ISSN : 0976-8505
Der Chemica Sinica
2-Aryldihydrobenzofuran Neolignans: Synthesis of Ficusal and Tomentosanan B
Siti Fadilah Juhan1, Siti Mariam Mohd Nor1*, Nur Kartinee Kassim1, Siti Munirah Mohd Faudzi1,2, Mohd Shukri Mat Ali3
1Department of Chemistry, Faculty of Science, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia
2Laboratory of Natural Products, Institute of Bioscience, Universiti Putra Malaysia,43400 Serdang, Selangor, Malaysia
3Genebank and Seed Centre, Malaysian Agricultural Research and Development Institute (MARDI), 43400 Serdang, Selangor, Malaysia
Abstract
The synthesis of 2-aryldihydrobenzofurans, ficusal and tomentosanan B was achieved via two different cross-coupling methods between aryl or iodoaryl with coniferyl alcohol. Both Pd(OAc)2 catalysed coupling reactions were performed in the presence of base with or without ligand; an oxidant or phase transfer agent. Tomentosanan B was also obtained directly from the reduction of ficusal using NaBH4. The structures of these 2-aryldihydrobenzofuran neolignans were confirmed by 1D and 2D NMR and HRMS.
Keywords
Coupling reaction, Palladium, Neolignan, Ficusal, Tomentosanan B
Introduction
Dihydrobenzofuran neolignans are interesting biologically active compounds that are found in plants. Some important activities that have been reported such as anti-inflammatory [1], antioxidant [2,3], antifungal [4], insecticidal [5] and anticancer against human cancer cell lines: HeLa (cervical), HepG2 (liver), A375-S2 (skin), HT1080 (tumor) and HL60 (leukemia) [6].
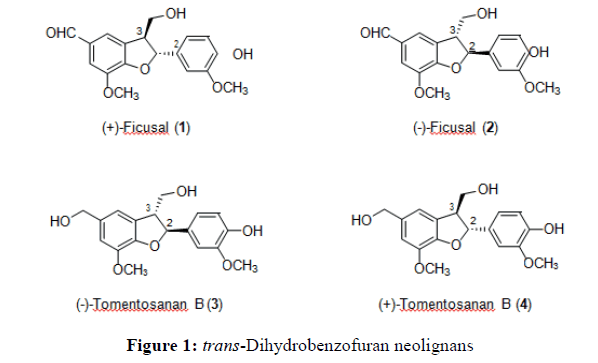
(+)-Ficusal is a 2-aryldihydrobenzofuran neolignan that has been isolated from various plants including leaves of Ficus microcarpa L. f. (Moraceace) [7], fruits of Vitex agnus-castus L. [8], Metasequoia glyptostroboides (Taxodiaceae) [9], seeds of Coix larchryma-jobi L. [10] and from leaves and stems of Manglietia insignis [11]. Recently this neolignan 1 was also isolated from Acanthopanax senticosus [12] and the seeds of Crataegus pinnatifida [13]. In contrast, (-)- ficusal and (-)-tomentosanan B were isolated from the seeds of Prunus tomentosa (Figure 1) [14]. Meanwhile no isolation work on (+)-tomentosanan B (4) has been previously reported.
Neolignans 2 and 3 have shown an effective antioxidant activity against both DPPH and ABTs radicals and antiinflammatory activity on nitric oxide (NO) production in murine microlia BV-2 [15]. Other biological activities that have been reported for neolignan 2 include antitumour against human tumor cell lines of HL-60 SMMC-7721, MCF-7 and SW480 [11], inhibition of tumor necrosis factor α (TNF-α) production by the PLS-induced murine macrophage cell line RAW264.7 [13] and in vitro inhibitory activities against protein tyrosine phosphatase 1B(PTP1B), human vaccinia H1 related protein (VHR) and protein phosphatase 1 (PP1) [12]. There is one pharmacological activity reported for neolignan 1, where it was found to show antiproliferative activity by inhibition of MDA-MB-231 cells at 50 μM by 69.3% [11].
The synthesis of benzofuran and trans-dihydrobenzofuran using Pd-catalysed reaction has already been discussed in the literature [16,17]; however, no synthetic work has been reported on ficusal (6) and tomentosanan B (7). Therefore the focus of this paper is to discuss the simple reaction steps for synthesising these two neolignans. Based on their biological potential and a very limited work focusing on insecticidal activity, all targeted synthesised neolignans and intermediates will be further studied on their potential in agriculture practices.
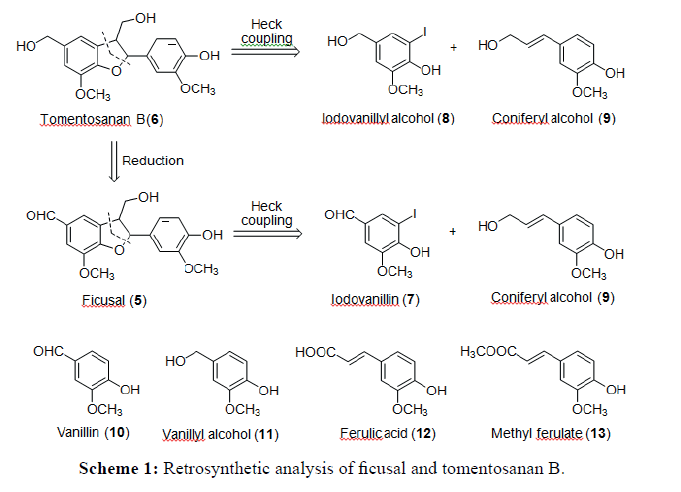
The retrosynthetic analysis showed that ficusal (6) and tomentosanan B (7) can be directly synthesised by the Heck coupling reaction between iodovanillin (5) or iodovanillyl alcohol (3) with trans-coniferyl alcohol (1). An alternative coupling reaction also can be perfomed between vanillin or vanillyl alcohol (2) with compound trans-coniferyl alcohol. Primary alcohols, iodovanillyl alcohol and vanillyl alcohol, can be produced by functional group interconversion (FGI) through the reduction of aldehydes iodovanillin and vanillin, whereas trans-coniferyl alcohol can be reduced from trans-ferulic acid or trans-methyl ferulate (4). Iodovanillin (5) and ester, trans-methyl ferulate, can be prepared through iodination and esterification reactions respectively. Due to the same core structure of ficusal (6) and tomentosanan B (7), it can be suggested that tomentosanan B can also be directly reduced from ficusal (Scheme 1).
Experimental
General
All chemicals and solvents are commercially available as analytical grades and used without purification unless otherwise stated. All reactions were monitored by thin layer chromatography (TLC), using Silica gel 60 F245 (Merck KGaA) precoated aluminium backed plates which were visualised with ultraviolet light (UVP, UV Lamp UVGL- 58) and then stained with KMnO4 solution. Column chromatography was performed with 100-150 mesh of Silica gel 60 (0.040 mm - 0.063 mm). The organic extracts were dried over sodium sulfate (Na2SO4) and evaporated using Buchi Switzerland rotavapor R-215. The infrared spectra were measured by using the Fourier Transform Infrared Spectroscopy, Perkin-Elmer FT-IR Model Spectrum 100.
Reduction
Method 1: Ferulic acid or methyl ferulate (4) (1.0 mmol) was dissolved in Et2O (10 mL) followed by addition of LiAlH4 (1.1 mmol) under nitrogen atmosphere and stirred at room temperature for 6-72 hours. The reaction mixture was quenched by the addition of H2O (2 mL), 10% H2SO4 (2.5 mL) and then extracted with Et2O (3x10 mL). The combined organic layer was dried, evaporated and purified by column chromatography (hexane:EtOAc, 3:2).
Method 2: An aldehyde (iodovanillin or vanillin) (1.0 mmol) was dissolved in 1 M NaOH (0.6 mL) and stirred homogenously at room temperature under a nitrogen atmosphere. NaBH4 (0.8 mmol) was added slowly to the reaction mixture and stirred for another 30 minutes. 1 M HCl (1 mL) was added dropwise to the reaction mixture and cooled in the ice bath. The precipitate form was filtered and further purified by column chromatography (8 = hexane:EtOAc, 1:1; 11=hexane:EtOAc, 3:2).
Coniferyl alcohol (1): White solid; 58%; mp 74-75°C (Lit. 68-73°C, Akita et al., 2006); IR (UATR) 3241, 2928, 1507, 1241 cm-1; δH (500 MHz, CDCl3) 6.87 (3H, m, H-Ar), 6.48 (1H, d, J 16.0 Hz, CH=C), 6.17 (1H, dt, J 5.7, 16.0 Hz, CH=C), 5.93 (1H, br. s, OH), 4.26 (2H, d, J 5.7 Hz, CH2), 3.84 (3H, s, CH3); δC (125 MHz, CDCl3) 146.2, 145.5, 131.2, 129.2, 126.0, 120.2, 114.5, 08.3, 63.7, 55.8; m/z (EIMS) 180 (M+, C10H12O3 requires 180).
Vanillyl alcohol (2): White solid; 93%; mp 110-112°C (Lit. 112-114°C, Naimi-Jamal et al., 2009); IR (UATR) 3433, 3148, 2947, 1605, 1499, 1226 cm-1; δH (500 MHz, CDCl3) 6.89 (1H, s, H-Ar), 6.86 (1H, d, J 8.0 Hz, H-Ar), 6.81 (1H, d, J 8.0 Hz, H-Ar), 5.69 (1H, br. s, OH), 4.57 (2H, s, CH2), 3.87 (3H, s, CH3); δC (125 MHz, CDCl3) 146.7, 145.3, 132.9, 120.2, 114.3, 110.0, 65.4, 55.9; m/z (EIMS) 154 (M+, C8H10O3 requires 154)
Iodovanillyl alcohol (3): White solid; 98%; mp 118-119°C; IR (UATR) 3433, 3148, 2948, 1605, 1477, 1226, 444 cm-1; δH (500 MHz, CDCl3) 7.25 (1H, s, H-Ar), 6.86 (1H, s, H-Ar), 6.10 (1H, br. s, OH), 4.55 (2H, s, CH2), 3.87 (3H, s, CH3); δC (125 MHz, CDCl3) 146.2, 145.2, 134.5, 129.0, 109.9, 80.8, 64.5, 56.3; m/z (EIMS) 280 (M+, C8H9IO3 requires 280).
Esterification
Concentrated H2SO4 (0.025 mL) was added to the solution of ferulic acid (trans-ferulic acid) (1 mmol) in MeOH (10 mmol) and refluxed for 3 hours. The reaction mixture was cooled to room temperature and ice-cold water (5 mL) was added. The solution was extracted with Et2O (3 × 10 mL) and the combined organic layer was washed with a saturated NaHCO3 solution (3 × 30 mL), brine (3 × 30 mL), dried, evaporated and purified by column chromatography (petroleum ether:EtOAc, 3:2).
Methyl ferulate (4): White solid; 98%; mp 64-65°C (Lit. 65°C, Prasat and Chaudhury, 1958); IR (UATR) 3402, 2949, 1700, 1597, 1514, 1440, 1169 cm-1; δH (500 MHz, CDCl3) 7.58 (1H, d, J 16.0 Hz, C=CH), 7.02 (1H, dd, J 2.3, 8.0 Hz, H-Ar) 6.98 (1H, s, H-Ar), 6.88 (1H, d, J 9.2 Hz, H-Ar), 6.25 (1H, d, J 16.0 Hz, C=CH), 6.15 (1H, br. s, OH), 3.86 (3H, s, CH3), 3.76 (3H, s, CH3); δC (125 MHz, CDCl3) 167.7, 148.0, 146.8, 144.9, 126.7, 122.9, 114.9, 114.8, 109.4, 55.8, 51.5 m/z (EIMS) 208 (M+, C11H12O4 requires 208).
Iodination
Vanillin (1 mmol) and I2 (2 mmol) were dissolved in H2O (5 mL) followed by the addition of 30% H2O2 (4 mmol). The reaction mixture was stirred at 50°C for 24 hours. The saturated Na2S2O3 solution (5 mL) was added to the reaction mixture followed by extraction with EtOAc (3x10 mL). The combined organic layer was dried, evaporated and purified by column chromatography (hexane:EtOAc, 3:2).
Iodovanillin (5). White solid; 90%; mp 179-181°C (Lit. 179-182°C, Choi et al., 2008); IR (UATR) 3421, 3158, 2918, 1735, 1664, 1570, 1410, 1153 cm-1; δH (500 MHz, CDCl3) 9.75 (1H, s, CHO), 7.80 (1H, s, H-Ar), 7.35 (1H, s, H-Ar), 6.67 (1H, br. s, OH), 3.95 (3H, s, CH3); δC (125 MHz, CDCl3) 189.5, 151.4, 146.5, 136.2, 131.1, 108.7, 80.5, 56.6; m/z (EIMS) 278 (M+, C8H7O3I requires 278).
Heck coupling for the synthesis of ficusal and tomentosanan B
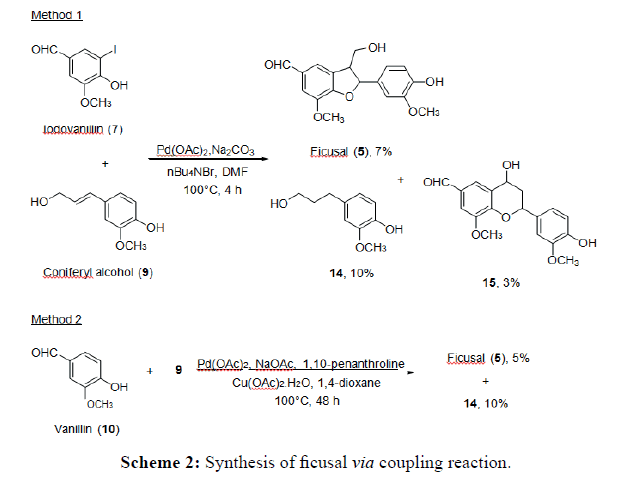
Method 1: Iodovanillin (5) (1.0 mmol), coniferyl alcohol (1) (2.0 mmol), Pd(OAc)2 (0.05 mmol), Na2CO3 (1.0 mmol) and n-Bu4NBr (0.15 mmol) were dissolved in DMF (2.3 mL) and heated to 100°C for 2-3 days. The reaction mixture was cooled to room temperature and extracted with EtOAc (3x10 mL). The combined organic layer was washed successively with saturated NH4Cl (3x30 mL) and brine (3x30 mL). The organic layer was dried, evaporated and purified by column chromatography (hexane:EtOAc, 1:1).
Method 2: Vanillin (5.0 mmol) and coniferyl alcohol (1) (1.0 mmol) were dissolved in 1,4- dioxane (8 mL) followed by addition of Pd(OAc)2 (0.05 mmol), NaOAc (5.0 mmol), 1,10- phenanthroline (0.10 mmol) and Cu(OAc)2. H2O (2.0 mmol). The reaction mixture was stirred at 100°C for 2-3 days. After cooling to room temperature, the reaction mixture was filtered and washed with CHCl3 (1x20 mL). The filtrate was evaporated and purified by column chromatography (hexane:EtOAc, 1:1).
Ficusal (6): Yellow oil; 5-7%; IR (UATR) 3413, 3005, 1704, 1429, 1222 cm-1; δH (500 MHz, (CD3)2CO) 9.83 (1H, s, CHO), 7.52 (1H, s, H-Ar), 7.41 (1H, s, H-Ar), 7.05 (1H, s, H-Ar), 6.88 (1H, dd, J 8.0, 2.3 Hz, H-Ar), 6.82 (1H, d, J 9.2 Hz, H-Ar), 5.69 (1H, d, J 6.9 Hz, CH-C), 3.90-3.94 (2H, m, CH2), 3.91 (3H, s, CH3), 3.82 (3H, s, CH3), 3.67 (1H, m CH-C); δC (125 MHz, (CD3)2CO) 190.9, 154.7, 148.5, 147.6, 145.8, 133.3, 132.2, 131.1, 121.4, 119.8, 115.7, 113.4, 110.6, 89.8, 64.2, 56.3, 56.2, 53.8; δH (500 MHz, CDCl3) 9.79 (1H, s, CHO), 7.40 (1H, s, H-Ar), 7.36 (1H, s, H-Ar), 6.87 (3H, s, H-Ar), 5.74 (1H, br. s, OH), 5.68 (1H, d, J 8.0 Hz, CH-C), 3.90-3.94 (2H, m, CH2), 3.92 (3H, s, CH3), 3.84 (3H, s, CH3), 3.67 (1H, m CH-C); δC (125 MHz, CDCl3) 190.6, 154.0, 146.8, 146.0, 145.0, 132.0, 131.3, 128.7, 120.9, 119.4, 114.5, 112.1, 108.7, 89.4, 63.8, 56.1, 56.0, 52.7; m/z (HRMS) 330.1108 [M]+ (Calcd for C18H18O6 requires 330.1103).
Tomentosanan B (7): Colourless oil; 5-30%; IR (UATR) 3388, 2923, 1728, 1609, 1461, 1138 cm-1; δH (500 MHz, CD3OD) 6.97 (1H, s, H-Ar), 6.92 (1H, s, H-Ar), 6.90 (1H, s, H-Ar), 6.84 (1H, d, J 6.9 Hz, H-Ar), 6.78 (1H, d, J 8.0 Hz, H-Ar), 5.55 (1H, d , J 5.7 Hz, CH-C), 4.55 (1H, s, CH2), 3.89 (3H, s, CH3), 3.77-3.87 (2H, m, CH2), 3.83 (3H, s, CH3), 3.52 (1H, m, CH-C); δC (125 MHz, CD3OD) 147.7, 147.4, 146.1, 144.0, 134.8, 133.3, 128.5, 118.3, 115.8, 114.7, 111.7, 109.1, 87.7, 64.0, 63.6, 55.3, 54.9, 53.9; m/z (HRMS) 332.1275 [M]+ (Calcd for C18H20O6 requires 332.1260), 355.1169 [M+Na]+ (Calcd for C18H20O6Na requires 355.1158).
Results and Discussion
All intermediate compounds for the coupling reaction were synthesised by established reduction, iodination and esterification methods [18-21]. Two different Heck coupling methods involving reaction between C-I or C-H bonds of aryl with alkenyl alcohol were applied for the synthesis of ficusal and tomentosanan B. For method 1, Pd(OAc)2, Na2CO3 and a phase transfer agent, nBu4NBr were used. In contrast, Pd(OAc)2 and NaOAc in the presence of ligand, 1,10- phenanthroline and co-catalyst/oxidant, Cu(OAc)2.H2O were used in method 2. Both coupling reactions were performed at 100°C in DMF and 1,4-dioxane for methods 1 and 2 respectively.
Synthesis of ficusal
The Heck coupling reaction between iodovanillin (5) and coniferyl alcohol (1) via method 1 successfully gave the targeted neolignan, ficusal (6) together with 4-(3-hydroxypropyl)-2- methoxyphenol and dihydrobenzopyran. Meanwhile, only Ficusal and 4-(3-hydroxypropyl)-2- methoxyphenol were produced from method 2 (Scheme 2). Increasing the reaction time did not give significant changes in the production of Ficusal. As the time increased, the yield of 4-(3-hydroxypropyl)-2- methoxyphenol reduced as it was readily reacted with iodovanillin to form compound dihydrobenzopyran.
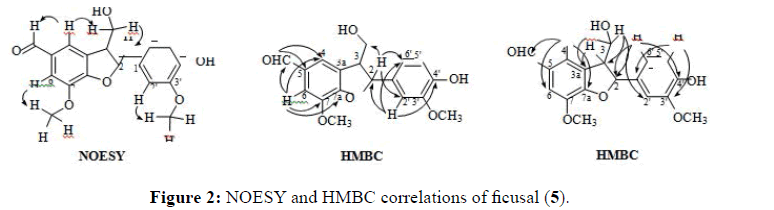
The IR spectrum of ficusal showed the presence of hydroxyl (3413 cm-1), carbonyl (1704 cm-1), aromatic double bonds (1429 cm-1) and ether (1222 cm-1). The molecular formula of Ficusal was established as C18H18O6 by HRMS at m/z 330.1108 [M]+ (calculated for C18H18O6, 330.1103). The coupling constant, 3J2,3=6.9 Hz [14,22,23] and no correlation between H2 (δ 5.69, d) and H3 (δ 3.67, q) in NOESY spectrum indicated that these two hydrogens are in trans-configuration (Figure 2).
The 1H NMR and 13C NMR of synthesised ficusal were found similar to the reported data [7]. In the 1H NMR spectrum, three singlet protons corresponding to an aldehyde (δ 9.83, 5-CHO) and two methoxy groups (δ 3.91, 7-OCH3 and δ 3.82, 3’-OCH3) suggested that this compound was successfully formed through the coupling reaction. Meanwhile the replacement signal of the olefinic carbons in trans-coniferyl alcohol with methine carbons (δ 89.8, C2 and δ 53.8, C3) proved the formation of dihydrofuran ring in the structure of Ficusal. The HMBC analysis further confirmed the structure as it gave a correlation between H2 (δ 5.69, d, J 6.9 Hz) with C2’ (δ 110.6), C6’ (δ 119.8) and 3-CH2 (δ 64.2); H3 (δ 3.67, m) with C3a (δ 131.1); 3-CH2 (δ 64.2) with C3a (δ 131.1), 3-CH2 (δ 64.2) with C3a (δ 131.1), C3 (δ 53.8) and C2(δ 89.8); also H2’ (δ 7.05, s) and H6’ (δ 6.88, dd, J 8.0, 2.3 Hz) with C2 (δ 89.8) (Figure 2). The mechanism of the formation of ficusal for methods 1 and 2 was proposed to follow Emrich and Larock’s [24] and Kuram’s mechanisms [25].
Synthesis of tomentosanan B
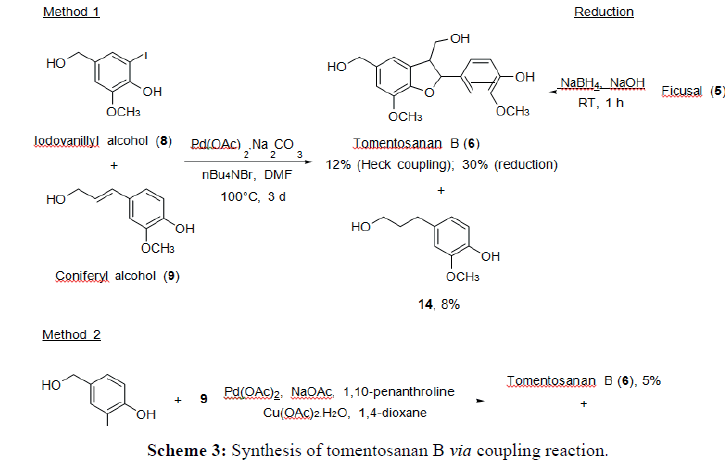
Both Heck coupling reaction between iodovanillyl alcohol (3) and vanillyl alcohol (2) with coniferyl alcohol produced tomentosanan B (7) and by-product 4-(3-hydroxypropyl)-2- methoxyphenol in low yields even though the reactions were prolonged up to three days. Increasing the reaction time will only lead to the formation of by-products and decomposition of starting material. In a separate reaction, neolignan, tomentosanan B, was also produced in a 30% yield from the direct reduction of aldehyde group of Ficusal using NaBH4 (Scheme 3). The formation of tomentosanan B using both methods of Heck coupling reaction was believed to follow the same mechanism as the formation of Ficusal. Tomentosanan B was obtained as colorless oil and the molecular formula was confirmed by HRMS at m/z 355.1169 [M + Na]+ (calculated for C18H20O6Na, 355.1158). The IR spectrum exhibited the absorption of hydroxyl (3388 cm-1) and aromatic rings (1609 and 1461 cm-1).
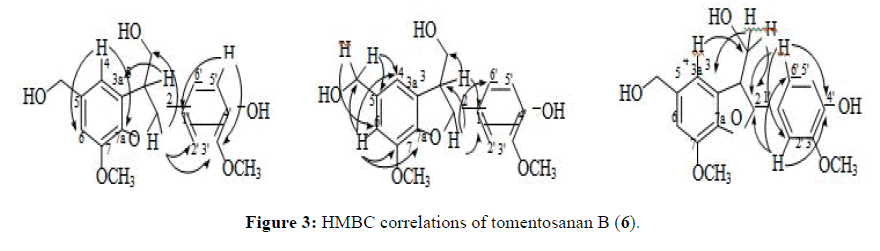
The 1D NMR analyses of tomentosanan B showed a multiplet peak at δ 3.52 ppm referring to the H3 (C3 at δ 53.9 ppm) and a doublet peak at δ 5.55 ppm referring to H2 (C2 at δ 87.7 ppm) which proved the formation of the dihydrofuran ring. The synthesised neolignan, tomentosanan B, showed a similar NMR data to the isolated (-)-tomentosanan B [14] thus, assured its structure. The HMBC spectrum showed correlations between H2 (δ 5.55, d, J 5.7 Hz) with C3 (δ53.9), 3-CH2 (δ 63.6), C1’ (δ 133.3), C2’ (δ 109.1), C3’ (δ 147.7) and C6’ (δ 118.3); H3 (δ 3.52, m) with 3-CH2 (δ 63.6), C3a (δ 128.5) and C1’ (δ 133.3); and the aromatic protons H2’ (δ 6.97, s) and H6’ (δ 6.84, d, J 6.9 Hz) with C2 (δ 87.7) and C4’ (δ 146.1). These correlations confirmed the hydrofuran ring was fused to the aromatic ring of vanillyl and the coniferyl fragment was substituted to C-2 position of the furan ring (Figure 3).
Conclusion
The ficusal and tomentosanan B were synthesised in 3-5 steps starting from vanillin and ferulic acid involving iodination, reduction, esterification and coupling reactions. The formation of dihydrofuran ring of both ficusal and tomentosanan B were achieved via Heck coupling reaction using Pd(OAc)2 as a catalyst. Tomentosanan B can also be obtained through simple mild reduction reaction of ficusal.
Acknowledgements
We are grateful to the Malaysia Government for the FRGS grant (Vot No.5524651) and the Department of Chemistry, Faculty of Science, UPM for the research facilities.
References
- Liu YQ, Liu Y, Xiao H, Gao R, Tian X (2008) Synthesis and insecticidal activities of novel derivatives of podophyllotoxin: Part XII. Pest Biochem Phys91: 116-121.
- Kang DGH, Oh H, Sohn EJ, Hur TY, Lee KC et al. (2004) Lithospermic acid B isolated from Salvia miltiorrhiza ameliorates ischemia/reperfusion-induced renal injury in rats. Life Sci75: 1801-1816.
- Jin CJ, Yu SH, Wang X, Woo SJ, Park HJ et al. (2014) the effect of lithospermic acid, an antioxidant, on development of diabetic retinopathy in spontaneously obese diabetic rats. Pub Lib Sc9: 1-10.
- Stoessl A (1996) The antifungal factors in barley – the constitutions of hordatine A and B.Tetrahedron Lett21: 2287-2292.
- González CA, Reina M, Gutiérrez C, Fraga BM (2002) Natural Insecticides: Structure Diversity, Effects and Structure-activity Relationships. A Case Study. Stud Natu Produ Chemi26: 849-879.
- Huang XX, Zhou CC, Li LZ, Peng Y, Lou LL, et al. (2013) Cytotoxic and antioxidant dihudrobenzofuran neolignans from the seeds of Crataegus pinnatifida. Fitoterapia91: 217-223.
- Li Y, Kuo YH (2000) Four new compounds, ficusal, ficusesquilignan A, B and ficusolide diacetate from the heartwood of Ficus microcarpa. Chem Pharm Bull48: 1862-1865.
- Chen S, Friesen JB, Webster D, Nikolic D, Breemen RB et al. (2011) Fitoterafia phytoconstituents from Vitex agnuscastus fruits. Fitoterapia82: 528-533.
- Zeng Q, Guan B, Cheng X, Wang C, Jin H et al. (2013) Chemical constituents from Metasequoia glyptostroboides Hu et Cheng. Biochem Syst Ecol50: 406-410.
- Chung CP, Hsu CY, Lin JH, Kuo YH, Chiang W (2011) Antiproliferative lactams and spiroenone from adlay bran in human breast cancer cell lines. J Agric Food Chem 59: 1185-1194.
- Shang SZ, Kong LM, Yang LP, Jiang J, Huang J, et al. (2013) Bioactive phenolics and terpenoids from Manglietia insignis. Fitoterapia84: 58-63.
- Li JL, Li N, Xing SS, Zhang N, Li BB, et al. (2015) New neo-lignan from Acanthopanax senticosus with protein tyrosine phosphatase 1B inhibitory activity. Arch Pharmacal Re40: 1265-1270.
- Huang XX, Bai M, Zhou L, Lou LL, Liu QB, et al. (2015) Food byproducts as a new and cheap source of bioactive compounds: lignans with antioxidant and anti-inflammatory properties from Crataegus pinnatifida seeds. J Agric Food Chem63: 7252-7260.
- Liu QB, Huang XX, Yan XJ, Bai M, Yu LH, et al. (2014) Neolignans from the seeds of Prunus tomentosa (Rosaceae) and their chemotaxonomic interest. Biochem Syst Ecol55: 236- 240.
- Liu QB, Huang XX, Bai M, Chang XB, Yan XJ, et al. (2014) Antioxidant and anti- inflammatory active dihydrobenzofuran neolignans from the seeds of Prunus tomentosa.J Agric Food Chem 62: 7796-7803.
- Larock RC, Yum EK (1996) Palladium-catalyzed Annulation of Vinylic Cyclopropanes and Cyclobutanes. Tetrahedron52: 2743-2758.
- Coy BED, Jovanovic L, Sefkow M (2010) One-Pot, Pd-Catalyzed Synthesis of trans- Dihydrobenzofurans from o-Aminophenols. Org Lett12: 1976-1979.
- Brown HC, Krishnamurthy S (1979) Forty years of hydride reductions. Tetrahedron 64: 567-607.
- Fowler RG (1992) Microscale reactions of vanillin. J Chem Educ69: A43.
- Moussouni S, Saru ML, Ioannou E, Mansour M, Detsi, A et al. (2011) Crude peroxidase from onion waste as a tool for organic synthesis. Part II : oxidative dimerisation- cyclisation of methyl p-coumarate. Tetrahedron Lett. 52: 1165-1168.
- Gallo RDC, Ferreira IM, Casagrande GA, Pizzuti L, Oliveira SD, et al. (2012) Efficient and eco-friendly synthesis of iodinated aromatic building blocks promoted by iodine and hydrogen peroxide in water : a mechanistic investigation by mass spectrometry. Tetrahedron Lett53: 5372-5375.
- Matsuda N, Kikuchi M (1996) Studies on the constituents of Lonicera species. X. Neolignan glycosides from the Leaves of Lonicera gracilipes var. glandulosa MAXIM. Chem Pharm Bull44: 1676-1679.
- Maeda S, Masuda H, Tokoroyama T (1994) Studies on the preparation of bioactive lignans by oxidative coupling reaction. III. Synthesis of polyphenolic benzofuran and coumestan derivatives by oxidative coupling reaction of methyl (E)-3-(4-Hydroxy-2- methoxyphenyl)propenoate and their inhibitory effect on liquid peroxidation. Chem Pharm Bull42: 2536-2545.
- Emrich DE, Larock RC (2004) Palladium-catalyzed Heteroannulation of Cyclic Alkenes by Functionally Substituted Aryl Iodides. J Organomet Chem689: 3756-3766.
- Kuram MR, Bhanuchandra M, Sahoo AK (2013) Direct Access to Benzo[b]furans through Palladium-catalyzed Oxidative Annulation of Phenols and Unactivated Internal Alkynes. Angew Chem Int Ed 52: 4607-4612.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences