Pneumonia Due to Klebsiella pneumoniae Complicated with Acute Myocarditis after Bone Marrow Transplant
Marcì M1*, Carmina MG1, Raspanti G1, Indovina A2, Tringali S2, Magrin S2,Teisè L3 and Sanfilippo N1
1Cardiology Department, Azienda Ospedaliera Ospedali Riuniti Villa Sofia – Cervello, Palermo, Italy
2Haematology Department, Azienda Ospedaliera Ospedali Riuniti Villa Sofia – Cervello, Palermo, Italy
3Radioloy Department, Azienda Ospedaliera Ospedali Riuniti Villa Sofia – Cervello, Palermo, Italy
- *Corresponding Author:
- Marcì M
Cardiology Department, Azienda Ospedaliera
Ospedali Riuniti Villa Sofia – Cervello, Palermo, Italy
Tel: +39 091 780 8111
E-mail: marcellomarci60@gmail.com
Received date: October 22, 2016; Accepted date: November 08, 2016; Published date: November 14, 2016
Citation: Marcì M, Carmina MG, Raspanti G, et al. Pneumonia Due to Klebsiella Pneumoniae Complicated with Acute Myocarditis after Bone Marrow Transplant. J Clin Med Ther. 2016, 1:1.
Keywords
Myocarditis; Bone marrow transplant; Klebsiella Pneumoniae
Introduction
Klebsiella pneumoniae is a common cause of nosocomial pulmonary infection, particularly in “immuno-compromised” subjects who are hospitalized from severe diseases, such as malignancy, neutropenia, end-stage heart or renal failure [1]. It is estimated that each year this Gram negative pathogen can determine about 8% of infectious disease among patients in western countries hospitals [2].
We report a case of K. pneumoniae induced myocarditis associated to pulmonary infection in a patient receiving bone marrow transplant.
Myocarditis caused by bacterial pathogens is rare in immunocompetent individuals. Moreover, to the best of our knowledge, only few cases of myocarditis due to Klebsiella have been reported in literature so far.
Case Report
A 37-years old man, who had been diagnosed with hemophagocytic lympho-histiocytosis syndrome, on the basis of bone-marrow biopsy, underwent allogenic bone marrow transplant. He had been treated with corticosteroids therapy and before transplant underwent a reduced conditioning regimen with fludarabine, melphalan and anti-thymocyte globuline. Pre-transplant electrocardiogram (ECG) and echocardiogram were normal, blood cell count demonstrated: 3,400,000 red cells, 10,500 white cells (WBC) and 88,000 platelets.
Complete donor chimerism was achieved after bone marrow transplant. With cyclosporine therapy there was not any sign of graft-versus-host disease such as skin rash, vomiting, diarrhea or abdominal pain. After a week the patient was not yet completely immunocompetent (WBC were 1,280 with 82% neutrophils, 7.4% monocytes and only 9% lymphocytes), in addition the IgA and the IgM were normal while the IgG concentration was reduced to 403 mg/dl (751-1560 mg/dl).
About ten days after transplant he developed acute dyspnea at rest, with fever, cough and chest pain. On physical examination the heart rate was 130 beats/min and the blood pressure was 140/80 mmHg. The heart sounds were normal and a 2/6 Levine murmur was audible on the right sternal border; the breath sounds were diminished bilaterally and rales were audible in both lung bases, the peripheral oxygen saturation was 72%. A chest X-ray revealed cardiomegaly, pulmonary congestion and a nodule in the right lower lung field.
ECG showed synusal tachycardia, and new onset of negative T waves in a VL, V1 and V2.
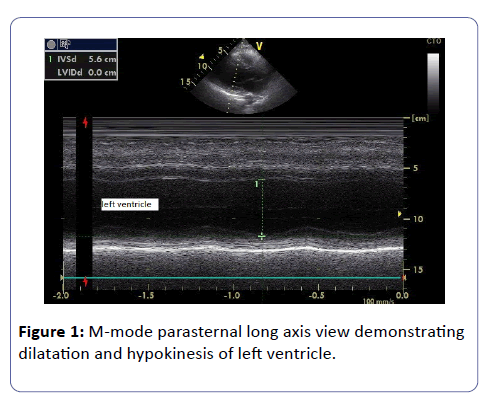
Echocardiogram demonstrated dilatation of left ventricle (end-diastolic diameter was 56 mm and end-diastolic volume was 150ml) with global impaired contraction (ejection fraction=40%); mild mitral regurgitation was also demonstrated by colour Doppler. No pericardial fluid was detected (Figure 1).
Initial troponin T was markedly elevated up to 2,911 ng/L (0.00-40.00 ng/L).
In addition, the initial level of pro-hormone of B natriuretic peptide (BNP) increased to 47,490 pg/mL (0.0-100 pg/mL) and it was reduced to 30,700 pg/mL after a week.
As Klebsiella pneumoniae was detected both in the blood and in the sputum, he was diagnosed with presumed bacterial myocarditis complicating pneumonia and was treated with intravenous Inipenem for 14 days (until haemocoltures became negative) as well as Furosemide and Enalapril 10 mg, with a significant clinical benefit.
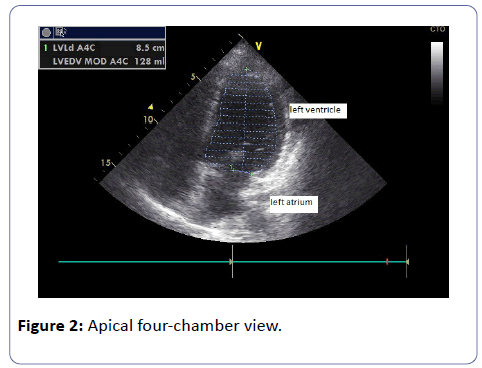
As matter of fact, the ventricular end-diastolic volume was reduced to 128 mL, and ejection fraction was 46%, furthermore the patient did not complain of dyspnea (Figure 2).
Endo-myocardial biopsy (EMB) was deemed too dangerous for presence of thrombocytopenia and leukopenia. Moreover, the therapy probably should not have been modified by the result of biopsy for the good prognosis of this case.
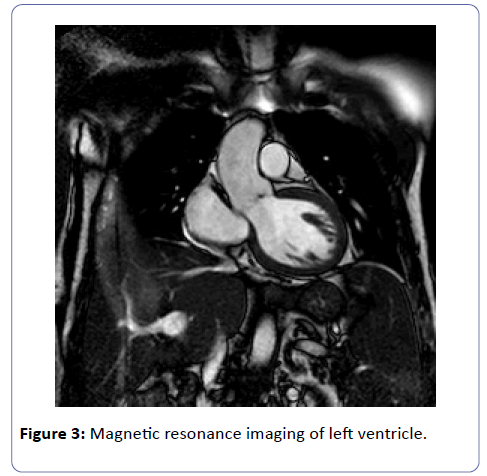
As the priority was to stabilize the patient and to treat the infection, cardiac resonance magnetic imaging (CMRI) was postponed of about three weeks after the onset of myocarditis.
The images confirmed the mild left ventricular dilatation. But CMRI did not show findings consistent with myocarditis such as myocardial hyperaemia and focal edema, nor late gadolinium enhancement (Figure 3).
Follow up echocardiograms demonstrated that left ventricular dimensions became normal and systolic function improved gradually with an ejection fraction of 50% by a month, so diuretics were discontinued. The patient was instructed by cardiologists and nurses to detect early symptoms of heart failure worsening, such as onset of dyspnoea, tachycardia, ankle swelling or weight gain [3].
Discussion
Acute myocarditis is an inflammatory disease of the cardiac muscle that frequently causes heart failure and sometime can evolve to dilated cardiomyopathy. Acute myocarditis can be caused by infections with various pathogens, autoimmune disorders, drugs and toxins. The majority of cases of myocarditis are idiopathic or result from viral infection. Instead, bacterial myocarditis is very rare, with a prevalence ranging from 0.2 to 1.5% [4], and it usually occurs in immuno compromised patients [5].
Generally, it can be a complication of bacteremia discovered at post-mortem examination without significant clinical symptom.
Pathogenesis is largely unknown. A direct bacterial invasion of myocardium from blood has been hypothesized as well as indirect damage by release of cytokines and myocardial depressant substances.
Myocarditis can affect individuals of all ages, although it is most frequent in the young adults. The actual incidence in the general population is hard to determine but it is estimated ranging from 1.06 to 5.0% [6,7].
Myocarditis is a common but challenging diagnosis. In fact clinical manifestations may vary widely, ranging from mild symptoms of chest pain associated with transient ECG anomalies to cardiogenic shock, death or life-threatening arrhythmias.
Even though the diagnostic golden standard is EMB, it has many limitations such as lack of availability, low sensitivity and specificity, not to mention the potential risks.
For these reasons EMB is infrequently performed, especially in those patients with mild forms of suspected acute myocarditis, because they usually do not need a specific treatment and have a favourable prognosis. Recently, EMB has been recommended only for a selected group of critically ill patients with a rapid worsening, that could benefit of specific treatments [4,7].
Recently CRMI has proved to be an alternative, non-invasive diagnostic tool without the risks connected to EMB [4].
The diagnosis of myocarditis can be particularly difficult if it occurs in the context of overwhelming sepsis. In our case the diagnosis was suspected from the new onset of repolarization disorders at ECG associated to heart failure that prompted echocardiographic examination.
Although, we cannot directly demonstrate with EMB that myocarditis was caused by KP infection, the close temporal association between the diagnosis of pneumonia and the onset of cardiac symptoms suggests with every likelihood that pulmonary infection induced the myocarditis probably through a toxic mechanism. Furthermore, it has been demonstrated that tracheal aspirates may be a useful substrate for identification of causative agents by Polymerase Chain Reaction (PCR) analysis in young patients with myocarditis and pneumonitis. In fact all PCR obtained by tracheal aspirate demonstrated identical results to those performed on EMB specimens [8].
A systematic review of literature yielded only four case reports of Klebsiella associated myocarditis so far [9-12].
In conclusion, myocarditis should be a rare but severe complication of Klebsiella pneumonia infections. However, it requires a high grade of suspicion by clinicians in order to recognize early signs and symptoms of this serious complication. Chest pain and ECG changes should prompt to perform echocardiogram and Troponin level measurement in order to diagnose heart inflammation [13].
References
- Podschun R, Ullmann U (1998) Klebsiella spp. As nosocomial pathogens: epidemiology, taxonomy, typing methods and pathogenicity factors. ClinMicrobiolRev 11:589-603.
- Stamm WE, Weinstein RA, Dixon RE (1981) Comparison of endemic and epidemic nosocomial infections. Am J Med 70: 393-397.
- Ciccone MM, Aquilino A, Cortese F, Scicchitano P, Sassara M, et al. (2010) Feasibility and effectiveness of a disease and care system for patients with heart failure and diabetes (Proiect Leonardo). Vasc Health Risk Manag6: 297-305.
- Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, et al. (2013) Current state of knowledge on aetiology, diagnosis, management and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 34: 2636-2648.
- Haddad F, Berry G, Doyle RL, Martineau P, Leung TK, et al. (2007) Active bacterial myocarditis: a case report and review of the literature. J Heart Lung Transplant 26:745-749.
- Calabrese F, Thiene G (2003) Myocarditis and inflammatory cardiomyopathy: microbiological and molecular biological aspects. Cardiovasc Res 60:11-25.
- Feldman AM, McNamara D (2000) Myocarditis. N Engl J Med 343:1388-1398.
- Magnani JW, Dec GW (2006) Myocarditis: current trends in diagnosis and treatment. Circulation 113: 876-890.
- Akhtar N, Ni J, Stromberg D, Rosenthal GL, Bowles NE, et al. (1999) Tracheal aspirate as a substrate for polymerase chain reactiondetection of viral genome in childhood pneumonia and myocarditis. Circulation99:2011-2018.
- Chuang TY, Lin CJ, Lee SW, Chuang CP, Jong YS, et al. (2012) Rapidly fatal community-acquired pneumonia due to Klebsiellapneumoniae complicated with acute myocarditis and accelerated idioventricular rhythm. J Microbiol Immunol Infect45: 321-323.
- Jagadish JS, Mankani HL, Shadaksharappa BK, Donkari VSJ (1987) Acute myocarditis in association with Klebsiella pneumonia. J Assoc Physicians India 35: 652-654.
- Scothorn DJ, Winick NJ, Timmons CF, Aquino VM (2002) Rapidly fatal acute bacterial myocarditis in a non-neutropenic child with acute lymphoblastic leukemia in remission. J PediatrHematolOncol24:662-665.
- Zou Y, Lin L, Xiao H, Xiang D (2016) A Rare Case of Toxic Myocarditis Caused by Bacterial Liver Abscess Mimicking Acute Myocardial Infarction. Am J Case Rep 17: 1-5.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences