ISSN : 2393-8854
Global Journal of Research and Review
The Role of Inorganic Salts on the Tio2 based Photocatalysis of Rodamin B (RB) in Aqueous Solutions
Fatemeh Maghami*, Abdolraouf Samadi-Maybodi and Mohammd Javad Chaich
Department of Chemistry, University of Mazandaran, Babolsar, Iran
- *Corresponding Author:
- Fatemeh Maghami
Department of Chemistry, University of Mazandaran, Babolsar, Iran
E-mail: ottuhpeter@gmail.com
Received Date: June 08, 2021;Accepted Date: June 23, 2021;Published Date: June 30, 2021
Citation: Maghami F, Samadi-Maybodi A, Chaich MJ (2021) The Role of Inorganic Salts on the Tio2 based Photocatalysis of Rodamin B (RB) in Aqueous Solutions. Glob J Res Rev Vol.8 No.4: 78.
Abstract
The photo-catalytic bleaching of industrial dyes using Tio2 has appeared promising in laboratory studies, but little attention has been focused on whether other species might be found in wastewater have a harmful or useful effects on the photo-bleaching. This study highlights effects of some ionic species on the photo-bleaching of Rodamin B (RB) dye in the aqueous acidic and basic solutions. The rate of RB photo-catalytic oxidation was studied at different pH values. The effect of solvents on photo-degradation of RB was also investigated. Various metals doped titanium dioxide (Tio2) photocatalysis have been studied intensively for the photodegradation of dye in wastewater treatment. The Ag particles were photodeposited on Tio2 powder surface. Nanostructure Ag-Tio2 nanoparticles were synthesized by photo reduction method.The X-ray diffraction (XRD), Scanning Electron Microscope (SEM), Energy dispersive X-ray spectroscopy (EDXS), and photoluminescence (PL) spectrophotoscopy were applied to investigate the
structure and morphologies of the samples. It was found that the loaded Ag particles have no effect on the XRD patterns.Photodegradation capacity of Tio2 nanoparticleswas compared to AgTio2 photoactivity. Above the optimum silver ion loading, the activity of Ag/Tio2 particles decreased. The use of electron acceptors, such as S2O8-2 in Tio2 system increased dye photo-degradation.
Keywords
Photo catalytic oxidation; Tio2; Rodamin B; Ionic species
Introduction
Rhodamine dyes (RB) are important class of xanthene dyes. They have high quantum yield of fluorescence and high absorption factors. They have been widely studied due to their properties and practical applications in dye lasers [1]. Furthermore, RB dyes have been used for some specific applications, such as biological stains, electrochemical luminescence sensitizer, water- tracing agents, molecular probes, chromo-ionophores in optical chemical sensors and solar collectors and many others devices so treatment extra dye in wastewater is very important [2]. Clearly, wastewater is full of different material with different range of the pH values. Photo-degradation efficiency of dyes is affected by pH of the solution in the presence of Tio2.
Heterogeneous photo-catalysis is a processing which the irradiation of an oxide semi-conductor produces photo-excited electrons (e−) and positive charged holes (h+) to the form hydroxyl radical and superoxide radical anion, that both of them play as the oxidizing species in the photo-catalytic oxidation method. The efficiency of photo-excitation of the semi-conductor is dependent on the energy state of the electrons valence band and conduction band. The extent of dye adsorption depends on several factors such as, dye nature of the dye, surface area of the used photo-catalyst, concentration and pH of the solution. The pH controls the surface charge of the photo-catalyst and dye. Adsorption of the dye is minimum when the pH of the solution is at the isoelectric point (point of zero charge). The surface of the photo-catalyst is positively charged below isoelectric point and carries a negative charge above that. As a result, the structure of dye is important to be found in due course, i.e., the efficiency of adsorption on the surface of a photo-catalyst can be low or high in acidic and basic media [3].
In Tio2/UV system positive charged holes can be scavenged by the negatively charged anions and therefore depends on the positive charged or negatively charged adsorption of dyes on the surface of Tio2 is considerably inhibited or promoted [4]. Previous studies indicated that inorganic anions can scavenge •OH to form the corresponding anion radicals [5-7]. The anionradicals may themselves oxidize organic and inorganic compounds [5,8,9], which can also influence on the total rates of the photo- catalytic oxidation. It is also reported that competitive adsorptionof the inorganic anions for active sites on the Tio2 surface may contribute to the photo-catalytic degradation of organic compounds [10-13]. For instance, Cl− decreases the degradationrate of 2-chlorophenol and 2-nitrophenol at pH values lower thanthe Tio2 point of zero charge (pHpzc), while Cl− had no inhibitory role at pH values greater than the pHpzc due to slight adsorptionto the negatively charged Tio2 surface [12]. Inorganic anions canalso change the rates of dye photo-catalytic oxidation in one of the following ways: (i)•OH scavenging by inorganic anions, (ii)direct oxidation of dye by anion radicals, or (iii) adsorption ofinorganic anions to the Tio2 surface [14,15].
The photo-catalytic activity of Tio2 for the oxidative degradation of (RB) may be enhanced by first, surface modification using metals, second, transition metal doping and third, coupled photo-catalysts. In this research Ag metals were used for increasing in photo-degradation of (RB) dyes. Improving the photocatalytic activity by Ag deposition may be associated to different mechanisms:
(1) Ag nanoparticles deposited on Tio2 act as electron traps, enhancing the electron-hole separation and the later transfer of the trapped electron to the adsorbed O2 acting as an electron acceptor [1-3].
(2)self-photosensitization pathway, that means,extendthe lightabsorption into the visible range and enhancesurface electronexcitation by plasmon resonances excitedby visible light, injectingan electron into the conduction band of the Tio2 semiconductor,so, the injected electron on the Tio2 particle reacts with adsorbedoxidants to produce reactive oxygen radicals and dye is degradedby these oxygen radicals. Under visible light irradiation, thesemiconductor Tio2 acts only as an electron-transfer mediatorand.
(3)modify the surface properties of photocatalyst [4-8].Zakerhamidi and co-author studied solvent effects on thedipolemoment and photophysical properties of rhodamine dyes.This study describes the effective parameters such as pH of thesolution, type of solvent and cationic and anionic species on thephoto-bleaching of (RB) in the presence of Tio2 nanoparticles[16,17]. The amount of degradation of RB were discussed indifferent circumstances.In this experience it was investigated theeffects of pH and common salts and solvent on photo degradationof dye Rhodamine (RB).
Materials and Methods
Titanium dioxide nanoparticles (purity>99.5 %,) with average particle size 30 nm and specific surface of 50 m2/g was purchased from Degussa Corporation (USA).
Compounds of CuCl2. 2H2O, Cu(NO3)2. 3H2O, Al(NO3)3, Co(NO3)2, Fe(NO3)3 .9H2O, CoCl2 .6H2O and FeCl2 were obtained from Fluka (Buchs, Switzerland) and used without further purification. The pH (2,6 and 12) values of the solutions were adjusted using HCl (0.01 M) and KOH (0.01 M) solutions. RB dye (10-5 M) was prepared from Fluka (Buchs, Switzerland). The photo-catalyst was added to dye solution and the suspension was irradiated under UV-Vis light. Then fluorescence spectra of the dye solutions were recorded and de-colorization process was monitored in terms of change in intensity at λmax excitation (550 nm) and λmax emission (580 nm) of the dye after 0, 2, 5, 8, 10 minute irradiation.
Ag-Tio2 was synthesized by photo-reduction of AgNO3. In this way, Ag+ ions are deposited as Ag metal on the Tio2 surface by photo-reduction of AgNO3 under UV irradiation [8]. One gram of Tio2 was added into 250 mL of double-distilled water and then irradiated with UV-Vis light for 10 min to remove any impurities present on Tio2 surface. Then approperiate amount of AgNO3 was added into the suspension of Tio2 solution and the suspension adjusted to pH 3.5, finially it was irradiated using UV light for 40 min with continuous stirring under N2 atmosphere. The suspension was then filtered,washed,dried and then ground into a fine powder [9,10].
General procedure
The photo-catalytic reaction was carried out in an inner irradiation type reactor. The photo-catalyst powder was dispersed by magnetic stirrer. A 300 W high-pressure mercury lamp from Oriel GMBH, Model 8500 (Cheltenham, England) was used as the light source and spectro-fluorimeter of Perkin-Elmer LS-3B Luminescence (USA) was used. The cell was made with Quartz (3 mL content) that it was put it in the close space in front of the light in reactor. Analysis of the crystalline structures was performed by XRD Diffracto-meter (GBC MMA made in Australia) with wavelength of Cu Ka radiation in 2Ө range from 15° to 80° , SEM analysis was performed by a VEGA TESCAN (USA) instrument.
Results
The effect of pH
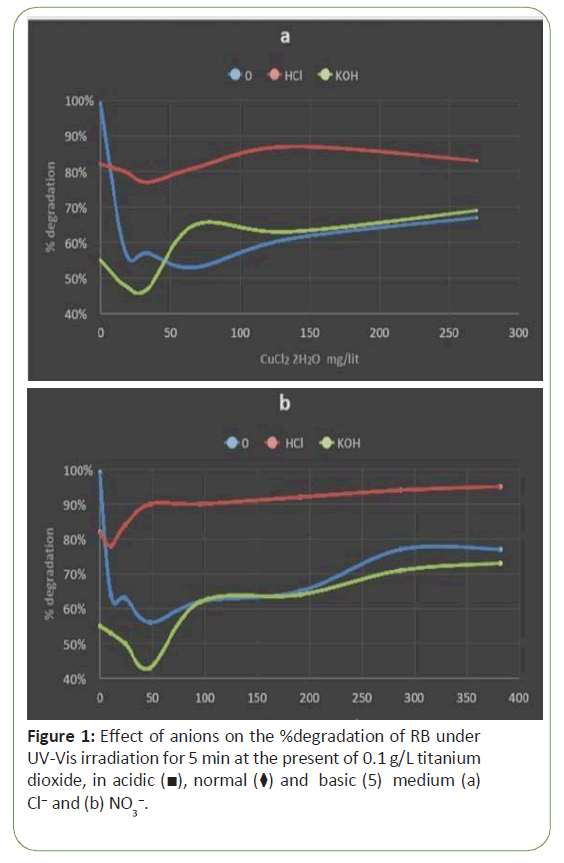
To study the effect of pH on photo-degradation, several experiments were done using 1.0 × 10-5 M dye concentration and it was exposed under UV-Vis light in the presence of nanoparticles of Tio2. Figure 1 shows increasing of % degradation of RB in acidic (pH=2), normal (ca. pH=6) and basic medium (ca. pH=12) with increasing of anionic species, after ten minute UV-Vis irradiation in every experiments and in present of 0.1 g/L titanium dioxide. As can be seen acidic, normal and basic medium is significantly depended on the concentration of anions either NO3- or Cl-. The behavior of dye degradation in acidic, normal and basic medium are nearly the same so that in higher concentration of anions the percentage of dye degradation as function of anion concentration is smooth (Figure 1).
Effect of cations on the titanium dioxide-based photo-catalytic oxidation of aqueous R
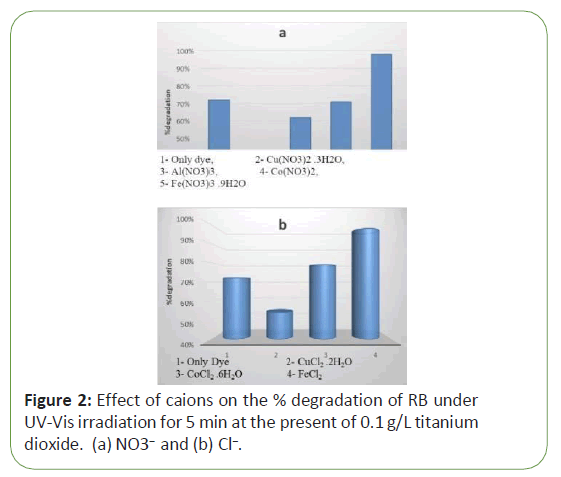
Wastewater is full of material, effect anion and cation for increase adsorption dye on catalyst was investigated in different pH, however the kind of cation is important. Salts of NO3- and Cl- are very common. In a quartz cell, 3 mL of the solution containing 0.01 M of (a) NO3- and (b) Cl- are exposed by UV-V irradiation for five minutes in the presence of 0.1 g/L titanium dioxide. Experiments were performed using 50 mL of solution with constant initial concentration of dye RB (10-5 M) and in the present of salts nitrate and chloride. Figure 2 shows the effect of cations on % degradation of RB in normal medium with increasing of anionic species. The counter ions are the same, i.e., (a) NO3- and (b) Cl- for the cations of Al3+, Cu2+ and Co2+ and Fe3+. Figure 2 illustrates the influence of the cations on the % degradation of RB. Results reveal that % degradation of RB is highly enhance in the presence of Fe2+ and in contrast the degradation is very low in the presence of Cu2+. The other cations (Al3+, Co2+ ) are also have differences effects, in this case both cations of Al3+, Co2+ have almost similar effect on the % degradation of RB so that % degradation almost have the nearly the same values. The order of % degradation for the cations are as follows Fe3+ > Co2+ > Al3+ >Cu2+ (Figure 2).
Effect of solvent
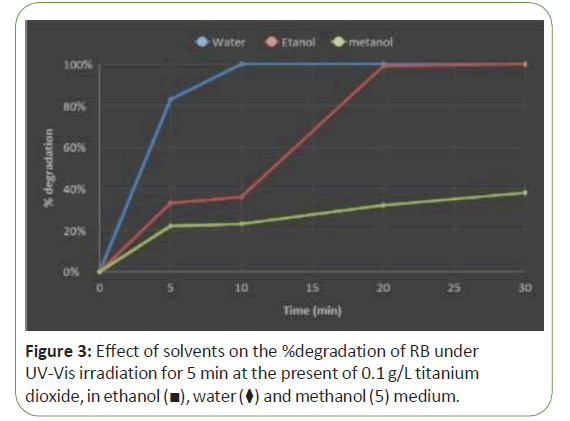
The percentage of dye degradation was also examined using different of solvents i.e., water, methanol and ethanol. Figure 3 illustrate the effect of dye degradation in aqueous (water) and nonaqueous (alcoholic medium). Results indicated that rate of % degradation in water is the highest amount (Figure 3).
Characterization of Tio2 and Ag-Tio2 nanoparticles
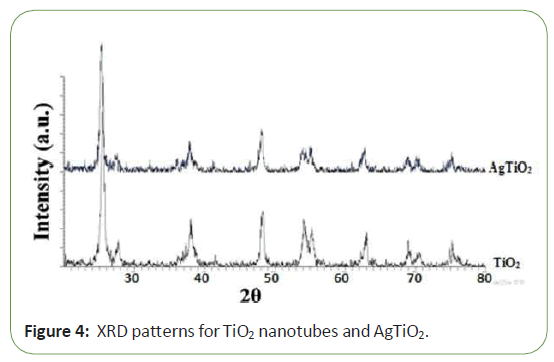
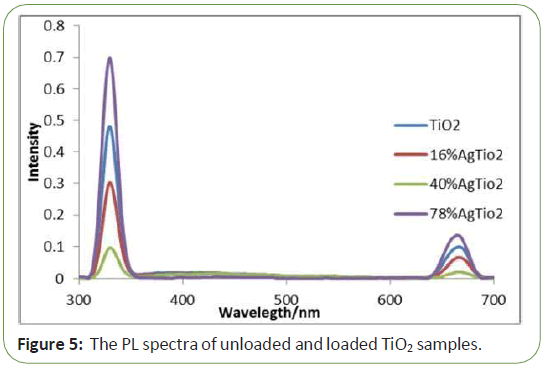
In order to investigate the changes in the crystal structure due to silver deposit, XRD measurements were taken in the range of 2Ө 15-80 for pure Tio2 and Ag-Tio2 nanoparticles (Figure 1). It is seen that the XRD patterns of Ag-Tio2 sample are almost the same as for pure Tio2, except for the intensities of the peaks. Also, theXRD patterns show no diffraction peaks due to silver deposition.This may be due to the fact that deposition alters the crystallinitybut not the crystal structure of Ag-Tio2 and thus suggest that thesilver deposits are merely placed on the surface of the crystals.Diffractions that are attributable to anatase phase of Tio2 crystals Tio2samples. The signal intensity of unloaded sample is very strong and shows evident characteristics of the radiative recombinations of electrons and holes. The PL peak at 367 nm arises from the band-to-band recombination which evidently decreases with the loaded Ag particles. Two factors affect the PL intensity after Ag deposition. One is the migration of the electrons from Tio2 to Ag particles; the other is the Ag plasmon absorption. The PL band from 390 to 440 nm is from the recombination transition related to the surface structure, which also decreases with the loaded Ag, indicating the photo- deposited Ag has affected the surface structure of Tio2, leading to the decrease of surface recombination but if silver amount became above the optimum loading, silver particles can also act as recombination centers so The signal intensity of loaded sample is very strong [11,12] (Figure 5).
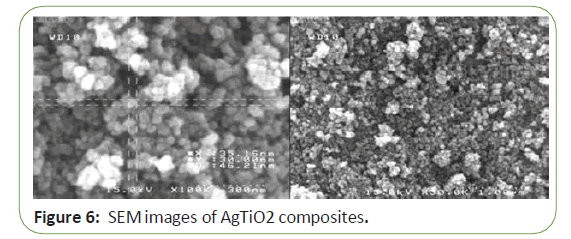
Figure 2 shows SEM images of the AgTio2 composites. The general morphology of the AgTio2 can be clearly observed in these micrographs. Tio2 particles were uniformly distributed. It can be considered that the better dispersion provides a larger number of active catalytic centers for the photocatalytic reaction (Figure 6).
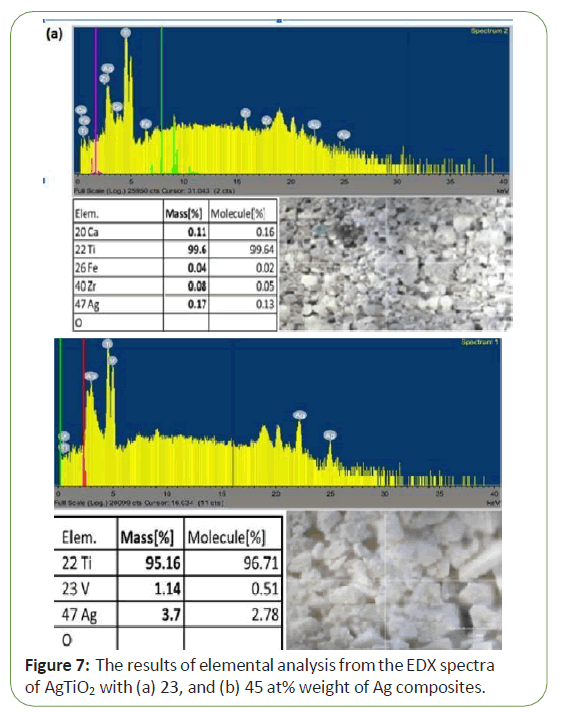
Figure 4 shows the results of elemental analysis from the EDX spectra of AgTio2 with 23, 45 at % weight of Ag composites. These spectra show the presence of peaks from Ag and Ti. The elemental composition analysis of the composite series is listed in Tables. Ti was the major elements in the composite series. Figure 7a is 23 at % weight of Ag composites and Figure 7b is 45 at % weight of Ag composites. As expected, it was observed that the Ag content of the AgTio2 composites showed an increase with increasing the at % weight of Ag (Figure 7).
Photo-catalytic degradation of RB
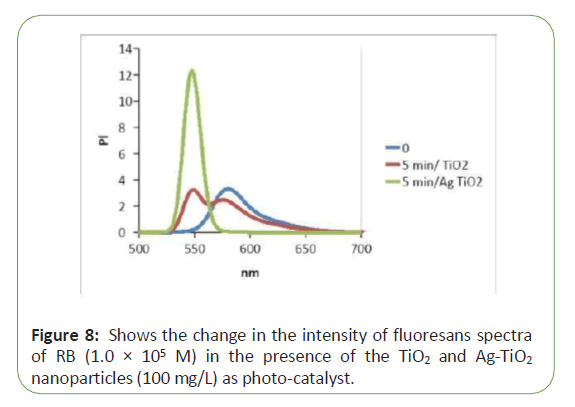
Figure 5 shows the change in the intensity of fluoresans spectra of RB (1.0 × 105 M) in the presence of the Tio2 and Ag-Tio2nanoparticles (100 mg/L) as photo-catalyst. After irradiation with UV-Vis light, the intensity of spectra decreased due to decolouration of the dye. Figure 5 indicated that speed of de- colouration, after 5 min irradiation in present of AgTio2 was faster than in present of Tio2 (Figure 8).
Effect of catalyst amount
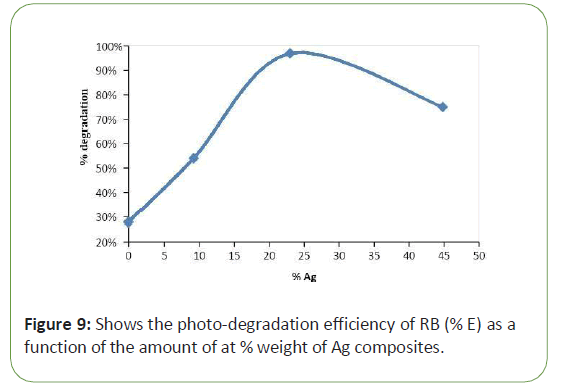
The effect of Ag-Tio2 amount on the photo-catalytic degradation of RB was studied at fixed concentration of RB M in aqueous solutions. Figure 6 shows the photo-degradation efficiency of RB (% E) as a function of the amount of at % weight of Ag composites. An optimum silver ion loading of 23 at % weight of Ag was found. For all silver ion loadings used, Ag/Tio2 performed significantly better than bare Tio2 .Above the optimum silver ion loading, the activity of Ag/Tio2 particles decreased. There are several reasons for the existence of an optimum metal loading. It is also possible that above the optimum loading, silver particles can also act as recombination centres [5,6,13] (Figure 9).
Effect of electron acceptors in dye photo- degradation
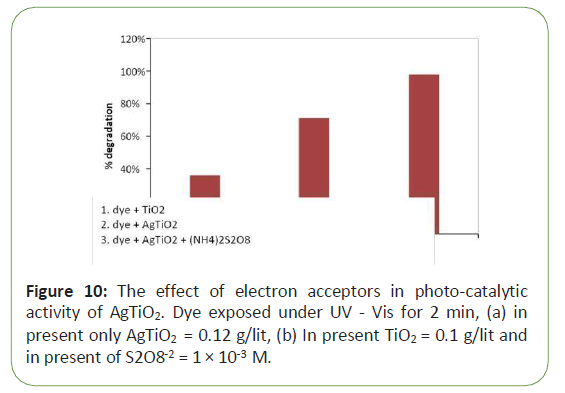
The use of inorganic oxidants, such as S2O8-2 in Tio2 system increased the quantum efficiencies either by inhibiting electron- hole pair recombination through scavenging conduction band electron at the surface of Tio2 or by offering additional oxygen atom as an electron acceptor to from the superoxide radical ion and along reaction electron acceptor with AgTio2 due production of active radicals. Figure 7 gives the effect of electron acceptors in photo-catalytic activity of AgTio2. Dye exposed under UV-Vis for 2 min, (a) in present only AgTio2=0.12 g/lit, (b) In present Tio2=0.1 g/lit and in present of S2O8-2=1 × 10-3 M [10] (Figure 10).
Discussion
The effect of pH
The pH of the solution is an important parameter which strongly influences the surface charge properties in aqueous dispersion. Generally, surface of the net Tio2 is positively charged in acid media and negatively charged in alkaline media with an isoelectric point of around pH 6~7 [18]. Therefore, adsorbates in the aqueous solution tend to adsorb on the surface of Tio2by the negatively charged or electron abundant group in acidic solution and by positive charged in alkaline solution because of the electrostatic interaction [19]. The ζ-potential of Tio2 with different pH amounts was investigated before. Photo-degradation efficiency of dyes is affected by pH of the solution in the presence of Tio2. The amount of pH solution changes the surface charge of Tio2 particle [3]. As a result, the adsorption of dye on the surface is altered thus causing a change in the reaction rate. Under acidic or alkaline condition the surface of Tio2 can be protonated or deprotonated (Eq. 1 and 2) respectively according to the following reactions, therefore, the pH effect is often dependent on the nature of the dye. To study the effect of pH on photo- degradation, several experiments were done using 1.0 × 10-5 M dye concentration and it was exposed under UV-Vis light in the presence of nanoparticles of Tio2 [20]. Figure 1 shows increasing of % degradation of RB in acidic (pH=2), normal (ca. pH=6) and basic medium(ca. pH=12) with increasing of anionic species, after ten minute UV-Vis irradiation in every experiments and in present of 0.1 g/lit Titanium dioxide. Figure 1 shows the effect of anions (i.e., NO3- and Cl-) on the % degradation of RB under UV-Vis irradiation (for 5 min) at the present of 0.1 g/L titanium dioxide in acidic, normal and basic medium. As can be seen acidic, normal and basic medium is significantly depended on the concentration of anions either NO3- or Cl-). The behavior of dye degradation in acidic, normal and basic medium are nearly the same so that in higher concentration of anions the percentage of dye degradation as function of anion concentration is smooth. It is pertinent to point out that in both normal and basic medium the concentration of anion at concentration of about 25 mg/L has lowest value of degradation either in the presence of NO3- or Cl-. Results also revealed that the % degradation of RB is hardly depends on the concentration of anion concentration in acidic media. As a result the % degradation of RB is depended on medium, i.e., acid, neutral and basic. This evidence can be attributed to the surface of nanoparticles as well as the chemical structure of the dye in question.
TiOH + H+→ TiOH2+ (1)
TiOH +OH-→ TiO- + H2O (2)
Acidic medium
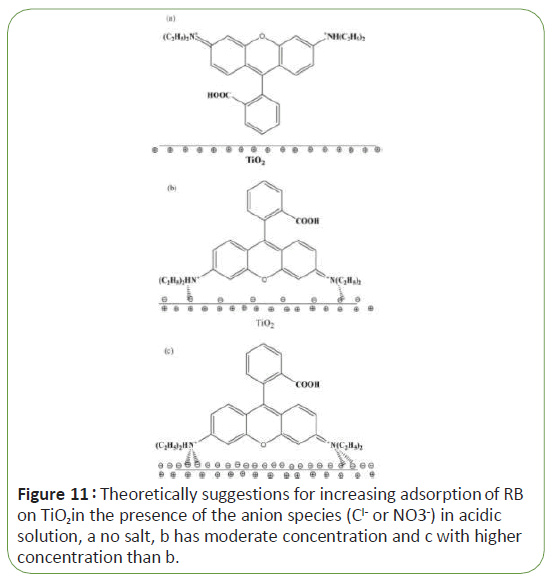
In acidic medium, the rationale behind this is that at low pH values, more H+ are available for adsorption to mask the surface of the catalyst thus inhibiting the photo-excitation of semiconductor particles, [3]. In addition, adsorption of dye on the surface positively charged of Tio2 is not favorite, results indicated that adsorption of RB on Tio2 is significantly increased in the presence of the anionic species in acidic medium and hence increasing RB % degradation. Figure 11 shows theoretically suggestions for increasing adsorption of RB on Tio2 in the presence of the anion species (Cl- or NO3-) in acidic solution with 10-2 molar of HCl with different concentrations of salt (Figure 11).
Neutral medium
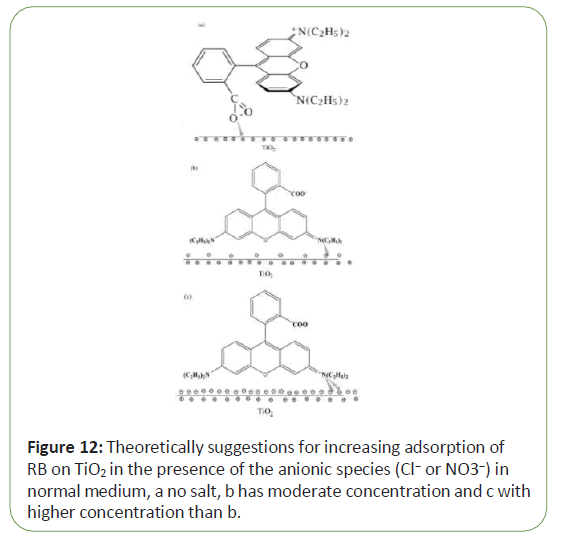
In neutral solution, the pH value of the reaction solutions under the experiment conditions was controlled at about 6, in which the surface of Tio2 was positively charged. The acid dissociation constant of the carboxyl group of RB is about 4.1 ± 0.1. Thus, under the experimental conditions, most of the carboxyl group of RB was dissociated and negatively charged at −COO− state and RB tends to adsorb on the surface positively charged of Tio2 by the negatively charged carboxyl group of RB [19]. So rate of photo degradation of dye (RB) without salt in neutral medium is the highest. Therefore, RB Dyeis zwitter-ionic form when the solution pH is higher than the acid dissociation constant of RB [21]. Adsorption of RB molecules through the positively charged significantly increased in the presence of the anionic species in normal medium. Figure 12 shows theoretically suggest ions for increasing adsorption of RB on Tio2 in the presence of the anionic species (Cl- or NO3-) in normal medium (Figure 12).
Basic medium
In basic solution, RB is zwitter-ionic and the surface of Tio2 is negatively charged. As a result, adsorption of RB molecules through the positively charged is chosen on the surface negatively charged of Tio2. Besides H2O2 molecules that are formed during photo-degradation process could easily self-decomposed in basic medium. The species of •OOH could also react with both •OH radical as well as H2O2 in the basic medium. Thus, it woulddecrease the concentration of •OH radicals and affect degradationefficiency of RB. The degradation rate was found to increasewith oxidation potential of ·OH radical in the acidic medium asreported by Wang additionally in the alkaline conditions there isa competitive adsorption between hydroxyl groups and the dyemolecule [3,22,23].
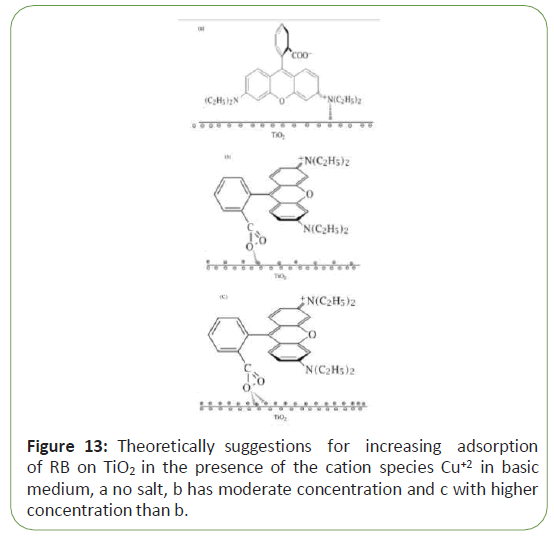
It is pertinent to point out that the adsorption of RB molecules through the negatively charged carboxyl group on the surface negatively charged of Tio2 was also significantly increased in the presence of the cationic species in basic medium. Results indicated that positively copper complex, leads to increase adsorption of RB on Tio2 and hence increasing color removal from the solution. Figure 13 shows theoretically suggest ions for increasing adsorption of RB on Tio2 in the presence of the cation species Cu+2 inbasic medium using 10-2 M of KOH with different concentrations of salt (Figure 13).
Effect of cations on the titanium dioxide-based photo-catalytic oxidation of aqueous RB
It can be justified that suppression of OH• radicals takes place due to trapping of the conduction band electrons by the adsorbed metal ions and decreasing color removal might be expected [24]. Results indicated that significant decreasing in the initial degradation rate of photo-catalysis, is occurred in the presence of cations, among them Cu+2 has the largest effect (Figure 2). It can deduce that the retardation is due to Cu+2 ions, which it scavenges the electrons required for the formation of hydroxyl radicals. Results specified the Fe ions have an important role (increasing of % degradation) in the photo-degradation process which also has been reported by many researchers [25-27]. Fe+3 ions can accelerate the decomposition of many organic compounds in water using hydroxyl radicals formed in the following process [28].
Fe+3+OH-+h+→Fe+2+OH (7)
This effect could be attributed to the scavenging of OH radicals by solvents or solubility of electrons, which are vital for generating OH radicals. If latter is the reason, one can expect higher degradation in more polar solvents than in less polar solvents. The dielectric constants of methanol and ethanol are 32.6 and 24.3, respectively. Therefore the possibility of generation OH radical in protic solvent like methanol is much higher compared to that of ethanol and consequently increases the rate of the degradation [29].
This is not surprising since excited electrons are less solvated in organic solvents than in aqueous solution. It may be that as the organic portion of the reaction mixture is increased, free electrons becomes less easily solvated, and the possibility of recombination with the positive hole (h+) increases. Another possibility is that the acetonitrile (and the other solvents examined) scavenge surface holes, which might be expected to retard photo-oxidation [30,31].
As a result, solvent also can play a significant role in photo- degradation process. These effects are closely related to the nature and degree of the solute dipole moment changes in the process of solute solvent interactions [32]. In organic solvents, free electrons are less solvated and hence recombination of electron with hole is increased. Dye (RB) is zwitterionic form in neutral medium. Results specified that the degradation of dye in methanol is less than the others, this probably due to more solvation of dye in methanol. Since solvation can inhibit adsorption of dye on the surface of nano particles since solvation of dye in ethanol is less and hence degradation of dye in ethanol is more than methanol. Figure 3 presents the effect of solvents (Water, Ethanol and methanol) on the % degradation of RB by use Tio2 and in present of 0.1 g/lit Titanium dioxide.
In previous studies, the effect of parameters such as pH, adsorbent amount and initial dye concentration and the adsorption of (RB) dye were carried out using sodium montmorillonite [16]. Results obtained from the present work specified that using Tio2nanoparticles, the photodegridation of RB is significantly.
Conclusion
Photo-degradation of (RB) using nanoparticle Tio2 under UV light was investigated. The basis of reaction is photoredox process. Different parameters have important role in this process. Results showed that pH of the solution, type of solvent and the type of ions species can influence on dye degradation. The amount of pH solution changed the surface charge of Tio2 particle. As a result, the adsorption of dye on the surface is altered thus causing a change in the reaction rate. In neutral medium, the adsorption of dye on the surface positively charged of Tio2 is favourite, therefore % degradation of (RB) in this medium was the most amount. Adsorption of (RB) molecules on the surface of Tio2 significantly changed in the presence of more amounts of the ionics in the normal, acidic and basic solutions so was influenced on the photo-bleaching of (RB) dye. Plus, in the normal solutions and in presence of the same amount of different salts, kind of salt was very important. Results indicated that significant decreasing in the initial degradation rate of photo-catalysis, is occurred in the presence of various cations, among them Cu+2 has largest effect but Fe+2 increased photo degradation of (RB). Results indicated that, solvent also can play a significant role in variation of photo- physical properties in solutions, and results indicated that rate of % degradation in water is the highest amount between ethanol and methanol and water.
References
- Deshpande AV,Namdas EB (1996) Efficient lasing action of rhodamine 6G in Nafion membranes. Chem Phys 263: 449-455.
- Haugland RP (2002) Handbook of Fluorescent Probes and Research Products (9th edn) Molecular Probes, Eugene, United States.
- Rauf MA, Hisaindee S (2011) An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 276(3): 13-27.
- Sökmen M, Özkan A (2002) Decolourising textile wastewater with modified titania: the effects of inorganic anions on the photocatalysis. J Photochem Photobiol A 147(1): 77-81.
- Neta P, Huie RE, Ross AB (1988) Rate constants for reactions of inorganic radicals in aqueous solution. J Phys Chem 17(3): 1027-1284.
- Jayson GG, Parsons BJ, Swallow AJ (1973) Swallow, Some simple, highly reactive, inorganic chlorine derivatives in aqueous solution. Their formation using pulses of radiation and their role in the mechanism of the Fricke dosimeter. J Chem 69: 1597-1607.
- Kochany J, Lipczynska-Kochany E (1992) Application of the EPR spin-trapping technique for the investigation of the reactions of carbonate, bicarbonate, and phosphate anions with hydroxyl radicals generated by the photolysis of H2O2. Chemosphere 25(12): 1769-1782.
- Maruthamuthu P, Neta P (1917) Phoshate radicals. Spectra, acid- based equilibria, and reactions with inorganic compounds. J Phys Chem 82(6): 710-713.
- Neta P, Maruthamuthu P, Carton PM, Fessenden RW (1978) Formation and reactivity of the amino radical. J Phys Chem 82: 1875-1878.
- Abdullah M, Low GKC, Matthews RW (1990) Effects of common inorganic anions on rates of photocatalytic oxidation of organic carbon over illuminated titanium dioxide. J Phys Chem 94(17): 6820-6825.
- Chen H, Zahraa O, Bouchy M (1997) Inhibition of the adsorption and photocatalytic degradation of an organic contaminant in an aqueous suspension of TiO2 by inorganic ions. J Photochem Photobiol A Chem 108(1): 37-44.
- Wang K, Hsieh Y, Chou M, Chang C (1999) Photocatalytic degradation of 2-chloro and 2-nitrophenol by titanium dioxide suspensions in aqueous solution. Appl Cata B: Environ 21(1): 1-8.
- Zhang W, An T, Cui M, Sheng G, Fu J (2005) Effects of anions on the photocatalytic and photoelectrocatalytic degradation of reactive dye in a packed-bed reactor. J Chem Technol Biotechnol 80(2): 223-229.
- Zhu X, Nanny MA, Butler EC (2007) Effect of inorganic anions on the titanium dioxide-based photocatalytic oxidation of aqueous ammonia and nitrite. J Photochem Photobiol A: Chem 185(2): 289-294.
- Zhao J, Wu T, Wu K, Oikawa K, Hidaka H, Serpone N (1998) Photoassisted degradation of dye pollutants. 3. Degradation of the cationic dye rhodamine B in aqueous anionic surfactant/TiO2 dispersions under visible light irradiation: evidence for the need of substrate adsorption on TiO2 particles. Environ Sci Technol 32(16): 2394-2400.
- Panneer SP, Preethi S, Basakaralingam P, Thinakaran N, Sivasamy A, Sivanesan S (2007) Removal of rhodamine B from aqueous solution by adsorption onto sodium montmorillonite. J Hazard Mater 155(2): 39-44.
- Zakerhamidi MS, Moghadam M,Ghanadzadeh A, Hosseini S (2012) Anisotropic and isotropic solvent effects on the dipole moment and photophysical properties of rhodamine dyes. Journal of Luminescence 132(4): 931-937.
- Zhao J, Hidaka H, Takamura A, Pelizzetti E, Serpone N (1993) Photodegradation of Surfactants. 11 z-Potential Measurements in the Photocatalytic Oxidation of Surfactants in Aqueous TiO2 Dispersions. Langmuir 9(7): 1646-1650.
- Chen F, Zhao J, Hidaka H (2006) Highly selective deethylation of Rhodamine B: adsorption and photooxidation pathways of the dye on the TiO2/SiO2 composite photocatalyst. Inter Journal of Photoenergy 5: 209-217.
- Konstantinou IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations. Appl Catal B-Environ 49(1): 1-14.
- Merouani S, Hamdaoui O, Saoudi F, Chiha M (2010) Sonochemical degradation of Rhodamine B in aqueous phase: effects of additives. J Eng Chem 158(3): 550–557.
- Wang XK, Wang JG, Guo PQ, Guo WL, Li GL (2007) Chemical effect of swirling jet-induced cavitation: Degradation of Rhodamine B in aqueous solution. Ltrason Sonochem 15(4): 357-363.
- Tang SK, Teng T, Alkarkhi A, Li Z (2012) Sonocatalytic Degradation of Rhodamine B in Aqueous Solution in the Presence of TiO2 Coated Activated CarbonInte. J Environmental Science and Development 3(1): 66-65.
- Aarthi T, Narahari P, Madras G (2007) Photocatalytic degradation of Azure and Sudan dyes using nano TiO2. J Hazard Mater 149(3): 725-734.
- Pera-Titus M, Garcia-Molina V, Baños M, Giménez J, Espluga S, et al. (2004) Degradation of chlorophenols by means of advanced oxidation processes. Appl Catal B 47(4): 219-256.
- Spacek W, Bauer R, Heisler G (1995) Heterogenous and homogeneous wastewater treatment-comparrson between photodegradation with TiO2 and photo fenton reaction. Chemosphere 30(3): 477- 484.
- Bauer R, Waldner G, Fallmann H, Hager S, Klare M, et al. (1999) The photo-Fenton reaction and the TiO2/UV process for wastewater treatment-novel developments. Catal Today 53(1): 131-144.
- Kan Z, Zeda M, Wonchun O (2010) Degradation of Rhodamine B by Fe-Carbon Nanotubes/TiO2 Composites under UV Light in Aerated Solution. Chinese J Catal 31(7): 751-758.
- Aarthi T, Narahari P, Madras G (2007) Photocatalytic degradation of Azure and Sudan dyes using nano TiO2. J Hazard Mater 149(3): 725-734.
- Warman IM, DeHaas MP, Pichat P, Serpone N (1991) Effect of isopropyl alcohol on the surface localization and recombination of conduction-band electrons in Degussa P-25 TiO2. A pulse-radiolysis time-resolved microwave conductivity study. J Phys Chem 95(22): 8858-8861.
- Epling GA, Lin C (2002) Investigation of retardation effects on the titanium dioxide photodegradation system. Chemosphere 46(6): 937-944.
- Zakerhamidi MS, Ghanadzadeh A, Moghadam M (2011) Effect of anisotropic and isotropic solvent on the dipole moment of coumarin dyes Spectrochim. Acta A 78(3): 961-966.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences