ISSN : 2634-7806
Journal of Clinical and Molecular Pathology : Open Access
Reversion-Inducing Cysteine-Rich Protein With Kazal Motifs (RECK) In Angiogenesis Of Microvessels In Glioblastoma
Kohei Kanaya1, Keiichi Sakai1,2,*, Kazuhiro Hongo1, Mana Fukushima3, Masatomo Kawakubo3 and Jun Nakayama3
1Department of Neurosurgery, Shinshu University School of Medicine, 3-1-1 Asahi, Matsumoto, 390-8621, Japan
2Department of Neurosurgery, Shinshu Ueda Medical Center, 1-27-21, Midorigaoka, Ueda, 386-8610, Japan
3Department of Molecular Pathology, Shinshu University Graduate School of Medicine, 3-1-1 Asahi, Matsumoto, 390-8621, Japan
- *Corresponding Author:
- Keiichi Sakai
Department of Neurosurgery
Shinshu University School of Medicine, Japan
Tel: +81-263-37-2690
E-mail: skeiichi@shinshu-u.ac.jp
Received Date: March 19, 2017; Accepted Date: April 20, 2017; Published Date: April 27, 2017
Citation: Sakai K, Kanaya K, Hongo K, Fukushima M, Kawakubo M, et al. (2017) Reversion-Inducing Cysteine-Rich Protein with Kazal Motifs (RECK) in Angiogenesis of Microvessels in Glioblastoma.
Copyright: © 2017 Kanaya K et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Reversion-inducing cysteine-rich protein with Kazal motifs (RECK) may play a role in tumor angiogenesis. Glioblastomas, the most malignant type of primary brain tumor, are highly vascularized. Little is known about the role of RECK in angiogenesis of glioblastomas. The aims of this study were to characterize RECK expression and investigate the role of RECK in glioblastomas.
Methods and Findings: Tumor samples from 50 patients with glioblastoma and brain tissue samples from neurologically normal controls were immunohistochemically stained for RECK, CD34, and vascular endothelial growth factor (VEGF). RECK was expressed in endothelial cells but not tumor cells. A significant correlation between microvessel density (MVD) according to RECK and CD34 was revealed. However, RECK expression was remarkably weak in glomeruloid vessels. MVD according to RECK staining was significantly lower than MVDto CD34 staining in the group with glomeruloid vascular proliferation, which was related with high expression of VEGF.
Conclusions: RECK expression was observed in endothelial cells of microvessels in normal brain and the patients with glioblastoma. However, RECK expression was remarkably downregulated in endothelial cells of glomeruloid vessels. Thus, RECK may act during angiogenesis in glioblastomas, especially in forming simple vessels as microvessels rather than glomeruloid vessels.
Keywords
RECK; Glioblastoma; Angiogenesis; Microvessel density; Microvascular pattern
Introduction
Reversion-inducing cysteine-rich protein with Kazal motifs (RECK) was first identified as a cDNA clone, the encoded protein of which induces morphological reversion (‘flat reversion’) in NIH3T3 cells transformed with the v-K-ras oncogene [1]. In addition, RECK acts as a membrane-anchored metalloproteinase regulator and negatively regulates matrix metalloproteinase family members (e.g., MMP-2, MMP-9, and MT1-MMP), which contribute to tumor growth, invasion, angiogenesis, and metastasis [1-4]. RECK mRNA is expressed ubiquitously in normal human tissues [5], but RECK is down regulated in many types of solid tumors. Down regulation of RECK often correlates with poor prognosis and recurrence of tumors [5-7].
On the other hand, RECK was known as having the function of angiogenesis. Oh et al. reported that embryos of RECK-deficient mice have severe developmental defects in the vascular network [2]. In tumor models in which HT1080 cells and human umbilical vein endothelial cells are transfected with RECK siRNA, the vessel density and tumor weight are significantly lower compared with control tumor models. This result suggested that RECK induction in vascular endothelial cells is required for tumor growth [8]. Positive RECK staining is also seen in endothelial cells of human neuroblastomas and osteosarcomas, indicating the role in tumor angiogenesis [8,9]. Chandana et al. reported that large and irregular dilated vessels are present in Reckinactivated mice, suggesting that Reck may play a role in intussusception and pruning rather than sprouting angiogenesis [10]. Recent study has found that RECK modulates Wnt signaling pathways that acts in endothelial cells during angiogenesis in the central nervous system [11,12].
Glioblastomas are highly vascularized and the most aggressive type of malignant glioma. Despite adequate surgical treatment, radiation, and chemotherapy, tumors often recur, and the prognosis is quite poor. Few reports have examined the role of RECK in gliomas. The RECK expression profile in gliomas is not known. Our study reported that RECK is expressed in endothelial cells in gliomas and that microvessels density (MVD) expressed by RECK is positively correlated with MVD expressed by CD34 and the glioma grade [13]. The aim of this study was to characterize RECK expression in microvascular morphology pattern and investigate the role of RECK in glioblastomas.
Methods
Patients
This study was approved by the local Ethical Committee of Shinshu University School of Medicine under registration number 2604. Formalin-fixed and paraffin-embedded tissue samples from 50 patients with glioblastoma and neurologically normal brain tissues as a normal control from donor bodies.
Immunohistochemistry analysis
A formalin-fixed paraffin-embedded block containing the representative tumor tissue was selected. Four-micron-thick sections were cut for immunohistochemistry, which was performed with antibodies recognizing RECK (R&D Systems, Inc., Minneapolis, MN, USA), CD34 (Beckman Coulter, Inc., Brea, CA, USA), and vascular endothelial growth factor (VEGF) (Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Paraffin-embedded tissue samples were washed free of fixative with Tris-buffered saline (TBS; pH 7.6) and treated with 0.3% H2O2 in TBS for 10 min to inactivate endogenous peroxidases. For CD34, samples were pretreated with 0.01 M citrate buffer for 30 min and microwaved. No antigen retrieval was used before RECK and VEGF immunostaining. The sections were then reacted with primary antibody for 1 hr in a humid chamber at room temperature. Dilutions of primary antibodies were 1:50 for anti- RECK IgG, 1:100 for anti-CD34 IgG, and 1:50 for anti-VEGF IgG. Polyclonal goat IgG antibody, as the secondary antibody for RECK, polyclonal mouse IgG antibody for CD34, and polyclonal rabbit IgG antibody for VEGF were applied to the sections for 30 min. The color was developed with 0.02%, 3,3’ diaminobenzidine tetrahydrochloride (DAB) and 0.006% H2O2 in TBS. The sections were washed three times in TBS and counterstained with Mayer’s hematoxylin.
RECK- and CD34-stained regions were counted to determine MVD, which was obtained by manually counting the positive foci in sections counterstained with hematoxylin. MVD was scored as the number of vessels in the field, and the final score was the average of the three most highly vascularized areas in the highpower field (200×). All areas of the specimens were screened, and we divided the microvascular formations into two types of microvascular patterns (MVP): glomeruloid vascular proliferation (GVP), defined as more than three clustered vessels with connective stroma as described by Chen et al. [14], and no GVP. In addition, paraffin-embedded tissue samples were double-stained to more specifically determine co-expression of RECK and CD34 in endothelial cells. Paraffin-embedded double staining was performed using the EnVisionTM G|2 Doublestain System (Dako Denmark A/S, Glostrup, Denmark) with two different chromogens: DAB+ Chromogen (brown color) for RECK and Permanent Red Chromogen (red color) for CD34. VEGF was evaluated according to both the proportion of stained cells (PS) and the intensity of staining (IS). The PS was graded into four levels: undetectable, PS grade 0; detectable but ≤ 33%, PS grade 1; 34–66%, PS grade 2; and ≥ 67%, PS grade 3. IS was also graded into four levels: undetectable, IS grade 0; weak, IS grade 1; moderate, IS grade 2; and strong, IS grade 3. The VEGF score was obtained by multiplying the IS and PS grades, which was the same way of immunohistochemical evaluation defined by Takeuchi et al. [7].
Statistical analysis
Correlation of MVD according to RECK and MVD according to CD34 was assessed using Spearman’s correlation. The comparison of MVD according to RECK staining, MVD according to CD34 staining, and VEGF was performed using the Student’s t test for each MVP. Probability values of less than 0.05 were considered significant. Xlstat® for Mac (Addinsoft SARL, Paris, Paris, France) was used for the statistical analysis.
Results
Correlation between MVD by RECK and CD34
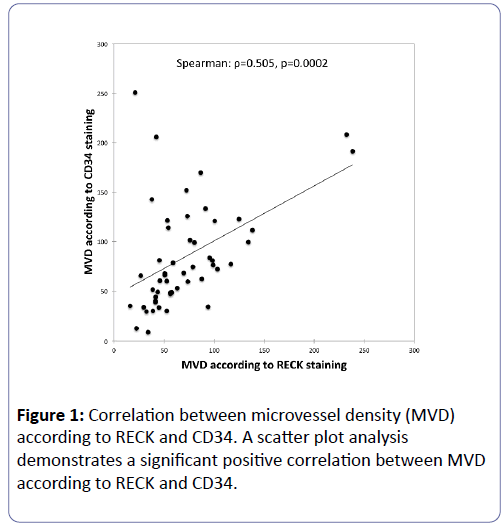
MVD was counted in 50 patients with glioblastoma by RECK and CD34 immunohistochemical staining. Average score of MVD according to RECK was 71.5 (range 16.0-238.3) and average score of MVD according to CD34 was 84.6 (range 9.0–250.7), respectively. A scatter plot analysis demonstrates a significant positive correlation (ρ=0.505, p=0.0002) between MVD according to RECK and CD34 (Figure 1).
Expression pattern of RECK and CD34 in normal brain and glioblastoma
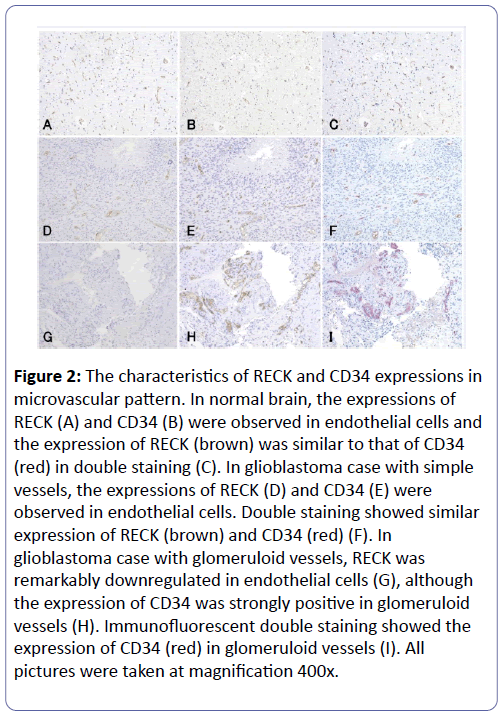
In normal brain, RECK (Figure 2A) and CD34 (Figure 2B) were expressed in endothelial cells, and the expression of RECK was similar to that of CD34 in double staining (Figure 2C). In glioblastoma, GVP was detected in 56% (28/50) glioblastoma cases and 44% (22/50) glioblastoma cases had simple vessels without GVP. RECK was expressed only in endothelial cells, but no expression was seen in tumor cells (Figure 2D and 2G). The expression of RECK (Figure 2D) was similar to that of CD34 (Figure 2E) and double staining disclosed the similar location of the expression between RECK and CD34, which was the same pattern as normal brain (Figure 2F). However, the expression of RECK was remarkably lower in glomeruloid vessels (Figure 2G) compared to vessels labeled with CD34 (Figure 2H) and immunofluorescent double staining revealed the low expression of RECK and high expression of CD34 in the glomeruloid vessels (Figure 2I).
Figure 2: The characteristics of RECK and CD34 expressions in microvascular pattern. In normal brain, the expressions of RECK (A) and CD34 (B) were observed in endothelial cells and the expression of RECK (brown) was similar to that of CD34 (red) in double staining (C). In glioblastoma case with simple vessels, the expressions of RECK (D) and CD34 (E) were observed in endothelial cells. Double staining showed similar expression of RECK (brown) and CD34 (red) (F). In glioblastoma case with glomeruloid vessels, RECK was remarkably downregulated in endothelial cells (G), although the expression of CD34 was strongly positive in glomeruloid vessels (H). Immunofluorescent double staining showed the expression of CD34 (red) in glomeruloid vessels (I). All pictures were taken at magnification 400x.
Comparison of MVD according to RECK staining, MVD according to CD34 staining, and VEGF score in each MVP
MVD according to RECK and CD34, and the VEGF score were evaluated in no GVP group and GVP group (Figure 3).
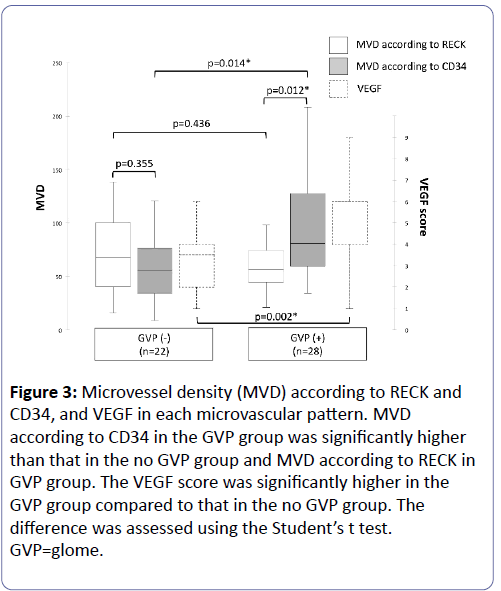
Figure 3: Microvessel density (MVD) according to RECK and CD34, and VEGF in each microvascular pattern. MVD according to CD34 in the GVP group was significantly higher than that in the no GVP group and MVD according to RECK in GVP group. The VEGF score was significantly higher in the GVP group compared to that in the no GVP group. The difference was assessed using the Student’s t test.GVP=glome.
Considering MVPs, we found no significant differences between MVD according to RECK staining and MVD according to CD34 staining in the no GVP group (p=0.355). However, MVD according to CD34 staining was significantly higher than MVD according to RECK staining in the GVP group (p=0.012). The VEGF score was significantly higher in the GVP group compared to that in the no GVP group (p=0.002). MVD according to RECK in the no GVP group was no significant difference with that in the GVP group (p=0.436), although MVD according to CD34 in the GVP group was significantly higher than that in the no GVP group (p=0.014). MVD according to CD34 was higher in the GVP group compared to the no GVP group, however MVD according to RECK had no difference between that in the GVP group and that in the no GVP group, because the expression of RECK in the endothelial cells was significantly decreased in the glomeruloid vessels. These results suggest that glomeruloid vessels supported by VEGF express less RECK in endothelial cells.
Discussion
RECK expression is a good prognostic marker for patient survival in a wide variety of tumors (e.g., peripheral T-cell lymphoma [15], hepatocellular [16], pancreatic [17], gastric [18], breast [19], nasopharyngeal [20], and non-small cell lung cancers [21,22]). Although RECK is expressed in normal human tissues and many tumor samples [1,5], our studies have demonstrated that negative expression of RECK was found in glioblastoma cells, but RECK was expressed in endothelial cells, especially in simple vessels. However, the expression of RECK was remarkably downregulated in glomeruloid vessels. We speculate that vessel fusion progresses and vessels become larger with decreased RECK expression. This speculation is consistent with a study by Chandana et al., which reported the presence of large and dilated vessels were observed in RECKinactivated mice and RECK plays a role in non-sprouting angiogenesis, such as intussusception and pruning [10]. Noda et al. also reported that microvessels are destabilized; permitting lateral fusion and ectopic anastomoses when Reck is absent or reduced [23]. RECK is crucial for angiogenesis and plays an important role in the process of embryonic development and RECK is also implicated in the proliferation of vascular endothelial cells during both physiological and pathological angiogenesis [2,8]. Our results suggest that RECK functions in stabilization of normal brain vasculature and acts during angiogenesis in glioblastomas in forming microvessels. Glomeruloid vessels as advanced vascular stage in relation to VEGF [24] might appear when RECK expression is absent or decreased. In other words, RECK may be necessary to maintain the vessels in small size and to prevent vessel fusion and being large vessels such as glomeruloid vessels.
In summary, RECK expression was found only in endothelial cells especially in microvessels in glioblastomas. The expression of RECK was remarkably decreased in glomeruloid vessels which was related with high expression of VEGF. RECK may act during angiogenesis of microvessel formation; however the mechanism of RECK-mediated angiogenesis remains unclear. Further research examining RECK expression in tumor cells and other cells, especially vascular endothelial cells, is needed, and the research of RECK might help still more to elucidate physiological angiogenesis and pathological angiogenesis in involving cancer development.
Acknowledgements
We would like to thank Ms. Y. Kanai and Ms. Y. Sato for excellent technical support of our laboratory experiments.
Funding
This study was supported in part by the Research Fund for Health Care Promotion Plan in Jyosho Area, Nagano Prefecture, from the Ministry of Health, Labour and Welfare of Japan.
References
- Takahashi C, Sheng Z, Horan TP, Kitayama H, Maki M, et al. (1998) Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK. ProcNatlAcadSci USA 95: 13221-13226.
- Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, et al. (2001) The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell 107: 789-800.
- Miki T, Takegami Y, Okawa K, Muraguchi T, Noda M, et al. (2007) The reversion-inducing cysteine-rich protein with Kazal motifs (RECK) interacts with membrane type 1 matrix metalloproteinase and CD13/aminopeptidase N and modulates their endocytic pathways. J Biol Chem. 282: 12341-12352.
- Omura A, Matsuzaki T, Mio K, Ogura T, Yamamoto M, et al. (2009) RECK forms cowbell-shaped dimers and inhibits matrix metalloproteinase-catalyzed cleavage of fibronectin. J Biol Chem. 284: 3461-3469.
- Noda M, Takahashi C (2007) Recklessness as a hallmark of aggressive cancer. Cancer Sci. 98: 1659-1665.
- Rahmah NN, Sakai K, Nakayama J, Hongo K (2010) Reversion-inducing cysteine-rich protein with kazal motifs and matrix metalloproteinase-9 are prognostic markers in skull base chordomas. Neurosurg Rev. 33: 167-173.
- Takeuchi T, Hisanaga M, Nagao M, Ikeda N, Fujii H, et al. (2004) The membrane-anchored matrix metalloproteinase (MMP) regulator RECK in combination with MMP-9 serves as an informative prognostic indicator for colorectal cancer. Clin Cancer Res. 10: 5572-5579.
- Miki T, Shamma A, Kitajima S, Takegami Y, Noda M, et al. (2010) The β1-integrin-dependent function of RECK in physiologic and tumor angiogenesis. Mol Cancer Res. 8: 665-676.
- Clark JC, Akiyama T, Thomas DM, Labrinidis A, Evdokiou A, et al. (2011) RECK in osteosarcoma: a novel role in tumour vasculature and inhibition of tumorigenesis in an orthotopic model. Cancer 117: 3517-3528.
- Chandana EP, Maeda Y, Ueda A, Kiyonari H, Oshima N, et al. (2010) Involvement of the Reck tumor suppressor protein in maternal and embryonic vascular remodeling in mice. BMC Dev Biol. 10: 84.
- Vanhollebeke B, Stone OA, Bostaille N, Cho C, Zhou Y, et al. (2015) Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/β-catenin pathway during brain angiogenesis. elife 4: e06489.
- Ulrich F, Carretero-Ortega J, Menéndez J, Narvaez C, Sun B, et al. (2016) Reck enables cerebrovascular development by promoting canonical Wnt signaling. Development 143: 147-159.
- Rahmah NN, Sakai K, Sano K, Hongo K (2012) Expression of RECK in endothelial cells of glioma: comparison with CD34 and VEGF expressions. J Neurooncol. 107: 559-564.
- Chen L, Lin ZX, Lin GS, Zhou CF, Chen YP, et al. (2015) Classification of microvascular patterns via cluster analysis reveals their prognostic significance in glioblastoma. Hum Pathol. 46: 120-128.
- Mao X, Liu L, Zhang B, Zhang D (2013) Reversion-inducing cysteine-rich protein with Kazal motifs gene expression and its clinical significance in peripheral T-cell lymphoma. OncolLett. 5: 1867-1871.
- Furumoto K, Arii S, Mori A, Furuyama H, Gorrin Rivas MJ, et al. (2001) RECK gene expression in hepatocellular carcinoma: correlation with invasion-related clinicopathological factors and its clinical significance. Reverse-inducing cysteine-rich protein with Kazal motifs. Hepatology 33: 189-195.
- Masui T, Doi R, Koshiba T, Fujimoto K, Tsuji S, et al. (2003) RECK expression in pancreatic cancer: its correlation with lower invasiveness and better prognosis. Clin Cancer Res. 9: 1779-1784.
- Song SY, Son HJ, Nam E, Rhee JC, Park C (2006) Expression of reversion-inducing-cysteine-rich protein with Kazal motifs (RECK) as a prognostic indicator in gastric cancer. Eur J Cancer 42: 101-108.
- Span PN, Sweep CG, Manders P, Beex LV, Leppert D, et al. (2003) Matrix metalloproteinase inhibitor reversion-inducing cysteine-rich protein with Kazal motifs: a prognostic marker for good clinical outcome in human breast carcinoma. Cancer 97: 2710-2715.
- Zhou DN, Deng YF, Li RH, Yin P, Ye CS (2014) Concurrent alterations of RAGE, RECK, and MMP9 protein expression are relevant to Epstein-Barr virus infection, metastasis, and survival in nasopharyngeal carcinoma. Int J Clin Exp Pathol. 7: 3245-3254.
- Takenaka K, Ishikawa S, Kawano Y, Yanagihara K, Miyahara R, et al. (2004) Expression of a novel matrix metalloproteinase regulator, RECK, and its clinical significance in resected non-small cell lung cancer. Eur J Cancer 40: 1617-1623.
- Takenaka K, Ishikawa S, Yanagihara K, Miyahara R, Hasegawa S, et al. (2005) Prognostic Significance of Reversion-Inducing Cysteine-Rich Protein With Kazal Motifs Expression in Resected Pathologic Stage IIIA N2 Non–Small-Cell Lung Cancer. Ann SurgOncol. 12: 817-824.
- Noda M, Vallon M, Kuo CJ (2007) The Wnt7’s Tale: A story of an orphan who finds her tie to a famous family. Cancer Sci. 98: 1659-1665.
- Bulnes S, Bilbao J, Lafuente JV (2009) Microvascular adaptive changes in experimental endogenous brain gliomas. HistolHistopathol. 24: 693-706.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences