To Evaluate the Efficiency of Char and Biochar for Waste Water Treatment
Ayesha Rasheed*, Saman Sana, Saif-ur-Rehman Kashif, Zeeshan Umer and Maria Khatoon
Department of Environmental Sciences, Faculty of Bio Sciences, University of Veterinary and Animal Sciences, Lahore, Pakistan
- *Corresponding Author:
- Ayesha Rasheed
Department of Environmental Sciences
Faculty of Bio Sciences
University of Veterinary and Animal Sciences
Lahore,Pakistan
Tel: +923214449199
Email: ayesha.rasheed20@yahoo.com
Received Date: June 21, 2017; Accepted Date: October 14, 2017; Published Date: October 23, 2017
Citation: Rasheed A, Sana S, Kashif S, Umer Z, Khatoon M (2017) To Evaluate the Efficiency of Char and Biochar for Waste Water Treatment. Resour Recycl Waste Manag Vol.2 No.2:7
Abstract
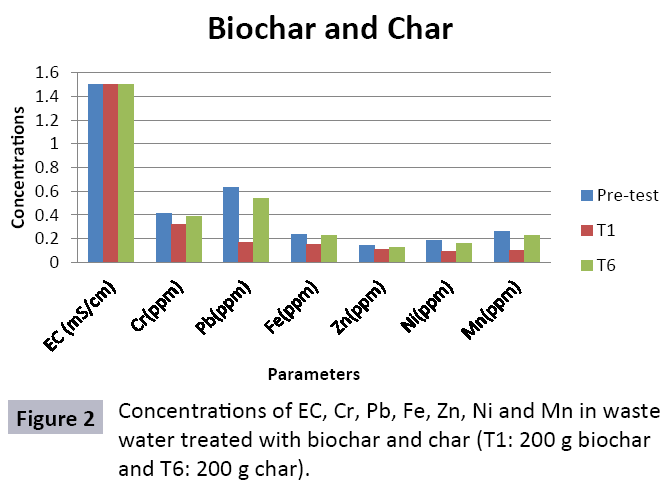
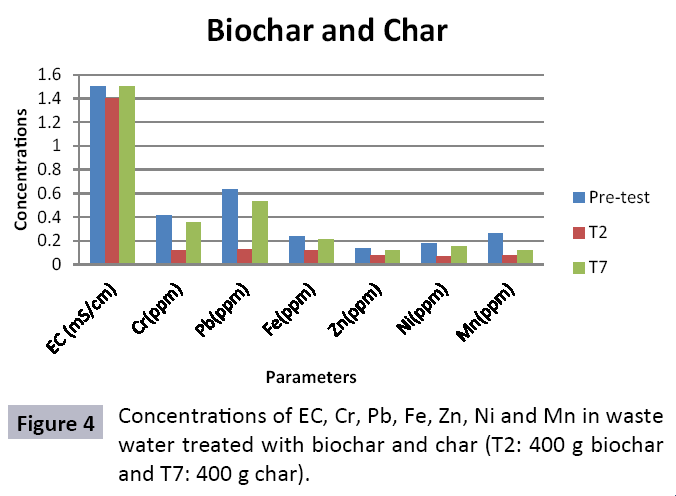
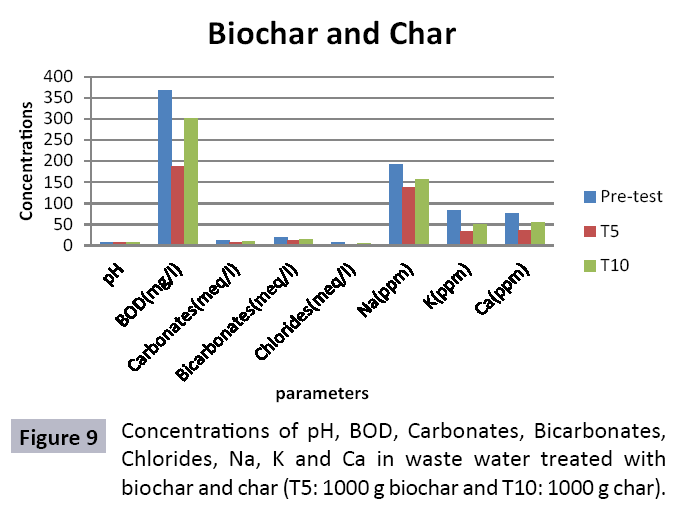
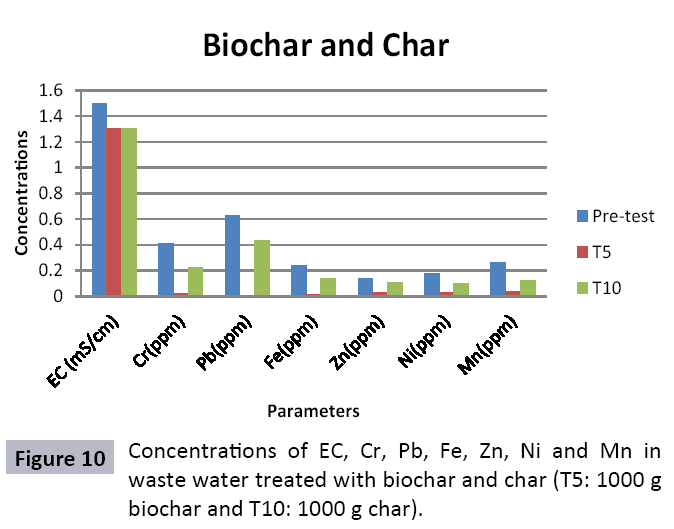
Appropriate waste administration is a basic key to ecological sustainability. Solid waste management and waste water treatment is a worldwide issue due to increment in both urbanization and industrialization. Water is one of significant common assets and is basic for maintainability of life. Toxic industrial effluents released into sewage systems and drains without treatment that deteriorating the water quality for drinking and irrigation. Char and Biochar produced from pyrolysis are stable carbon rich compounds which have various beneficial applications like soil conditioning, remediation, carbon sequestration and water treatment. To study efficiency of char and biochar for wastewater treatment, composite sample was collected from the Main Outfall Drain, Lahore. Ten treatments consisting of different quantities of biochar (T1=200 g, T2=400 g, T3=600 g, T4=800 g and T5=1000 g) and of char (T1=200 g, T2=400 g, T3=600 g, T4=800 g and T5=1000 g) were taken. Sample wastewater was analyzed (pre and post) for different heavy metals after being filtered through biochar and char filters. Significant decrease in concentration of heavy metals was observed. In biochar value of Cr decreased from (0.32 ppm to 0.02 ppm), Fe (0.15 ppm to 0.01 ppm), Zn (0.11 ppm to 0.03 ppm), Ni (0.09 ppm to 0.03 ppm), Pb (0.17 ppm to not detected) and Mn (0.10 ppm to 0.04 ppm). In char treated water also decrease in heavy metals was observed and significant results were observed in T10 (Cr=0.22, Pb=0.43, Fe=0.14, Zn=0.11, Ni=0.1 and Mn=0.12). It could be concluded from results that efficiency of biochar in treating wastewater is much better as compared to char as the reduction in heavy metals values is significant in case of aforementioned. Hence, biochar could be used as a sorbent for wastewater remediation.
Keywords
Char; Biochar; Waste water treatment; Althea rosea; Phytoremediation
Introduction
The environment in its most extensive sense incorporates everything, which is outer to the individuals. Environmental contamination is ascribed to the waste that can't be diminished by characteristic recycling because of their exorbitant amount, damaging quality and exclusive chemical formation [1].
Agrarian fields all through the world are getting waste water that directly being discharged to the water bodies stream channels and drains both from the municipal and industrial settlements. This waste water not only deteriorates the soil, and in addition, ground water with overwhelming metals which enter the natural way of life and cause a deadly risk to the life [2]. The metals having an atomic density higher than 6 g/cm3 are known as heavy metals. The extreme toxic and carcinogenic heavy metals include cadmium, arsenic, lead, mercury, nickel, copper and cadmium they are not only intense persistent pollutants but also industrial poisons in waste water. The contact of people with substantial metals can happen through an assortment of ways, which incorporate ingestion and inhalation. The adequate presence of heavy metals in water poses serious adverse effects to oceanic biological communities that cause the demise of aquatic life and also deteriorate the water quality due to severe chemical contaminants. Copious presence of heavy metals exhibit in soils, cause diminishment in both quantity and quality of food because it interferes with metabolic and physiological procedures of plants. To moderate the adverse effects of overwhelming metals on the health of people, creatures and nature, an assortment of remediation procedures exists [3]. Biochar is charcoal made from biomass (organic matter derived from the living source) via pyrolysis. The pyrolysis technique makes a fine-grained, exceedingly permeable charcoal that offers soils, significant assistance with retaining water and nutrients e.g. rice husk. The unique property of biochar i.e. highly porous nature and high surface area enable it to adsorb or retain nutrients and water, empowers it to adsorb or hold supplements and water [4]. RDF (refuse derived fuel) is made by changing over the combustible part of (Municipal Solid Waste) into a valuable fuel, taking after mechanical sorting and then prepared to improve the ignition and physical characteristics of the discarded material. For manufacturing of RDF the combustible portion of municipal solid waste convert into fuel by action of mechanical sorting that enhance the physical and ignition attributes of discarded waste, further the resultant RDF pellets used as a fuel in power stations and cement kilns that is technically viable [5].
Althea rosea is also known as garden Hollyhock while its local name is Gulekhera. It is well kno wn for its effective remedial and decorative properties. It has diversity of colors including red, pink and white color. it is inhabitant to china and grow well in welldrained, moist, and clay soil. It has potential to bear and accrue high concentrations of heavy metals, therefore, there use for the phytoremediation increasing day by day [6]. In Pakistan the waste containing industrial effluviums and household outflow is usually released to drain, close by field or into a sewage system. Major cities of Pakistan like Lahore, Karachi and Islamabad treat just a less extent of their wastewater before dumping, probably only 8% of urban waste water is treated. The waste water utilized for irrigation purposes is esteemed by agriculturists, for the most part due to its supplement contents and dependability of supply. Due to these intense water depleting conditions, Pakistan gradually changes from water surplus country to a water deficient nation [7] so there was need to introduce cost-effective and advantageous technique in the society for treatment of waste water that can be easily adopted by the society.
Materials and Methods
Present study was conducted to evaluate the efficiency of two types of pyrolysis chars (rice husk biochar, refuse derived fuel char) for treatment of waste water. Composite samples of waste water were taken from Main Outfall Drain, Lahore and stored in PET (polyethylene terephthalate) bottles. Rice husk was collected from rice mill, sample of rice husk was taken in pyrolysis unit and the reactor was closed. Nitrogen was given for 5-6 seconds before turning the unit on, in order to eliminate the oxygen gas that was already present within the unit. The pyrolysis unit was turned on and temperature of 400°C was set. When the temperature reached 400°C it was then left for 2 hours and unit was turned off, after cooling the resultant biochar was collected. Similarly RDF (refuse derived fuel char) was taken in pyrolysis unit and temperature of 500°C was set and the resultant char was collected. There were ten treatments T1 to T5 for biochar depending on different quantities, T6 to T10 for char depending on different quantities each with three replicates. PET bottles were cut from the bottom, inverted was fixed on wooden stand.
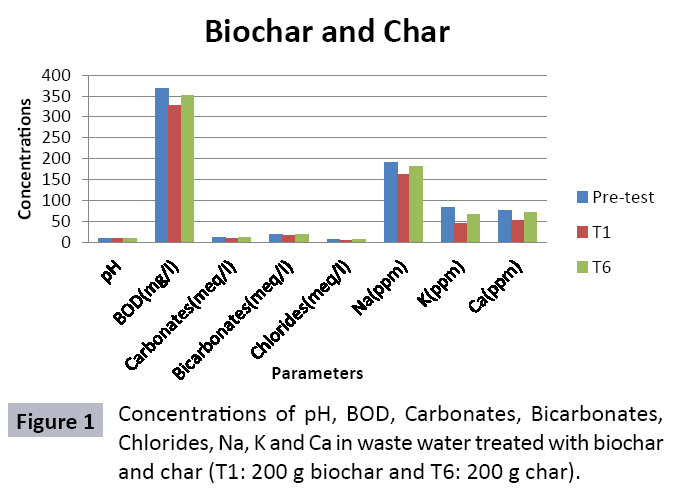
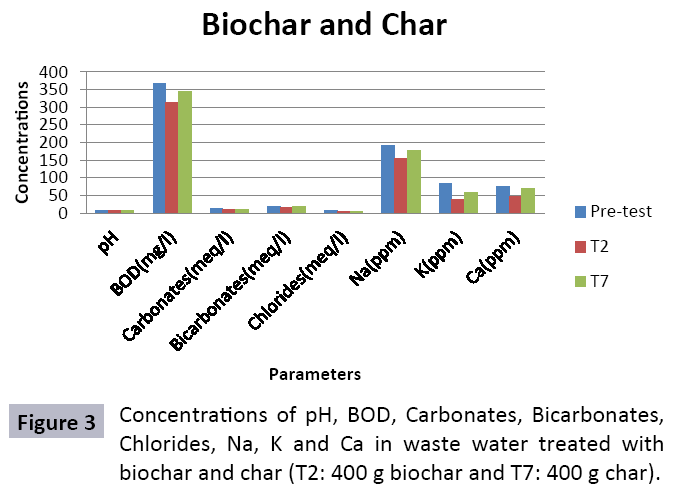
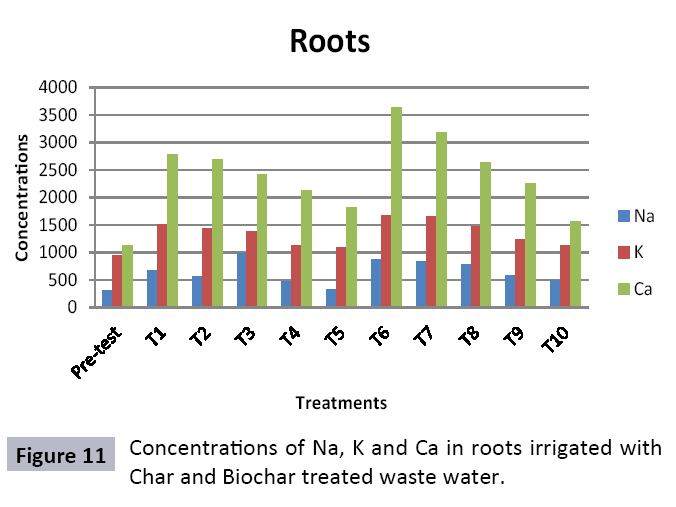
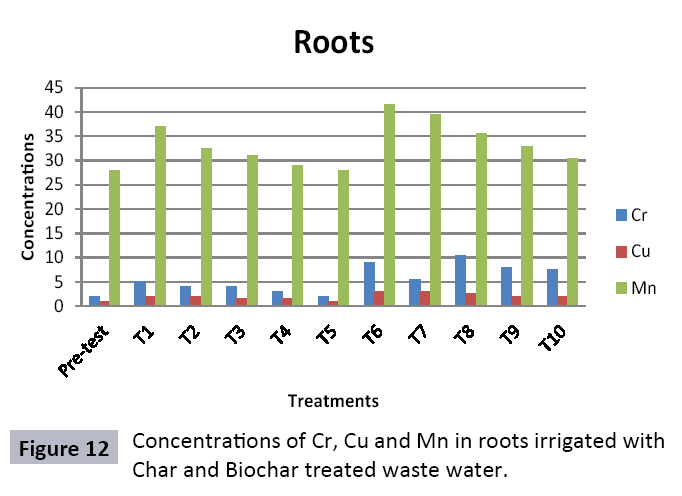
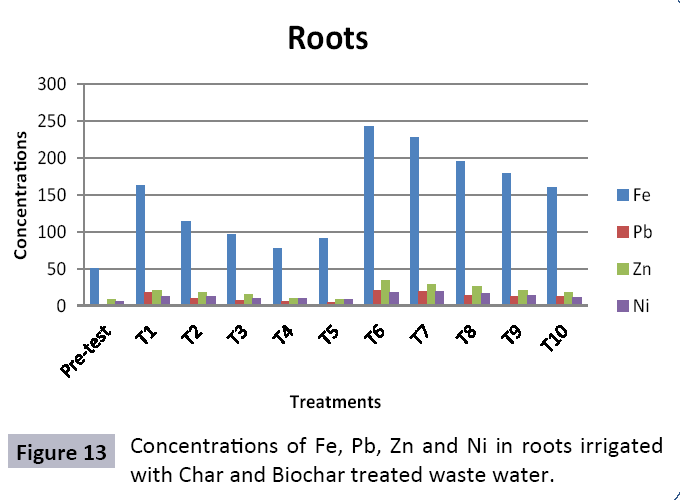
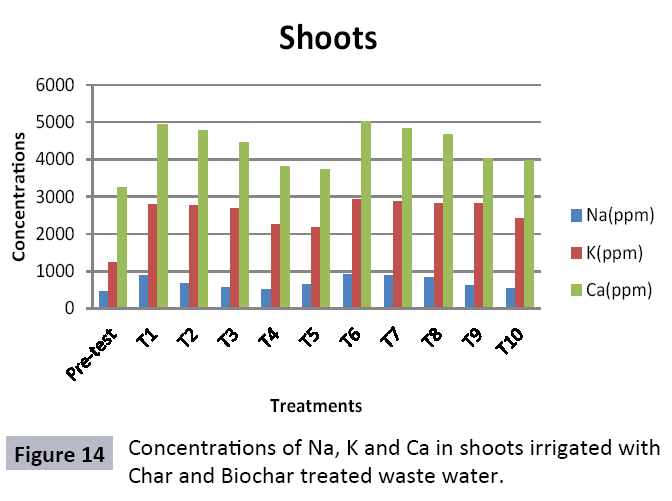
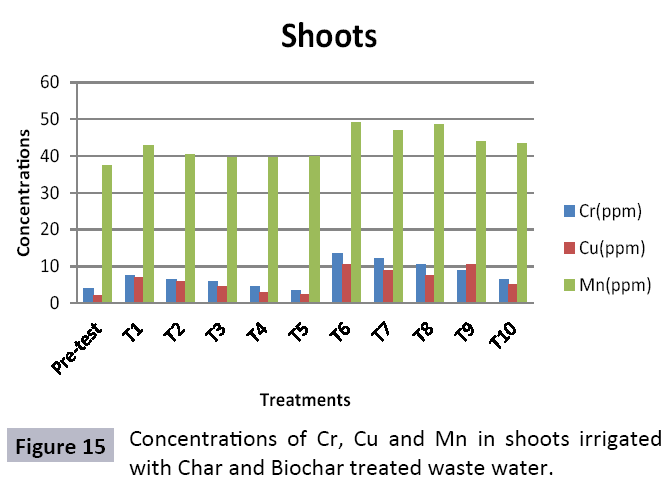
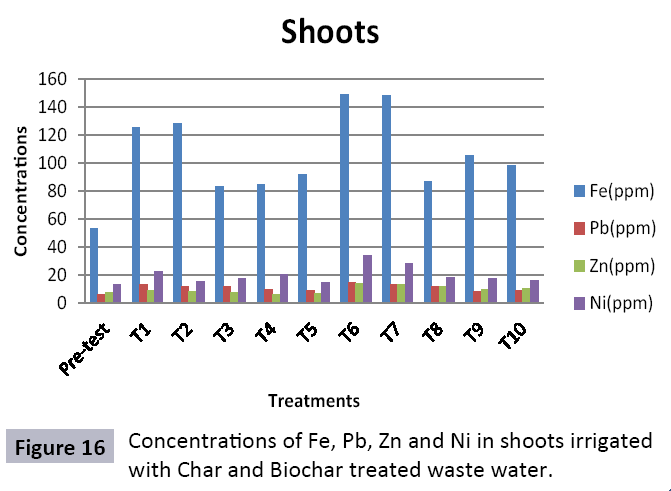
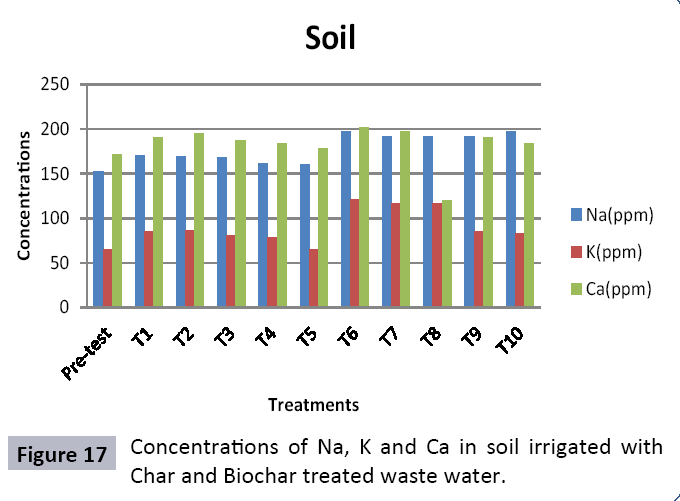
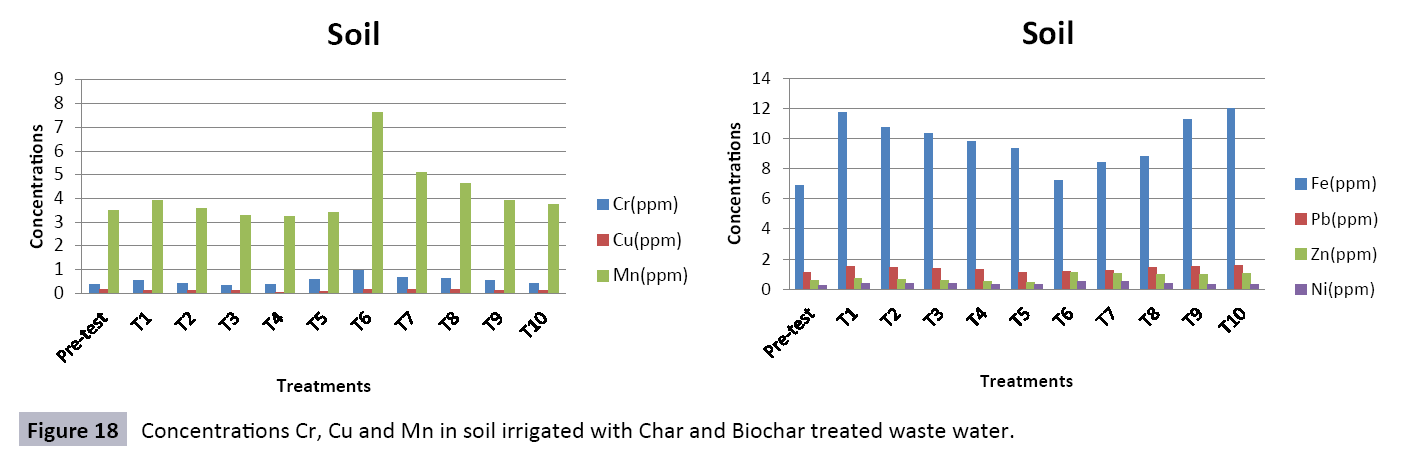
There were 10 groups of bottles each with 3 replicas. Bottles were filled with different column of char and biochar. 1.5 liters of waste water poured in each bottle and remain in it for 24 hours, after 24 hours the water was collected both the pre-analysis and post-analysis of waste water was directed that includes pH, electrical conductivity, BOD, carbonates, bicarbonates, chlorides, heavy metals and minerals. Electrical conductivity (EC) meter was used for the detection of electrical conductivity of water samples. To analyze the results the EC meter was dipped in distilled water, then in waste water samples to determine the EC. pH meter was used to determine the pH of water samples. First, the pH meter was calibrated, then pH meter was dipped into samples which have to be analyzed, and pH was recorded. The titration method was used to determine the carbonates and bicarbonates in water samples. Pipette 10 ml of waste water sample in a conical flask, 1-3 drops of indicator (phenolphthalein 1%) was added, appearance of pink colour showed the presence of carbonates, it was titrated against 0.1 N H2SO4 till the end point, colorless. To the same conical flask, 1-2 drops of indicator (methyl orange 0.1%) were added, it appeared golden yellow, then titrated against 0.1 N H2SO4 till the endpoint light pink or light orange. To the same conical flask, 3-4 drops of indicator (Potassium chromate 5%) were added, it was then titrated against 0.05 N silver nitrate, till end point brick red precipitates. DO meter was used to measure BOD of waste water. To determine the BOD of required sample, the DO meter was dipped in waste water sample and the initial reading D1 was taken, then the sample was incubated at 20°C for five days, after five days the final reading D2 was taken by using DO meter again dipped into same sample. Atomic Absorption Spectrophotometer (Model Hitachi 8200) was used for the analysis of heavy metals (Cu, Zn, Cr, Pb, Fe, Ni, Mn) and multi-channel flame photometer (Model from Biotech UK) for the analysis of crystalline earth metals (Na, K, Ca). The earthen pots were filled with soil and ornamental plant Althea rosea was grown, after one week of acclimatization the plants were irrigated with treated waste water from char and biochar. There were five pots per treatment and the plants were irrigated for one month. After one month, physiological estimation of plants was done, included root and shoot length, biomass of plants. Plants samples (root and shoots) were digested and analyzed for heavy metals and for alkaline earth metals (Na, K, Ca). Vegetative samples (root and shoots) were dried at 65°C, ground and passed through sieve. It was digested by diacid method. 1 g of vegetative sample was taken in kjeldahl tube, added 10 ml of diacids mixture (perchloric acid: nitric acid 1:3) and then placed on a hot plate for brisk heating, continue heating at 120°C then rise the temperature at 150°C until the emission of dense white fumes leaving behind the water clear solution, continue heating until the contents was reduced (Figures 1-18).
Results and Discussions
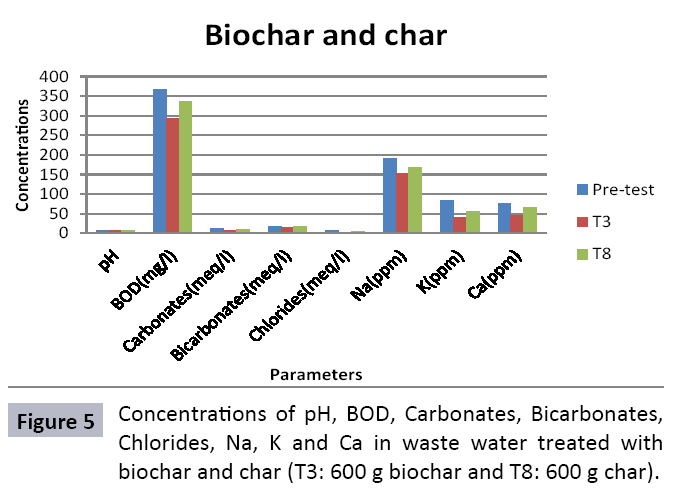
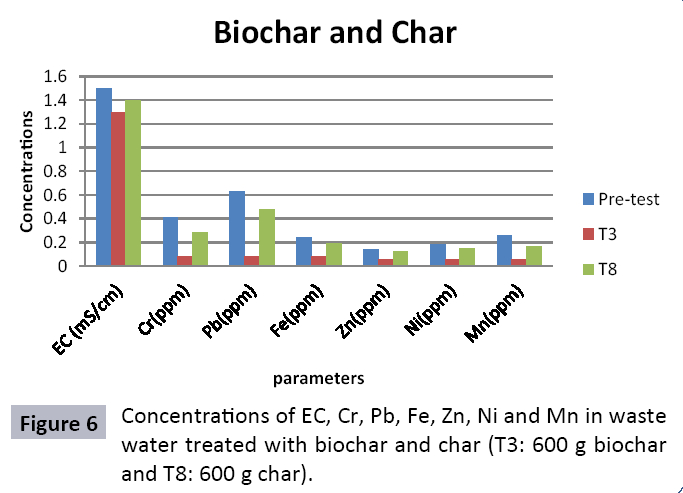
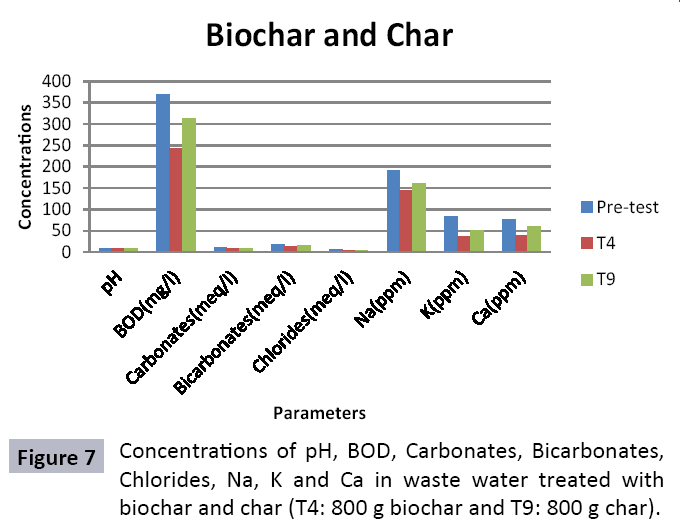
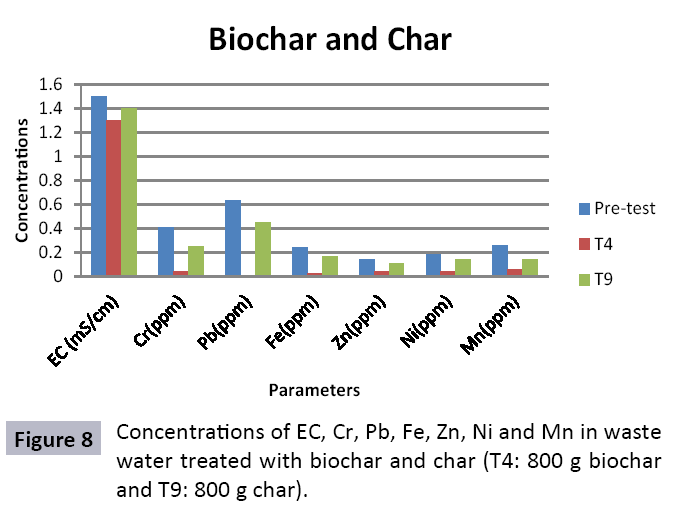
The study was conducted to evaluate the efficiency of char and biochar for waste water treatment using different quantities of char and biochar. In the present evaluation, different feedstock producing biochar and char was used for removal of overwhelming metals and other inorganic contaminants from waste water. The aftereffects of portrayal investigations and adsorption test demonstrated that biochar can be utilized as a promising adsorbent. Different concentrations of biochar were used in different treatments to evaluate its efficiency for waste water treatment, escalating the concentration of adsorbent resulted in improved removal efficiency of the heavy metals due to the increase in the total quantity of active sites. Biochar showed effective results for removal of heavy metals, mainly Cr, Pb, Zn, Fe, Cu, Ni. It was due to the effect that there are oxygen containing functional groups present on biochar surface like carboxylate, COOH and hydroxyl, OH group, these groups have high interactions with heavy metals and minerals that results in metal adsorption. Some of these interactions include ion exchange and electrostatic interactions. It is obvious from results that heavy metals removal efficiency from biochar is ninety percent in which recorded lead removal efficiency is a hundred percent. The Cr, Ni, Zn and Mn removal efficiency from biochar is near to eighty to ninety percent. The char produced from RDF (refused derived fuel) showed less effectiveness as compare to biochar the microspores in the char helps to bind metal ions and minerals so they can uptake contaminants from solution. The thermal degradation in absence of oxygen produced high surface area and high pore volumes that enabled char to act as an adsorbent that has the capacity to adsorb contaminants from aqueous solution.
The adsorbent capacity for Pb, Fe, Ni, Zn, Mn, Cr is effective up to some extent and it is less effective as compared to biochar. The char effectiveness is only somehow forty to fifty percent, it is evaluated that biochar is more efficient as compared to char. It is due to the fact that the efficiency depends on different types of feedstocks as rice husk and refused derived fuel is totally two different types of solid waste, their nature of the composition is different and that is why their reactivity towards adsorption is different. In current research, Althea rosea was under stress of treated waste water for one month. T1 to T5 groups of plants treated with biochar treated water while T6 to T10 groups treated with char treated waste water. After one month of stress the plants were harvested and the results were evaluated. Plants have great ability to handle topsoil without affecting its textures so in this context conserving soil fertility, preventing soil erosion and metal leaching. Plants have mechanism for solubilization of heavy metals in the soil. Rhizosphere microorganism’s that are fungi and bacteria appreciably rise the bioavailability of heavy metals. Althea rosea is one of that, exhibiting these advanced properties means have ability to accumulate astonishing amount of heavy metals in it, rapid translocation of heavy metals between shoots and roots and having a greater capacity to detoxify heavy metals derived fuel) showed less effectiveness as compare to biochar the microspores in the char helps to bind metal ions and minerals so they can uptake contaminants from solution. The thermal degradation in absence of oxygen produced high surface area and high pore volumes that enabled char to act as an adsorbent that has the capacity to adsorb contaminants from aqueous solution.
Conclusion
It is inferred from results that biochar treatment is cheap and effective than using char for treatment of waste water and use of ornamental plant at contaminated sites not only remediate the pollutants but also beautify the contaminated site at the same time.
References
- Rizwan TA, Chaudhary S, Ikram M (2013) Uptake of Some Toxic Metals in Spinach Crop Irrigated by Saggian Drain Water, Lahore, Biologia Pakistan183: 189.
- WWF (2001) Water Quality Monitering of Hudiara Drain Environmental Pollution Unit. Worldwide Fund for Nature, Lahore, Pakistan.
- Akpor OB, Ohiobor GO, Olaolu TD (2014) Heavy Metal Pollutants in Wastewater Effluents: Sources, Effects and Remediation. Advances in Bioscience and Bioengineering 37: 43.
- Ippolito JA, Laird DA, Busscher WJ (2012) Environmental Benefits of Biochar. Journal of Environmental Quality 967: 969.
- Global Alliance for Incinerator Alternatives (2013) Accesed on 2nd August, 2016.
- Liu JN, Zhoub QX, Sun T, Wang S (2008) Growth Responses of Three Ornamental Plants to Cd and Cd-Pb Stress and Their Metal Accumulation Characteristics. J Hazardous Materials 261: 267.
- Murtaza G, Munir H (2012) Wastewater Production, Treatment and Use in Pakistan. Disposal and Use of Sewage on Agricultural Lands in Pakistan: A Review. Pedosphere 15: 18.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences