100 MHZ, CDCl3) δ: 20.3, 21.1, 27.1, 31.2, 37.0, 117.0, 128.2, 128.8, 135.8, 141.6, 163.8, 196.6; Anal. Calcd.: C, 77.90; H, 6.54, (C20H20O3); Found: C, 77.88; H, 6.59.
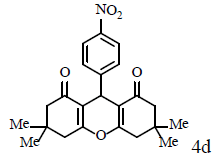
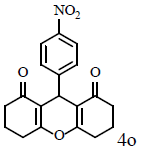
Compound 4o: mp 224-227°C; IR (KBr) νmax: 831, 1170, 1465, 1665, 2952, 3071 cm-1; 1H NMR (400 MHZ, CDCl3) δ (J, Hz): 2.07 (m, 4H, 2CH2), 2.35 (m, 4H, 2CH2) 2.61 (m, 4H, 2CH2), 4.88 (s, 1H, CH), 7.48 (d, J = 8.8, 2H, CHAr), 8.10 (d, J = 8.8, 2H, CHAr); 13C NMR (100 MHz, CDCl3) δ: 20.2, 27.1, 32.2, 36.8, 115.7, 123.4, 129.4, 145.5, 151.7, 164.6, 196.4; Anal. Calcd.: C, 67.25; H, 5.05; N, 4.13, (C19H17NO5); Found: C, 67.21; H, 5.11; N, 4.09.
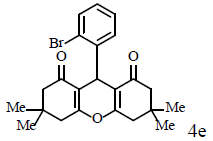
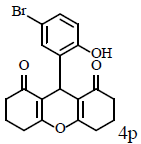
Compound 4p: mp 250-252°C; IR (KBr) νmax: 649, 813, 1004, 1034, 1085, 1179, 1214, 1288, 1368, 1462, 1564, 1620, 2959, 3096 cm-1; 1H NMR (400 MHz, CDCl3) δ (J, Hz): 1.86 (s, 2H, CH2), 2.05 (t, J=12.4, 2H, CH2), 2.15 (d, J=8.4, 1H, CH), 2.43 (m, 2H, CH2), 2.57 (t, J=9.2, 2H, CH2), 2.75 (d, J=8.8, 1H, CH), 4.58 (s, 1H, CH), 6.91 (d, J = 4.4, 1H, CHAr), 7.13 (s, 1H, CHAr), 7.26 (d, J=4.4, 1H, CHAr) 10.77 (s, 1H, OH); 13C NMR (100 MHZ, CDCl3) δ: 19.5, 19.9, 27.9, 28.0, 29.7, 35.9, 36.9, 112.0, 117.0, 117.3, 119.4, 126.9, 130.5, 130.7, 150.0, 170.7, 173.4, 197.1, 201.3; Anal. Calcd.: C, 58.63; H, 4.40, (C19H17BrO4); Found: C, 58.59; H, 4.36.
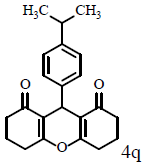
Compound 4q: mp 170-172°C; IR (KBr) νmax: 827, 1129, 1199, 1449, 1619, 1659, 3049 cm-1; 1H NMR (400 MHz, CDCl3) δ (J, Hz): 1.19 (d, J=6.8, 6H, 2CH3), 2.01 (m, 4H, 2CH2), 2.34 (m, 4H, 2CH2), 2.61 (m, 4H, 2CH2), 2.81 (t, J=7.2, 1H, CH), 4.80 (s, 1H, CH) 7.06 (d, J=8, 2H, CHAr), 7.19 (2H, d, J=8, H Ar); 13C NMR (100 MHZ, CDCl3) δ 20.3, 23.9, 27.1, 31.0, 33.6, 37.0, 117.0, 126.2, 146.5, 163.9, 196.6; Anal. Calcd.: C, 78.54; H, 7.19, (C22H24O3); Found: C, 78.55; H, 7.22.
Compound 4r: 252-255°C; IR (KBr) νmax: 802, 1001, 1165, 1210, 1425, 1460, 1623, 1665, 2950, 3038 cm-1; 1H NMR (400 MHz, CDCl3) δ (J, Hz): 2.29 (m, 8H, 4CH2), 2.39 (m, 8H, 4CH2), 2.57 (m, 4H, 2CH2), 2.67 (m, 4H, 2CH2), 4.74 (s, 2H, 2CH), 7.18 (d, 4H, J=7.6, CHAr) ppm; 13C NMR (100 MHZ, CDCl3) δ: 20.1, 27.1, 30.8, 36.9, 116.9, 128.0, 141.9, 164.0, 196.7 ppm; Anal. Calcd.: C, 75.28; H, 5.92, (C32H30O6); Found: C, 75.31; H, 5.89.
Results and Discussion
In the first view, however, there are some reports from condensation reaction between cyclic 1,3-diketones 1 and arylaldehydes 2, that lead to synthesize 2,2'-(arylmethylene)bis(3-hydroxycyclohex-2-enone) (3) [26,39-41], but under given conditions, 2,2'-(arylmethylene)bis(3-hydroxycyclohex-2-enone) 3 wasn’t obtained and reaction of cyclic 1,3-diketones 1 with arylaldehydes 2 leads to efficient synthesize of 9-aryl-substituted 1,8-dioxoöctahydroxanthenes 4.
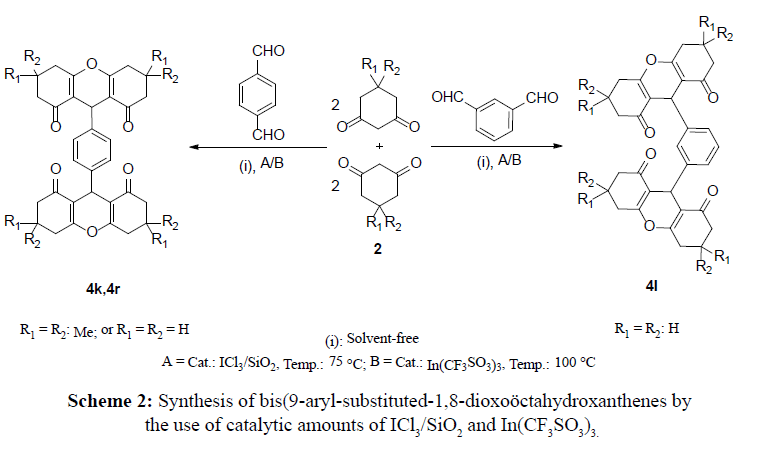
In the other investigation, using aryl-dialdehyde substrates, instead of monoaldehydes lead to condensation reaction with 1,3-cyclic diketones with 1:4 ratioes respectively to afford bisxanthenes as target products. In this five component reaction, 1,3-diketones (4 mmol) were reacted with dialdehyde (1 mmol) to afford bis(9-aryl-substituted-1,8- dioxoöctahydroxanthenes) (Scheme 2).
The chemical structure of the product 4 was characterized from their IR, 1H, and 13CNMR spectroscopic data. Also the melting points of products were compared and confirmed with reported ones in the literatures.
We are directed to immobilize iodine trichloride onto the common solid support in order to combine the properties such as catalyst selectivity, high activity with the ease of separation and catalyst reuse. It may be considered notable that the support materials play a significant role on catalyst activity when they are supported catalysts on their surface. For instance, silica gel was chosen as solid support due to its high surface area, excellent stability (chemical and thermal), good accessibility, recyclability, and ease of functionalization of the surface groups [42,43]. Iodine trichloride supported on silica gel (ICl3/SiO2) does not need activation and is recycled many times under the same conditions with fresh reactants to yield similar results without significant loss of activity [44]. In following of our study, In(CF3SO3)3 is handled as another effective and recoverable catalyst and is compared with ICl3/SiO2 in xanthene derivatives synthesis. However, a small part of this study, as an incomplete and partial results have been previously reported in a symposium [45], herein we wish to report developed and completed 1,8-dioxoöctahydroxanthenes synthesis protocol with highest yields in short reaction times under obtained best conditions.
To find the best conditions for catalytic (ICl3/SiO2 or In(CF3SO3)3) synthesis of xanthene derivatives, at first, synthesis of compound 4a was opted as a model.
By investigations on model reaction, the reaction carried out using ICl3/SiO2 or In(CF3SO3)3 as catalyst in different solvents including H2O, EtOH, CH3OH, CH3Cl, CH3CN, and solvent-free conditions. These experiments showed that the reaction is performed with shortest time and highest yield under solvent-free conditions (Table 2). Therefore, the reaction carries out under solvent-free conditions. On the other hand, to the best of our knowledge solvent-free condition has some advantage for chemical reactions such as reduce pollution, avoiding the use of harmful and toxic solvents, decreasing costs of solvent handling, easy and facile work up technique, and leading to save in labour [46].
| Entry | Solvent | A | B |
|---|---|---|---|
| Time (min)/Yield (%) | Time (min)/Yield (%) | ||
| 1 | H2O | 130/trace | 180/46 |
| 2 | EtOH | 130/60 | 130/50 |
| 3 | MeOH | 190/52 | 190/56 |
| 4 | CHCl3 | 190/46 | 190/41 |
| 5 | CH3CN | 190/33 | 190/27 |
| 6 | DMF | 190/35 | 190/30 |
| 7 | Dioxane | 65/76 | 65/81 |
| 8 | Solvent-less | 60/90 | 60/95 |
B) Reaction catalyzed by In(CF3SO3)
Table 2: Evaluation of solvent effect for model reaction.
The affords to evaluation of required catalysts in the synthesis 1,8-dioxoöctahydroxanthene derivatives for the model reaction, was revealed thet when the reaction was loaded with 5 mol% of ICl3/SiO2 and 2 mol% of In(CF3SO3)3, the maximum yield of product is obtained (Table 3).
| ICl3/SiO2 (mol%) |
Time (min) | Yielda(%) | In(CF3SO3)3 (mol%) |
Time (min) | Yielda (%) |
|---|---|---|---|---|---|
| 1 | 130 | 36 | 1 | 130 | 62 |
| 2 | 130 | 57 | 2 | 60 | 95 |
| 5 | 60 | 90 | 3 | 60 | 90 |
| 8 | 60 | 87 | 5 | 60 | 80 |
| 10 | 70 | 81 | 8 | 60 | 75 |
Table 3: Optimization of required catalysts for model reaction.
As the data of Table 3 are shown, the best quantities of required catalysts for this reaction were found to be 5 mol% for ICl3/SiO2 and 2 mol% for In(CF3SO3)3, whereas the use of larger amounts of the catalysts do not improve the yield.
In addition, the effect of temperature on reaction was studied. Examining of reactions progress at various temperatures in the presence of optimized amount of catalysts for model reaction, revealed that the maximum rate of reaction is achieved at 75°C when ICl3/SiO2 is used as catalyst, and at 100°C when In(CF3SO3)3 is used as catalyst (Table 4).
| Temp. (°C)a | Time (min)a | Yield (%)a | Temp. (°C)b | Time (min)b | Yield (%)b |
|---|---|---|---|---|---|
| r.t. | 120 | 25 | r.t. | 300 | 30 |
| 40 | 120 | 50 | 40 | 120 | 45 |
| 50 | 90 | 62 | 50 | 100 | 50 |
| 60 | 70 | 68 | 60 | 90 | 70 |
| 70 | 60 | 79 | 70 | 80 | 77 |
| 75 | 60 | 90 | 75 | 60 | 80 |
| 80 | 60 | 88 | 80 | 60 | 84 |
| 90 | 60 | 84 | 90 | 60 | 91 |
| 100 | 60 | 80 | 100 | 60 | 95 |
| 110 | 60 | 80 | 110 | 60 | 89 |
bIn(CF3SO3)as catalyst
Table 4: Evaluation of temperature effects on model reaction.
By a looking at Table 4, it is found that the reaction is completed slowly at room temperature. When ICl3/SiO2 is used as catalyst, by increasing temperature to 75°C [when In(CF3SO3)3 is used, it is 100°C], the yield of reaction is increased along with decreasing of the time of reaction. When, the reaction is heated above 75°C [above 100°C in the presence of In(CF3SO3)3], so more high temperatures do not further improve the yield and the time of reaction. Based on obtained optimal conditions, we run the xanthenes derivatives synthesis in the presence of ICl3/SiO2 (5 mol%, at 75°C) or In(CF3SO3) (2 mol%, at 100°C) in solvent-free conditions.
Considering optimal conditions, numerous arylaldehydes 2 bearing both electron-donating and electron-withdrawing groups were effectively condensed to give 9-aryl substituted 1,8-dioxoöctahydroxanthene derivatives 4. In all cases, corresponding xanthenes were isolated with good to excellent yields (Table 1) [13,29,30,47-58].
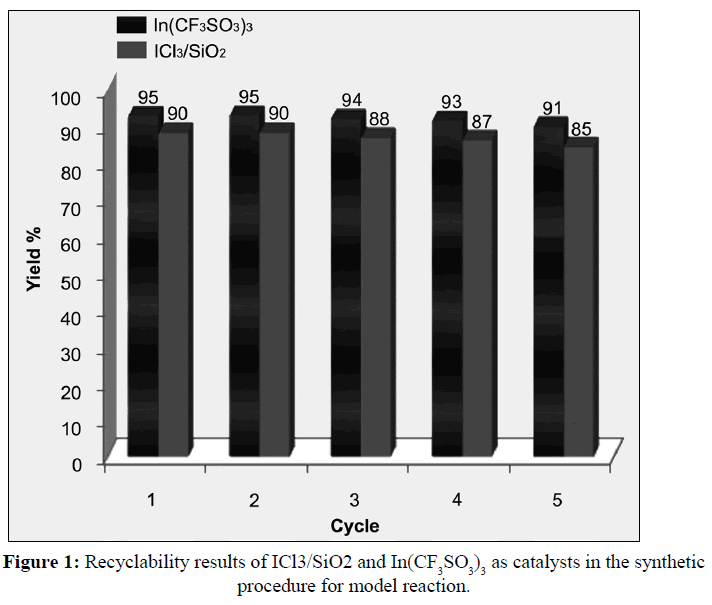
At the end of each run, the separated catalysts by filtration, washed with diethyl ether, In(CF3SO3)3 dried at 120°C for 1 h; and ICl3/SiO2 dried at 70°C for 8 h. The catalysts after drying reused in another reaction. It was found that both ICl3/SiO2 and In(CF3SO3)3 represent high catalytic activity wich lead to obtain products with good to excellent yields in short reaction times. Furthermore, the catalysts, can be recycled and reused four more times without considerable loss of their activity (Figure 1). All results of this evaluation for model reaction, are summarized in Table 5.
| Product | Total reusability | ICl3/SiO2 | In(CF3SO3)3 |
|---|---|---|---|
| Yield (%)a/Time (min) | Yield (%)a/Time (min) | ||
 |
1 | 90/60 | 95/60 |
| 2 | 90/60 | 95/60 | |
| 3 | 88/60 | 94/60 | |
| 4 | 87/60 | 93/60 | |
| 5 | 85/65 | 91/65 |
Table 5: Evaluation of catalysts recyclability to the synthesis of 4a.
Results comparison for the synthesis of 4a (model reaction) between current protocol and others previously reported, could be found in Table 6 [12-18,47,50,52,59-62]. As can be seen from Table 6, it was understood that, above used catalysts provided an excellent conditions to xanthenes synthesis than other that have been reported before. This protocol not only leads to obtain the xanthens with high yields but also eludes the issues dependent with environmental pollution, catalysts prices, and handling. Considering Table 6, in the most cases, the yield of obtained product in our work is higher than others. However, in certain instances, it can be seen that the reported yields are higher than our procedure, but those tolerate some disadvantages than our procedure such as longer reaction times [13,47-49], need to higher temperature [13,47,50,62], necessity of organic solvent [14,16,60], high-cost of catalyst or high amount of required catalyst [13,15,16,18,47,50,60-62], and less or non-reusability of catalyst [14-16].
| Entry | Catalyst | Catal. (mol %) | Solvent/Temp.(°C) | Time (min)/Yield% | [Ref.] |
|---|---|---|---|---|---|
| 1 | In(CF3SO3)3 | 2 | Solvent free/100 | 60/95 | This work |
| 2 | ICl3/SiO2 | 5 | Solvent free/75 | 60/90 | This work |
| 3 | DBSAa | 10 | H2O-Ultrasonic /30 | 60/89 | [57] |
| 4 | TMSClb | 100 | CH3CN/Reflux | 420/95 | [58] |
| 5 | TBAHSc | 10 | Dioxane, H2O/Reflux | 210/88 | [52] |
| 6 | DBSAa | 20 | H2O/Reflux | 180/91 | [59] |
| 7 | SelectfluorTM d | 10 | Solvent free/120 | 60/95 | [60] |
| 8 | PPA-SiO2e | 10 | Solvent free/140 | 30/93 | [45] |
| 9 | HClO4-SiO2 | 10 | Solvent free/140 | 180/32 | [45] |
| 10 | SbCl3-SiO2 | 10 | Solvent free/120 | 50/93 | [48] |
| 11 | LUS-Pr-SO3Hf | 0.02 g | Solvent free/140 | 15/90 | [12] |
| 12 | SmCl3 | 20 | Solvent free/120 | 540/98 | [13] |
| 13 | CANg / ultrasound irradiation (40 kHz) | 5 | 2-propanol/50 | 35/98 | [14] |
| 14 | [Bmim]ClO4h | 200 | Solvent free/100 | 40/92 | [15] |
| 15 | I2 | 20 | 2-propanol/70-80 | 18/90 | [16] |
| 16 | [cmmim][BF4]i MW j |
0.2 g | Solvent free/80 | 150/87 | [17] |
| 17 | p-Toluene sulfonic acid | 30 | Solvent free/80 | 30/99 | [18] |
Table 6: Comparison of results obtained with In(CF3SO3)3, and ICl3/SiO2 with other catalysts in xanthene synthesis (4a).
Conclusion
To conclude, a new protpol, green, and highly efficient method using ICl3 supported on silica and In(III) triflate as highly efficient and recyclable catalysts to 9-arylsubstituted-1,8-dioxoöctahydroxanthene synthesis was introduced. It is notable that, all obtained products by the use of catalytic amount of ICl3/SiO2 or In(CF3SO3)3 were achieved in excellent yields. The progress of reaction is strongly dependent in Lewis acidic virtues of ICl3/SiO2 and In(CF3SO3)3 as a key factor which efficiently catalysed reaction between cyclic 1,3-diketones and arylaldehydes. Using this highly efficient route without using any solvent not only provide an inexpensive and simple method but also lead to expanding the green chemistry aspects. The facile experimental procedure, short span of reaction times, use of neat procedure, recyclable catalysts, and readily available substrate are other advantages of this protocol.
Acknowledgment
Financial support from Yasouj University of Iran is gratefully acknowledged.
References
- Wang JX, Wang AQ, Xing YL, Zhu ZX, Wu XL, et al. (2015) Synthesis, characterization and properties of Ce-modified S2O82−/ZnAl2O4 solid acid catalysts.RSC Adv5: 105908-105916.
- Zillillah Z, Tan G, Li Z (2012) Highly active, stable, and recyclable magnetic nano-size solid acid catalysts: efficient esterification of free fatty acid in grease to produce biodiesel. Green Chem14: 3077-3086.
- MalleshamB, Sudarsanam P, Raju G, Reddy BM (2013) Design of highly efficient Mo and W-promoted SnO2 solid acids for heterogeneous catalysis: acetalization of bio-glycerol. Green Chem15: 478-489.
- Karami B, Eskandari K, Azizi M (2013) Tungstate sulfuric acid (TSA): a green and highly efficient catalyst for novel and known polysubstitutedimidazoles synthesis. Lett Org Chem10: 722-732.
- Poorali L, Karami B, Eskandari K, Azizi M (2013) New and rapid access to synthesis of novel polysubstitutedimidazoles using antimony trichloride and stannous chloride dihydrate as effective and reusable catalysts. JChemSci125: 591-599.
- WangQ, LiX (2016) Synthesis of 1H-Indazoles from Imidates and Nitrosobenzenes via Synergistic Rhodium/Copper Catalysis. Org Lett18: 2102-2105.
- Nandi RK, RégisGuillot N, Kouklovsky C, Vincent G (2016) Synthesis of 3,3-Spiroindolines via FeCl3-Mediated Cyclization of Aryl- or Alkene-Containing 3-Substituted N–Ac Indoles. Org Lett18: 1716-1719.
- Hassankhani A, Mossadegh E (2015) An efficient synthesis of tetrahydrotetrazolo[1,5-a]quinazoline derivatives by a three-component reaction of 5-aminotetrazole, arylaldehydes, and dimedone. Sci Iran C 22: 942-947.
- Heravi MM, Ghobadi N (2015) Nano-magnetite as an eco-friendly and magnetically separable catalyst for a one-pot synthesis of pyrano [2,3-c] pyrazoles and bis (4-hydroxycoumarin-3-yl) methane derivatives.Sci Iran 22: 911-918.
- Eskandari K, Karami B, Farahi M, Mouzari V (2016) Silica sodium carbonate catalyzed in water synthesis of novel benzylbarbiturocoumarin derivatives. Tetrahedron Lett57: 487-491.
- Karami B, EskandariK, Gholipour S, Jamshidi M (2013) Facile and rapid synthesis of 9-aryl 1, 8-dioxoöctahydroxanthenes derivatives using tungstate sulfuric acid. Org Prep ProcedInt45: 220-226.
- Rahimifard M, Mohammadi-Ziarani G, Badiei A, Asadi S, Abolhasani-Soorki A (2016) One-pot solvent-free synthesis of 1,8-dioxo-octahydroxanthene derivatives using sulfonic acid-functionalized LUS-1 and their antimicrobial activities. Res ChemInt42: 3847-3861.
- IlangovanA, Malayappasamy S, Muralidharan S, MaruthamuthuS (2011) A highly efficient green synthesis of 1, 8-dioxooctahydroxanthenes. ChemCentrJ5: 81-86.
- Mulakayala N, Kumar GP, Rambabu D, Aeluri M, Rao MVB, et al. (2012) A greener synthesis of 1,8-dioxo-octahydroxanthene derivatives under ultrasound. Tetrahedron Lett53: 6923-6926.
- Makone S, MahurkarSA (2013) Green Protocol for Efficient Synthesis of 1,8-Dioxo-Octahydroxanthenes Using Ionic Liquid. Green Sustainable Chem3: 27-32.
- Mulakayala N, Murthy PVNS, Rambabu D, Aeluri M, Adepu R, et al. (2012) Catalysis by molecular iodine: A rapid synthesis of 1,8-dioxo-octahydroxanthenes and their evaluation as potential anticancer agents. Bioorg Med ChemLett22: 2186-2191.
- Dadhania AN, Patel VK, Raval DK (2017) Ionic liquid promoted facile and green synthesis of 1,8-dioxo-octahydroxanthene derivatives under microwave irradiation. J Saudi ChemSoc21: S163-S169.
- Bayat M, Imanieh H, HossieniSH (2009) An Efficient Solvent Free Synthesis of 1,8-Dioxo-octahydroxanthene Using p-Toluene Sulfonic Acid. Chinese JChem27: 2203-2206.
- El-BrashyAM, Metwally ME, El-Sepai FA (2004) Spectrophotometric determination of some fluoroquinoloneantibacterials by binary complex formation with xanthene dyes.IlFarmaco59: 809-817.
- Chibale K, VisserM, Schalkwyk DV, Smith PJ, Saravanamuthu A, et al. (2003) Exploring the potential of xanthene derivatives as trypanothionereductase inhibitors and chloroquine potentiating agents. Tetrahedron59: 2289-2296.
- Bhowmik BB, Ganguly P (2005) Photophysics of xanthene dyes in surfactant solution. SpectChimActaA61: 1997-2003.
- Ion RM, Frackowiak D, Wiktorowicz K (1998) The incorporation of various porphyrins into blood cells measured via flow cytometry, absorption and emission spectroscopy. ActaBiochim Pol 45: 833-845.
- Knight CG, Stephens T (1989) Xanthene-dye-labelledphosphatidylethanolamines as probes of interfacial pH. Studies in phospholipid vesicles. Biochem J 258: 683-687.
- Ahmad MT, King A, Ko DK, Cha BH, Lee J (2002) Performance and photostability of xanthene and pyrromethene laser dyes in sol-gel phases. J Phys D: ApplPhys35: 1473-1476.
- Saint-Ruf G, Hieu HT, Poupelin JP (1975) The effect of dibenzoxanthenes on the paralyzing action of zoxazolamine. Naturwissenschaften62: 584-585.
- Al-Omran F, Mohareb RM, Abou-El-Khair A (2014) New route for synthesis, spectroscopy, and X-ray studies of 2-[aryl-(60-hydroxy-40,40-dimethyl-20-oxocyclohex-60-enyl)methyl]-3-hydroxy-5,5-dimethylcyclohex-2-enone and 1,8-dioxooctahydro-xanthenes and antitumor evaluation. Med Chem Res23: 1623-1633.
- Nisar M, Ali I, Shah MR, Badshah A, Qayum M, et al. (2013) Amberlite IR-120H as a recyclable catalyst for the synthesis of 1,8-dioxo-octahydroxanthene analogs and their evaluation as potential leishmanicidal agents. RSC Adv3: 21753-21758.
- Kinjo J, Uemura H, Nohara T, Yamashita M, Marubayashi N, et al. (1995)Novel yellow pigment from Pterocarpussantalinus: Biogenetic hypothesis for santalin analogs. Tetrahedron Lett36: 5599-5602.
- Casiraghi G, Casnati G, Cornia M (1973) Regiospecific reactions of phenol salts: reaction-pathways of alkylphenoxymagnesiumhalides with triethylorthoformate. Tetrahedron Lett14: 679-682.
- Bekaert A, Andrieux J, Plat M (1992)New total synthesis of bikaverin. Tetrahedron Lett33: 2805-2806.
- Knight DW, LittlePB (2001)The first efficient method for the intramolecular trapping of benzynes by phenols: a new approach to xanthenes. J ChemSoc Perkin Trans 1 14: 1771-1777.
- Kuo CW, Fang JM (2001) Synthesis of xanthenes, indanes, and tetrahydronaphthalenes via intramolecular phenyl-carbonyl coupling reactions. Synth Commun31: 877-892.
- Jha A, Beal J (2004) Convenient synthesis of 12Hbenzo[a]xanthenes from 2-tetralone. Tetrahedron Lett45: 8999-9001.
- Seyyedhamzeh M, Mirzaei P, Bazgir A (2008) Solvent free synthesis of aryl-14H-dibenzo[a,j]xanthenes and 1,8-dioxo-octahydro-xanthenes using silica sulfuric acid as catalyst. Dyes Pigm76: 836-839.
- Khodabakhshi S, Karami B, Eskandari K (2015) Molybdate sulfuric acid-catalyzed one-pot synthesis of substituted coumarins under solvent-free conditions. Res ChemInt41: 7263-7272.
- Eskandari K, Karami B (2016) Graphene oxide nanosheets-catalyzed synthesis of novel benzylbarbiturocoumarin derivatives under green conditions.MonatshChem147: 2119-2126.
- Karami B, Ferdosian R, Eskandari K (2014) New conditions for the effective synthesis of tri and tetrasubstitutedimidazolescatalysed by recyclable indium (III) triflate and magnesium sulfate heptahydrate. J Chem Res38: 41-45.
- Karami B, Eskandari K, Zare Z, Gholipour S (2014) A new access to 1,8-dioxooctahydro-xanthenes using yttrium (III) nitrate hexahydrate and tin (II) chloride dihydrate as effective and reusable catalysts.ChemHeterocyclCompd49: 1715-1722.
- Kaupp G, Naimi-Jamal MR, Schmeyers J (2003) Solvent-free Knoevenagel condensations and Michael additions in the solid state and in the melt with quantitative yield. Tetrahedron59: 3753-3760.
- Chen LH, Chung TW, Narhe BD, Sun CM (2016) A Novel Mechanistic Study on Ultrasound-Assisted, One-Pot Synthesis of Functionalized Benzimidazo[2,1‑b]quinazolin-1(1H)‑ones. ACS Comb Sci18: 162-169.
- Ramachary DB, Kishor M (2007) Organocatalytic Sequential One-Pot Double Cascade Asymmetric Synthesis of Wieland-Miescher Ketone Analogues from a Knoevenagel/Hydrogenation/Robinson Annulation Sequence: Scope and Applications of Organocatalytic Biomimetic Reductions. J OrgChem72: 5056-5068.
- Price PM, Clark JH,Macquarrie DJ (2000) Modified silicas for clean technology. J ChemSoc Dalton Trans2000: 101-110.
- Sharma RK, Mittal S, Koel M (2003) Analysis of Trace Amounts of Metal Ions Using Silica-Based Chelating Resins: A Green Analytical Method. Critical Rev Anal Chem33: 183-187.
- Yakaiah T, Reddy GV, Lingaiah BPV, Rao PS, Narsaiah B (2005) ZrOCl2.8H2O as a new solid phase and recyclable catalyst for an efficient Knoevenagel condensation under solventfree microwave irradiation conditions. Indian J Chem44B: 1301-1303.
- Ahmad-Hoseini M, Ansari G, Karami B, KhodabakhshiS (2010) Silica gel supported ICl3 as an efficient and reusable catalyst for the synthesis of 1, 8-dioxo-octahydroxanthene derivatives. 17th Iranian Seminar of Organic Chemistry, pp: 35-35.
- Reynoso-Soto EA, Rivero IA (2010) Synthesis of Peptides Histamine H2 Receptors in Solid-Phase Assisted by Microwave. J MexChemSoc54: 160-163.
- Kantevari S, Bantu R, Nagarapu L (2007) HClO4–SiO2 and PPA–SiO2 catalyzed efficient one-pot Knoevenagel condensation, Michael addition and cyclo-dehydration of dimedone and aldehydes in acetonitrile, aqueous and solvent free conditions: Scope and limitations. J MolCatal A: Chem269: 53-57.
- Venkatesan K, Pujari SS, Lahoti RJ, Srinivasan KV (2008) An efficient synthesis of 1,8-dioxo-octahydroxanthene derivatives promoted by a room temperature ionic liquid at ambient conditions under ultrasound irradiation. Ultr Sonochem Chem15: 548-553.
- Fan X, Hu X, Zhang X, Wang J (2005) InCl3·4H2Opromoted green preparation of xanthenedione derivatives in ionic liquids. Can J Chem83: 16-20.
- Zhang ZH, Lui Y (2008)Antimony trichloride/SiO2 promoted synthesis of 9-ary-3,4,5,6,7,9-hexahydroxanthene-1,8-diones. Catal Commun9: 1715-1719.
- Kozlov NG, Basalaeva LI (2005) Vanilline Alkanoates in the Synthesis of Hexahydrobenzacridine and Octahydroxanthene Derivatives. Russ J Chem75: 617-621.
- Horning EC, Horing MG (1946) Methone derivatives of aldehydes. J Org Chem11: 95-99.
- Niknam K, Damya M (2009) 1-Butyl-3-methylimidazolium Hydrogen Sulfate [Bmim]HSO4: An Efficient Reusable Acidic Ionic Liquid for the Synthesis of 1,8-Dioxo-Octahydroxanthenes.J Chin Chem Soc56: 659-665.
- Karade HN, Sathe M, Kaushik MP (2007) An efficient synthesis of 1,8-dioxo-octahydroxanthenes using tetrabutylammonium hydrogen sulfate. Arkivoc13: 252-258.
- Das B, Thirupathi P, Mahender I, Reddy VS, Rao YK (2006) Amberlyst-15: An efficient reusable heterogeneous catalyst for the synthesis of 1,8-dioxo-octahydroxanthenes and 1,8-dioxo-decahydroacridines. J Mol Catal A: Chem 247: 233-239.
- John A, Yadav PJP, Palaniappan S (2006) Clean synthesis of 1,8-dioxo-dodecahydroxanthene derivatives catalyzed by polyaniline-p-toluenesulfonate salt in aqueous media. J Mol Catal A: Chem248: 121-125.
- Tavakoli HR, Zamani H, Ghorbani MH, Etedali-HabibabadiH (2009) Solvent-free synthesis of 14-aryl(alkyl)-14H-dibenzo[a,j]xanthene, 9-aryl(alkyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-2H-xanthene-1,8-dione and 2-amino-5,6,7,8-tetrahydro-5-oxo-4-aryl-7,7-dimethyl-4Hbenzo-[b]-pyran derivatives using InCl3 as catalyst. Iran J Org Chem 2: 118-126.
- Sachar A, Sharma RL, Kumar S, Kaur D, Singh J (2006) Synthesis of novel bis-condensed heterocyclic ring assembly systems. J Heterocycl Chem 43: 1177-1181.
- Jin TS, Zhang JS, Wang AQ, Li TS (2006) Ultrasound-assisted synthesis of 1,8-dioxo-octahydroxanthene derivatives catalyzed by p-dodecylbenzenesulfonic acid in aqueous media. Ultr Sonochem Chem13: 220-224.
- Kantevari S, Bantu R, Nagarapu L (2006) TMSCl mediated highly efficient one-pot synthesis of octahydroquinazolinone and 1,8-dioxo-octahydroxanthene derivatives. Arkivoc16: 136-148.
- Bin LL, Shou JT, Sha HL, Meng L, Na Q, et al. (2006)The reaction of aromatic aldehydes and 1,3-cyclohexanedione in aqueous media. E-J Chem3: 117-121.
- Poor-Heravi MR (2009) SelectfluorTM promoted synthesis of 9-aryl-1,8-dioxooctahydro-xanthane derivatives under solvent-free conditions. J Iran Chem Soc6: 483-488.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences