Nonylphenol and Bisphenol A in Ambient Particulates: A Preliminary Approach in Italy
Angelo Cecinato*, Paola Romagnoli, Mattia Perilli and Catia Balducci
Institute of Atmospheric Pollution Research, National Research Council of Italy, Italy
- *Corresponding Author:
- Angelo Cecinato
National Research Council of Italy
Institute of Atmospheric Pollution Research (CNRIIA)
Via Salaria km 29.3, P.O. Box 10, I-00015
Monterotondo RM, Italy
Tel: +39-0690672260
Fax: +39-0690672660
E-mail: angelo.cecinato@iia.cnr.it
Received Date: October 16, 2017; Accepted Date: November 14, 2017; Published Date: November 24, 2017
Citation: Cecinato A, Romagnoli P, Perilli M, Balducci C (2017) Non ylphenol and Bisphenol A in Ambie nt Particulates: A Preliminary Approach in Italy. Environ Toxicol Stud J. 1:3.
Abstract
Similarly to other endocrine disruptors, nonylphenol (NOP) and bisphenol-A (BPA) are known to affect waters, soils, foods and biological matrices, and investigations have been performed to know their occurrence and behaviors in the environment. Nevertheless, studies in air remain quite few, and lacking in Italy. NOP and BPA were characterized in airborne particulates from Italy, as well as from Amsterdam, Netherland. Though limited, the study included a big Italian city (Rome) and a rural site (Rende), and in-field experiments were performed in different year seasons. Besides, dust collected indoors in Rome was analyzed. Particulates were extracted through sonication with a dichloromethane: acetone:methanol mixture, reduced close to dryness and fractionated by means of column chromatography on silica gel, from which NOP and BPA were eluted together with polar organic compounds. The dried residue was treated with methyl, tertzbutylsilyl-trifluoroacetamide (MTBSTFA) to form silyl derivatives of analytes, and instrumental analysis was carried out by means of gas chromatography coupled with mass spectrometric detection. NOP and BPA concentrations in the air ranged ~2-17 and <0.01-1.6 ng m-3. In the Rome dusts, NOP and BPA loads were equal to 2.62 and 1.09 μg g-1, respectively. The loads of both NOP and BPA in airborne particulates resulted heavily dependent on year season and site. Compared to ascertained toxicants (e.g., PAHs), in airborne particulates NOP was similar in concentration during the winter, and 3-8 times higher in the summer; meanwhile, in house dust it was less. BPA was all the time less than PAHs. Besides, compared to phthalate esters, i.e. the most important endocrine disruptors, NOP accounted for ca. 3.5-8.5%, and BPA for 0-1.7% (4.1% and 1.7%, respectively, in dust). Hence, the occurrence of NOP and BPA merits to be further investigated to improve knowledge of ambient toxicity in Italy.
Keywords
Nonylphenol (NOP); p,p'-Isopropylidenebisphenol (bisphenol-A or BPA); Airborne particulates; GC-MSD analysis; Methyl, tertzbutylsilyl-trifluoroacetamide (MTBSTFA); Endocrine disruptors (EDs)
Highlights
Nonylphenol (NOP) and bisphenol-A (BPA) were investigated in airborne particulates from Rome, Rende and Amsterdam. After silylation with MTBSTFA, the compounds were determined through GC-MS analysis.
NOPs and BPA were characterized in most of examined samples, and their concentrations depended on the site and time. BPA and NOP burdens in airborne particulates ranged <0.1-1.6 and 2-17 ng m-3, respectively.
NOP and BPA reached concentrations comparable with those of carcinogenic PAHs. NOP and BPA together accounted for up to ~8.5% of total phthalate esters.
Symbols and Abbreviations
bdl: Below Detection Limit; BPA: Bsphenol-A; EDs: Endocrine Disruptors; GC-MS: Gas Chromatography – Mass Spectrometry; i.d: Internal Diameter; MTBSTFA: Methyl, Tertzbutylsilyl-Trifluoroacetamide; m/z: Ion Trace (in mass spectrometry detection) corresponding to mass-to-charge ratio; n.d.: Not Determined; NOP: Nonylphenol; PAH: Polycyclic Aromatic Hydrocarbon; PEs: Phthalic Acid Esters Or Phthalates; TBPA: Tetrabromobisphenol-A.
Introduction
Present in the formulas of a lot of house and industry products, nonylphenol (NOP) and p,p'-isopropylidene bisphenol (known as bisphenol-A or BPA) have been recently subjected to restrictions of use [1-5], owing to their ascertained endocrine disrupting capacity [6-13].
Nonylphenol finds a number of applications as mixture of isomers in lubricating oils, detergents and emulsifiers; it is also used as stabilizer in rubber, and intermediate in the production of ethoxylate surfactants [14], which are back converted to NOPs when released into the environment and biota. BPA enters in the production of polycarbonate plastics and resins (e.g. for dentistry), in particular for beverage bottles, food cans, sport equipment and electronic devices manufacturing; it also acts as anti-oxidant and is used as industrial precursor of flame retardant tetrabromobisphenol-A (TBPA) [12,15,16].
NOP and BPA have been recognized as enough persistent [17,18] to accumulate in biota, household products and foods [19-22], and to fight the human organism [21,23-28]. Therefore, a number of researches have been implemented in water, wastes, soil, foods and biota dealing with the NOP and BPA occurrence under the form of native compounds and by-products [29-37]. Important investigations on BPA and NOP have been conducted with regard in USA, China, Germany, Korea, Belgium and Greece, aimed not only to determine the concentration ranges of both substances in the air and dust, but also to evaluate the gas-to-particulate partition and the daily intake trough ingestion [38-45]; hence, the environmental associated to NOP and BPA has been estimated for adults and children, as well as the contribution of ingestion to total intake. Indeed, both contaminants were investigated in the context of endocrine disruptors, which include phthalate esters (PEs), flame retardants and chlorinated compounds [38,42-44]. Most studies were focused on dusts of internal locations, where the two substances ranged <2-53 μg g-1 (NOP) and <0.05-22 μg g-1 (BPA), heavily depending on country and site type [41,42]. In particular, though not present everywhere, BPA was found to affect all of laboratories and daycare centers investigated [42]. The principal outcome of these studies was the confirm that the NOP and PBA behaviors in the environment seemed to merit further investigations. In fact, though the NOP and BPA related risk was usually calculated as well below the acceptable level established by USEPA, nonetheless owing to the endocrine disrupting capacity of both contaminants and to relatively high concentrations detected in interiors, their monitoring looked of both environmental and sanitary concern [40,41,43]. In particular, just considering plastics and surfactants and hypothesizing small percentages of NOP and BPA reach air and surfaces, nonetheless exposure of humans and biota is expected important for both substances. Besides, the knowledge of the NOP and BPA relationships with other environmental toxicants like polycyclic aromatic hydrocarbons and phthalate esters would help to understand the respective roles with regards to environmental quality of interiors, where concentrations of the two contaminants are presumably much higher and people pass most time; finally, this approach would provide information about the NOP and BPA sources indoors, which may change according to human presence and activity.
Despite their importance, NOP and BPA were not measured in Italy. Thus, this study was aimed at acquiring information about their occurrence and behavior in airborne particulate (PM10) and in dust, the former collected outdoors (both in summer and winter seasons), and the latter indoors. Though very preliminary, this study was conceived as baseline to address future researches in schools, homes and non-industrial locations in our country.
Several analytical procedures were optimized to determine NOP and BPA in various substrates. In general, the two substances were enriched from substrate and cleaned up from interferences by means of solvent extraction (e.g., repeated sonication with organic solvent mixtures), column chromatography or (micro) solid phase extraction [35-37]. After solvent evaporation, instrumental analysis was overall carried out by capillary gas chromatography [33,34,46-48] or high pressure liquid chromatography [37,45- 51], coupled with mass spectrometric detection; other method applied capillary zone electrophoresis [52,53], multi-dimensional GC-MS [54] and pyrolysis GC-MS [55-57]. Instrumental analysis of NOP and BPA was conducted on native compounds or the respective silyl derivatives. Finally, bio-sensors have been developed to measure NOP and BPA in aqueous solutions [56,57].
Experimental
Solvents (n-heptane, n-hexane, dichloromethane, chloroform, acetonitrile, acetone and methanol), all of residue analysis or far UV HPLC grade purity (ROMIL, Waterbeach, Cambridge, UK), were purchased from Delchimica Glassware, Naples, Italy. Standard solutions of NOP and BPA as well as of phthalate esters (analytes: diisobutyl, di-n-butyl, di-2-ethyhexyl, di-n-octyl and diisononyl; internal standards: di-n-propyl and diphenyl), each 1000 μg mL-1 in methanol, were purchased from Chemical Research, Rome, Italy or Superchrom, Cernusco sul Naviglio (MI), Italy, and calibration standard mixtures (0.02 to 2.0 μg mL-1) of analytes were obtained through dilution in chloroform stabilized with amylene. The silylating agent, namely N-methyl,N-(tertbutyldimethylsilyl) trifluoroacetamide MTBSTFA (containing 1% tert-butyldimethylchlorosilane), was purchased from Sigma. Standard mixture solutions of native PAHs (16 priority standards) and deuterated PAHs (phenanthrene-D10, pyrene-D10, chrysene-D12, perylene-D12, dibenz[a,h]anthracene-D14) were provided by Chemical Research.
The analytical procedure was adapted from that optimized for characterizing pharmaceutical compounds, described elsewhere [58]. Sample amounts corresponding to 160-430 m3 of air were examined for our purposes. The samples were spiked with n-heptandioic acid (D7) and diphenyl acid (DPA), chosen as reference compounds for analysis, respectively, for NoP and BPA, and were sonicated in dichloromethane:acetone:met hanol (5:3:2 in volume, three times with 12 mL of mixture, 18 min each). The extracts were filtered through a 0.45 μm PTFE membrane and lead close to dryness under ultra-pure nitrogen. The residue was back dissolved with small aliquots of isooctane (0.25-0.40 mL, four times) and transferred to the top of a silica gel column (1.3 g, i.d. = 6 mm, deactivated with 2.0% of water). Non-polar compounds were eluted first with n-heptane (5 mL), then PAHs and low-polar substances were eluted with n-hexane and dichloromethane in mixture (80:20, 5 mL); finally, 6 mL of the sample extraction mixture (6 mL) allowed collecting highly-polar organics. NOP and BPA, present in the third fraction, were converted into silyl derivatives through reacting with MTBSTFA (at 65°C, 40 min), reduced close to dryness and back dissolved into chloroform. Phthalates passed unaltered the silylation step and were co-eluted with NOP and BPA.
NOP and BPA were analyzed through capillary GC coupled with mass spectrometric detection operated in selected-ion-monitoring mode. A system formed by an Ultra gas chromatograph equipped with a manual cold-on column injector (OCI), and a DSQ Ultra mass spectrometer (both from Thermo, Rodano MI, Italy) and managed through the Xcalibur dedicated software, was adopted for the purpose. Gas chromatographic separation was obtained using a DB-1701 type column (50 m long, 0.32 mm i.d., 0.25 μm thick film, purchased from Agilent J&W, Folsom CA, USA). As for phthalate esters, the GC column and temperature program was the same of NOP and BPA, while MS detection was operated in spectrum scan mode; ion currents corresponding to m/z = 149 and 225, were used, respectively, to quantify di-n-propyl phthalate and diphenyl phthalate, and to m/z = 223, 279, 293, to identify dibutyl, dioctyl and dinonyl congeners, respectively. PAHs were determined using a DB-EUPAH type column was used (20 m x 0.18 mm x 0.14 μm, from Agilent J&W), operating in split-less injection mode (injector temperature=280°C).
All GC runs were performed in temperature gradient, according to the following program: T0=70°C, 1.2 min; + 15°C/min up to T1 =160°C, 2 min; +4°C/min up to T2 =280°C, 20 min; the transfer liner to MSD was set at 280°C. The OCI system was cooled with ultra-pure nitrogen during the first minute of run. In combination with retention times, four ion traces were chosen to identify NOP isomers that showed different mass spectrums, depending on structure of aliphatic moiety of molecule (m/z = 249, 263, 277 and 334), and three traces for BPA (m/z = 441, 249 and 207); finally, two ion traces were chosen for each of D7 (m/z = 331 and 373) and DPA (m/z = 456 and 441). Analyses were conducted in triplicate. Neither solvent nor field blanks showed the presence of the two target compounds; the use of OCI device allowed also minimizing the phthalate blank interference. The analytical procedure was optimized through processing 40-160 mg aliquots of a standard reference material, namely SRM-2585 house dust purchased from NIST, USA. SRM-2585 has been certified for numerous chemicals, including PAHs, polybromodiphenylethers and polychlorobiphenyls [59], though neither NOP nor BPA are in the list. Some SRM-2585 aliquots (ca. 150 mg each) were fortified with suitable amounts of analytes (ca. 30, 70 and 135 ng each) to evaluate the recovery percentages of substances and the occurrence of matrix effects. The examined ambient air PM2.5 or PM10 were from Amsterdam, Netherland, Rome and Rende (Cosenza), Italy (Table 1 for information about times and sites).
| City | No. of sites | site type | sampling period | year season | volume, m3 |
|---|---|---|---|---|---|
| Amsterdam | 4 | residential | 14-21/03/2013 | winter | 164 |
| Rome | 2 | residential | 25/07/-23/08/2010 | summer | 275; 436 |
| Rome | 2 | residential | 16-28/03/2013 | winter | 275; 436 |
| Rende | 2 | semi-rural | 28/06-03/07/2010 | summer | 330 |
| Rende | 2 | semi-rural | 06-11/02/2011 | winter | 330 |
Table 1: Types of air particulate sampling sites and corresponding periods of investigation.
The samples were collected through aspiration onto quartz fiber filters; 24-h samples, each corresponding to ca. 55 m3 (24 m3 in Amsterdam), and gathered into weekly pools.
Dusts were collected in Rome during March-April 2016 at two homes, one of which lived by smokers. Horizontal surfaces, 1.0 m2 each and sited at 1.8-2 m from ground in dining and bed rooms, were pre-cleaned with cotton wads. After 30 days, the settled dust was recovered manually by means of quarts filters and weighted by means of a micro-balance. Finally, dusts were combined to form a composite sample.
Results and Discussion
Instrumental chemical analysis of NOP and BPA
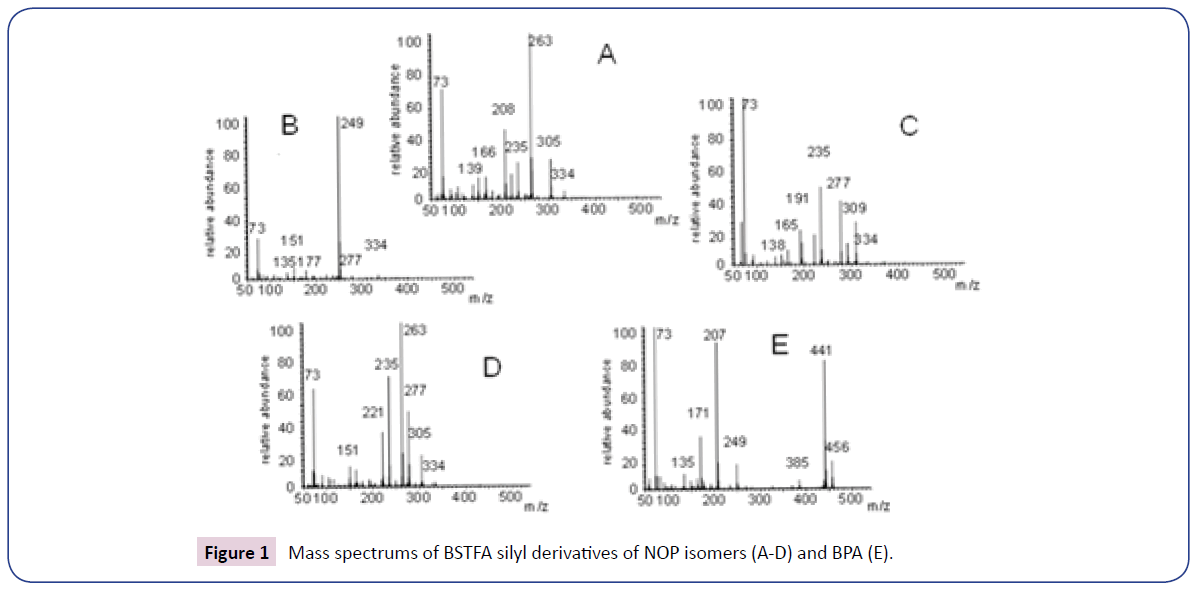
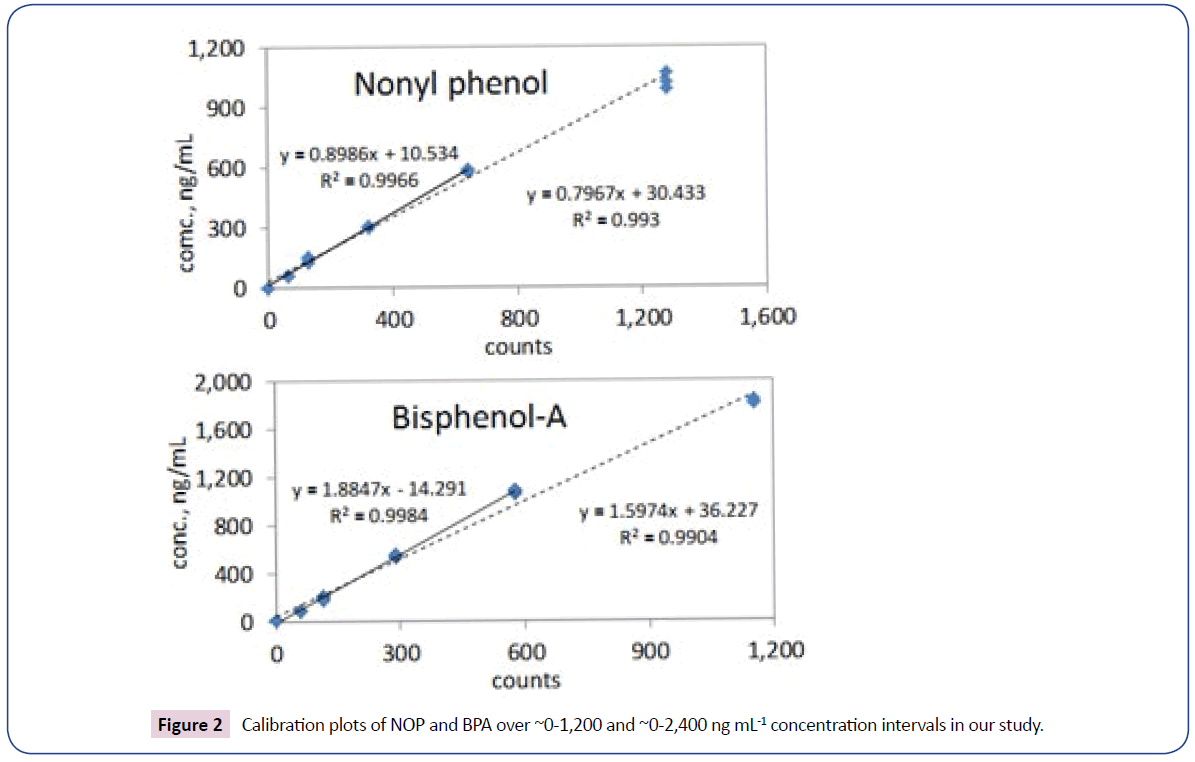
Molecular masses and calibration parameters of NOP and BPA according to the optimized method of analysis are provided in Table 2 and Figure 1. In all cases the signals corresponding to molecular ions were <10% of respective base M/Z traces). The mass spectrums of NOP and BPA MTBSTFA silyl derivatives are provided in Figure 2.
| compound | ret. time, min. |
M(MTBSTFA) | Mquant | LOD, µg mL-1 |
LOQ, µg mL-1 |
|---|---|---|---|---|---|
| nonylphenol | 30.0-33.4 | 334 | 235; 249; 263 | 0.006 | 0.020 |
| bisphenol A | 55.07 | 456 | 441 | 0.003 | 0.010 |
M(MTBSTFA)=molecular mass of MTBSTFA derivative.
Table 2: Retention times, molecular mass of MTBSTFA derivatives, LODs and LOQs of nonylphenol and bisphenol-A in this study.
Table 3 shows the average percent recoveries of NOP and BPA, calculated by processing SRM-2585 house dust. Recovery efficiencies resulted fine (83-109%; on the average, 100 ± 8% and 90 ± 11%, respectively, for NOP and BPA). Analogously, both repeatability and reproducibility were always better than 12.3% (on the average, better than 8% for both substances).
| mg of dust | 37 | 70 | 136 | average | |
|---|---|---|---|---|---|
| % recovery | nonylphenol | 98 ± 8 | 109 ± 6 | 93 ± 7 | 100 ± 8 |
| bisphenol-A | 100 ± 4 | 83 ± 11 | 87 ± 7 | 90 ± 11 | |
| mg of dust | 37 | 70 | 136 | % std. dev. | |
| % repeatability | nonylphenol | 9.9 | 6.6 | 10.6 | 8.0 |
| bisphenol-A | 0.9 | 6.2 | 9.1 | 5.0 | |
| % reproducibility | nonylphenol | 9.0 | 4.8 | 3.6 | 5.8 |
| bisphenol-A | 7.8 | 3.8 | 12.3 | 8.0 |
Table 3: Percent recovery, repeatability and reproducibility rates for nonylphenol and bisphenol-A analytical procedure at three levels of NIST SRM- 2585 spiking (37, 70 and 136 mg of dust).
Fine detector linearity was found within the range 0.02-1.200 μg mL-1, and even better within 0.02-0.60 μg mL-1, i.e. the working range of concentrations detected in the samples (Figure 2). Considering the calculated LOQs, the final volumes of sample solutions (100-250 μL), and air volumes passed to collect PM (from ca. 160 m3 up to 430 m3), in the case of suspended particulates the minimum quantifiable amounts of NOP and BPA were equal to 0.01 and 0.02 ng m-3, respectively.
The particulate and house dust samples were also characterized for the respective contents of polycyclic aromatic hydrocarbons (PAHs) and alkyl phthalates (PEs), according to consolidated methods [58,60]. Congeners with molecular weights ranging from 228 to 278 atomic mass units (benz[a]anthracene to dibenz[a,h] anthracene) were determined for PAHs, and diisobutyl, di-n-butyl, di-2-ethylhexyl, di-n-octyl, diisononyl and di-n-nonyl homologues for phthalates.
NOP and BPA in SRM-2585 house dust and in airborne particulates
Though standardization of SRM-2585 was out of our purposes, this dust was found to contain quantifiable amounts of both NOP mixture (1.22 ± 0.08 μg g-1, dry weight) and BPA (1.83 ± 0.04 μg g-1). A sight comparison with levels detected in biota, sewages and surface waters (≈μg kg-1 or ng L-1) showed that SRM-2585 was relatively rich of the two substances. Worth of note, the NOP and BPA contents in SRM-2585 were similar to that of benzo[a] pyrene (1.140 ± 0.010 μg g-1 [NIST, 2016]), which is internationally adopted as indicator of PM carcinogenicity. Unfortunately, phthalate esters could not be evaluated in this matrix due to contamination problems occurred during analysis.
In the Rome dusts, NOP and BPA loads were equal to 2.62 and 1.09 μg g-1, respectively. Besides, dusts contained 0.75 μg g-1 of benzo[a]pyrene, 20.6 μg g-1 of PAHs, 13.0 μg g-1 of diisobutyl phthalate, 9.4 μg g-1 of di-n-butyl phthalate, 33 μg g-1 of di-2- ethylhexyl phthalate, 0.90 μg g-1 of di-n-octyl phthalate and 7.4 μg g-1 of diisononyl phthalate (total phthalate esters = 63 μg g-1). The concentrations of NOP and BPA in airborne particulates of Amsterdam (Netherland), Rome and Rende (Italy) are reported in Table 4. The corresponding loads of PAHs and PEs are also provided.
| Compound | AMS (N=4) | ROM wi (N=4) | ROM su (N=4) | RND wi (N=2) | RND su (N=2) |
|---|---|---|---|---|---|
| NOP | 2.47 ± 0.32 | 12.0 ± 6.7 | 3.8 ± 0.8 | 7.3 ± 1.6 | 2.04 ± 0.63 |
| BPA | bdl | 2.39 ± 1.28 | 0.40 ± 0.23 | 0.35 ± 0.11 | 0.03 ± 0.03 |
| PEs | 41 ± 5 | 48 ± 14 | 70 ± 23 | 58 ± 7 | 169 ± 34 |
| PAHs | 2.35 ± 1.01 | 7.6 ± 3.8 | 0.48 ± 0.17 | 5.9 ± 2.9 | 0.65 ± 0.49 |
| NOP/PEs*1000 | 60 ± 40 | 250 ± 106 | 54 ± 6 | 126 ± 31 | 12 ± 7 |
| BPA/PEs*1000 | n.d. | 205 ± 43 | 5.6 ± 1.8 | 6.1 ± 1.1 | 0.20 ± 0.14 |
| NOP/PAHs | 1.05 ± 0.22 | 1.58 ± 1.23 | 7.9 ± 3.5 | 1.23 ± 0.39 | 3.1 ± 0.9 |
| BPA/PAHs | n.d. | 0.31 ± 0.09 | 0.83 ± 0.45 | 0.06 ± 0.03 | 0.05 ± 0.03 |
NOP=nonylphenol; BPA=bisphenol-A; PAHs=polycyclic aromatic hydrocarbons; PEs=phthalate esters (sum). AMS=Amsterdam; ROM=Rome; RND=Rende; wi=winter; su=summer; N=sample number; bdl=below detection limit; n.d.=not determined
Table 4: Nonylphenol, bisphenol-A, polycyclic aromatic hydrocarbon and phthalate ester concentrations (ng m-3) in the airborne particulates of Amsterdam (Netherland), Rome and Rende (Italy).
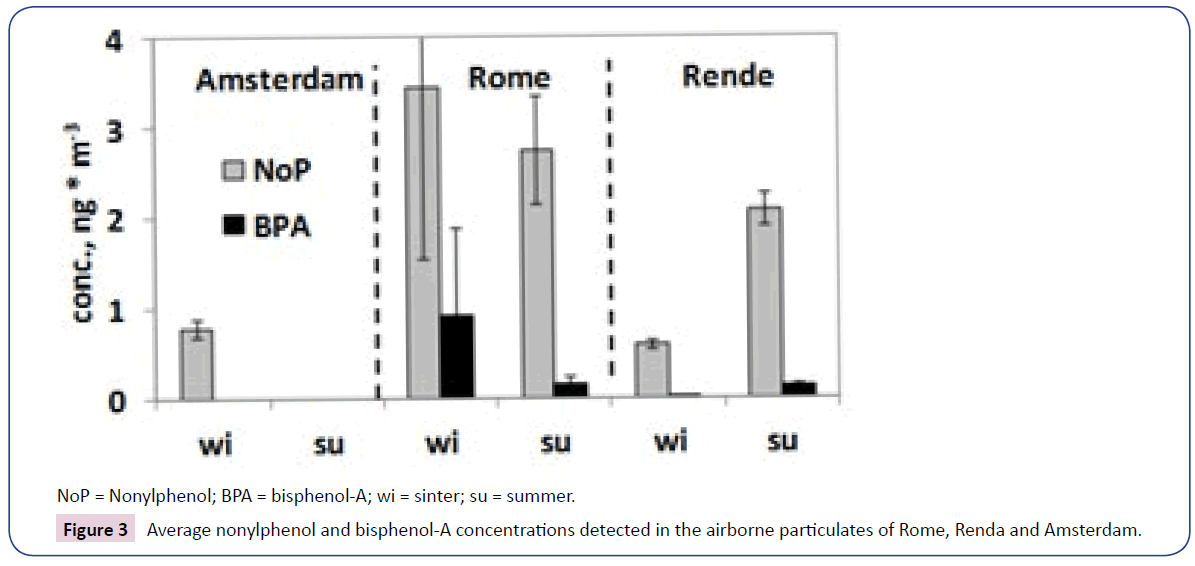
Nonylphenol (~0.6-17 ngm-3) was found in all samples examined, whilst bisphenol-A (affecting all samples from Italy, ca. 0.05- 4.5 ng m-3) was not detected in the Amsterdam particulate. It is worth to remark that NOP and BPA were identified as native compounds, not in the form of metabolites or decomposition products, both in SRM-2585 dust and in airborne particulates. The average concentrations of both substances in Amsterdam were lower than those found during the winter in Rome and Rende. Analogous behavior was followed by PAHs, but not by phthalate esters.
In Rome and Rende, NOP and BPA concentrations were higher in the winter than in the summer (winter vs. summer concentration ratios >1; see Figure 3); this was in accordance with most organic particulate contaminants, due to dispersion and decomposition of compounds favored by meteo-climatic situation occurring in the warm season (high mixing layer height, high temperature and vertical temperature gradients, more sunlight hours and intensity); PAHs showed analogous trends; by contrast, phthalates were more in the summer. That perhaps depended on the prevailing indoor origin of these substances, which are common components of plastics and most household products; meanwhile, the air exchange between interiors and open atmosphere is presumably much more pronounced in the summer.
Quite small NOP and BPA data bases are provided by scientific literature with regard to airborne particulates. Thus, just a short comparison could be attempted with our results. Anyway, Berkner and coworkers [61] reported that NOP and BPA concentrations were as low as <0.01 ng m-3 and ca. 0.02 ng m-3, respectively, in a rural area, reaching 0.03-0.15 and ≈0.01 ng m-3 in Bayreuth, Germany. On the other hand Salapasidou et al. [44], investigating NOP and BPA in Thessaloniki, Greece, found 5.1 ± 4.4 ng m-3 of NOP and 6.8 ± 6.5 ng m-3 of BPA in a residential zone, and 5.0±2.8 ng m-3 of NOP and 13 ± 18 ng m-3 of BPA at a traffic site; the same Authors reported PE concentrations equal to ca. 23 ± 12 ng m-3 and 5.3 ± 2.7 ng m-3, respectively, in the abovementioned city districts. It is worth noting, however, that NOP was found to occur in the atmosphere mainly (ca. 75%) in the gas phase, whilst BPA was overall associated to suspended particulate [44,62]. Indeed, Xie et al. [60] focused their investigation to vapor phase when examined the NOP partition between air and sea water.
On the other hand, much richer data archives are available for the two substances, which refer to characterization in dusts of interiors. Table 5 offers a sight overview of concentrations detected in USA, East Asia and Europe, in homes, offices and laboratories. According to them, the NOP and BPA in Rome dust rates were in agreement with those observed in U.S.A. Europe and Japan, though both falling in the low concentration ranges. A similar pathway was also observed for phthalate esters, taking in account that only particulate fraction were examined in this study; hence, both NOP and BAP seemed to account for ≈0.1-3% with respect to phthalates except for locations highly contaminates by plastic or gum residues. This further confirms the environmental relevance of these endocrine disruptors.
| Country | nonylphenol | bisphenol-A | phthalate esters |
|---|---|---|---|
| USA | 3.3-52.6 | bdl-17.6 | 94-1,100 |
| Japan | 3.1-42.3 | 0.4-21.8 | 225-10,800 |
| China | - | bdl-3.5 | 15-12,100 |
| Korea | - | 1.1-39.1 | - |
| Germany | - | 0.12-1.49 | 22-3,300 |
| Europe (a) | 3.3-13.1 | 4.7-8.3 | ~80-5,500 |
| Rome (this work) | 2.62 ± 0.63 | 1.09 ± 0.48 | 63 ± 28 |
bdl=below detection limit; a: European countries other than Germany.
Table 5: Concentration ranges of nonylphenol and bisphenol-A found in interiors (see Ma et al., and References herein). Data reported as μg g-1.
Conclusions
Both house dust (NIST SRM-2585) and airborne particulates examined in Italy were found as affected by quantifiable rates of nonylphenol and bisphenol-A. The compound concentrations widely varied with the site and year season, with higher values usually recorded in the winter. In comparison to Rome and Rende in Amsterdam, Netherlands, lesser NOP was contemporary found, while BPA was not detected. Meanwhile, PAHs followed a parallel trend and phthalates occurred at more similar extents. The concentration levels of both NOP and BPA in Italy were in agreement with those measured in other cities over the world, characterized by “low” contamination rates. Considering that airborne particulates account for ≈1-100 μg per cubic meter of air, the above amounts broadly correspond to ≈0.1-100 ppm, which usually means much more than in settled dusts. Compared to PAHs, NOP concentrations were all the time equal or higher, while BPA accounted for ~5-80%. On the other hand, NOP concentration could be equal to ~25% of that of phthalates, which are recognized as ubiquitous contaminants reaching high concentration levels all over the world.
This suggests implementing more extensive measurements of NOP and BPA in Italy, both in dusts and airborne particulates, in order to understand the respective behaviors in the environment and estimate the corresponding health risk for populations. Special attention, in particular, seems to be paid to indoor environments, because people spend there over 80% of their life, as well to children, whose intake of toxicants through dust is estimated much higher than that of adults [63].
References
- EC European Commission (2005) Commission Regulation No. 1895/2005 of 18 November 2005 on the restriction of use of certain epoxy derivatives in materials and articles intended to come into contact with foodstuffs, (2005). Official J European Union, L302, 28-32.
- EC European Commission (2011) Commission Regulation (EC) No. 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food, Official J European Union, L12, 1-89.
- USEPA, 2010. Assessing and managing chemicals under TSCA. Nonylphenol (NOP) and Nonylphenol Ethoxylates (NOPEs) Action Plan. Pp: 1-13.
- WHO-UNEP, 2013. State of the science of endocrine disrupting chemicals 2012. In: Bergman, A Heindel JJ, Jobling S, Kidd KA, Zoeller RT. (Eds.), WHO Press, Geneva, Switzerland.
- EFSA (European Food Safety Authority), 2015. Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J 13: 3978.
- Lee PC, Lee W (1996) In vivo estrogenic action of nonylphenol in immature female rats. Bull Environ Contam. Toxicol 57: 341-348.
- Chen MY, Ike M, Fujita M (2002) Acute toxicity, mutagenicity and estrogenicity of bisphenol-A and other bisphenols. Environ Toxicol 17: 80-86.
- Vandenberg LN, Chauhoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, et al. (2010) Urinary, circulating and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 118: 1055-1070.
- Rochester JR (2013) Bisphenol A and human health: a review of literature. Reprod Toxicol 42: 132-155.
- Lassen TH, Frederiksen H, Jensen TK, Petersen JH, Joensen UN, et al. (2014) Urinary bisphenol A levels in young men: association with reproductive hormones and semen quality. Environ Health Perspect 122: 478-484.
- Paulose T, Speroni L, Sonnenschein C, Soto AM (2015) Estrogens in the wrong place at the wrong time: Fetal BPA exposure and mammary cancer. Reprod Toxicol 54: 58-65.
- Huang YQ, Wong CKC, Zheng JS, Bouwman H, Bistáková J, et al. (2012) Bisphenol A (BPA) in China: A review of sources, environmental levels, and potential human health impacts. Environ Int 42: 91-99.
- Jambor T, Tvrdá E, Tušimová E, KováÄÂÂik A, Bistáková J, et al. (2017) In vitro effect of 4-nonylphenol on human chorionic gonadotropin (hCG) stimulated hormone secretion, cell viability and reactive oxygen species generation in mice Leydig cells. Environ Pollut 222: 219-225.
- Soares A, Guieysse B, Jefferson B, Cartmell E, Lester JN (2008) Nonylphenol in the environment: a critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ Int 34: 1033-1049.
- Staples CA, Dome PB, Klecka GM, Oblock ST, Harris LR (1998) A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 36: 2149-2173.
- Zhang C, Li Y, Wang C, Niu L, Cai W (2016) Occurrence of endocrine disrupting compounds in aqueous environment and their bacterial degradation: a review. Crit Rev Environ Sci Technol 46: 1-59.
- Sajiki J (2001) Decomposition of bisphenol-A (BPA) by radical oxygen. Environ Int 27: 315-320.
- Li Y, Duan X, Li X, Zhang D (2013) Photodegradation of nonylphenol by simulated sunlight. Marine Pollut Bull 66: 47-52.
- Guenther K, Heinke V, Thiele B, Kleist E, Hartmut Prast, et al. (2002) Endocrine disrupting nonylphenols are ubiquitous in food. Environ Sci Technol 36: 1676-1680.
- Cao XL, Corriveau J, Popovic S (2009) Levels of bisphenol A in canned soft drink products in Canadian markets. J Agricult Food Chem 57: 1307-1311.
- Niu Y, Zhang J, Duan H, Wu Y, Shao B (2015) Bisphenol A and nonylphenol in foodstuffs: Chinese dietary exposure from the 2007 total diet study and infant health risk from formulas. Food Chem 167: 320-325.
- Česen M, Lambropoulou D, Laimou-Geraniou M, Kosjek T, Blaznik U, et al. (2016) Determination of bisphenols and related compounds in honey and their migration from selected food contact materials. J Agric Food Chem 64: 8866-8875.
- Geens T, Roosens L, Neels H, Covaci A (2009) Assessment of human exposure to Bisphenol-A, triclosan and tetrabromobisphenol-A through indoor dust intake in Belgium. Chemosphere 76: 755-760.
- Ferguson KK, Peterson KE, Lee JM, Mercado-García A, Blank-Goldenberg C, et al. (2014) Prenatal and peripubertal phthalates and bisphenol A in relation to sex hormones and puberty in boys. Reprod Toxicol 47: 70-76.
- Covaci A, Den Hond E, Geens T, Govarts E, Koppen G, et al. (2015) Urinary BPA measurements in children and mothers from six European member states: Overall results and determinants of exposure. Environ Res 141: 77-85.
- Liu X, Miao M, Zhou Z, Gao E, Chen J, et al. (2015) Exposure to bisphenol-A and reproductive hormones among male adults. Environ Toxicol Pharmacol 39: 934-941.
- Wang PW, Chen ML, Huang LW, Wu KY, Huang YF (2015) Prenatal nonylphenol exposure, oxidative and nitrative stress, and birth outcomes: A cohort study in Taiwan. Environ Pollut 207: 145-151.
- Forte M, Di Lorenzo M, Carrizzo A, Valiante S, Vecchione C, et al. (2016) Nonylphenol effects on human prostate non tumorigenic cells. Toxicol pp: 357-358.
- Zgoła-Grześkowiak A, Grześkowiak T, Rydlichowski R, Ã…ÂÂukaszewski Z (2009) Determination of nonylphenol and short-chained nonylphenol ethoxylates in drain water from an agricultural area. Chemosphere 75: 513-518.
- Dong CD, Chen CW, Chen CD (2015) Seasonal and spatial distribution of 4-nonylphenol and 4-tert-octylphenol in the sediment of Kaohsiung Harbor, Taiwan. Chemosphere 134: 588-597.
- Lapworth DJ, Baran N, Stuart ME, Manamsa K, Talbot J (2015) Persistent and emerging micro-organic contaminants in Chalk groundwater of England and France. Environ Pollut 203: 214-225.
- Ömeroğlu S, Murdoch FK, Sanin FD (2015) Investigation of nonylphenol and nonylphenol ethoxylates in sewage sludge samples from a metropolitan wastewater treatment plant in Turkey. Talanta 131: 650-655.
- Zhang H, Bayen S, Kelly BC (2015) Co-extraction and simultaneous determination of multi-class hydrophobic organic contaminants in marine sediments and biota using GC-EI-MS/MS and LC-ESI-MS/MS. Talanta 143: 7-18.
- Czech T, Barco Bonilla N, Gambus F, González R, Marín-Sáez J, et al. (2016) Fast analysis of 4-tertoctylphenol, pentachlorophenol and 4-nonylphenol in river sediments by QuEChERS extraction procedure combined with GC-QqQ-MS/MS. Sci. Total Environ pp: 557-558.
- Gallart-Ayala H, Moyano E, Galceran MT (2011) Analysis of bisphenols in soft drinks by on-line solid phase extraction fast liquid chromatography-tandem mass spectrometry. Analyt Chim Acta 683: 227-233.
- Villar-Navarro M, Ramos-Payán M, Fernández-Torres R, Callejón-Mochón M, Bello-López MA (2013) A novel application of three phase hollow fiber based liquid phase microextraction (HF-LPME) for the HPLC determination of two endocrine disrupting compounds (EDCs), n-octylphenol and n-nonylphenol, in environmental waters. Sci Total Environ 443: 1-6.
- Asimakopoulos AG, Thomaidis NS (2015) Bisphenol A, 4-t-octylphenol, and 4-nonylphenol determination in serum by Hybrid Solid Phase Extraction-Precipitation Technology technique tailored to liquid chromatography-tandem mass spectrometry. J Chromatog B pp: 986-987.
- Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG (2003) Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol 37: 4543-4553.
- Abdallah MA, Harrad S, Covaci A (2008) Hexabromocyclododecanes and tetrabromobisphenol-A in indoor air and dust in Birmingham, U.K: implications for human exposure. Environ Sci Technol 42: 6855-6861.
- Mercier F, Glorennec P, Thomas O, Le Bot B (2011) Organic contamination of settled house dust, A review for exposure assessment purposes. Environ Sci Technol 45: 6716-6727.
- Liao C, Liu F, Guo Y, Moon HB, Nakata H, et al. (2012) Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ Sci Technol 46: 9138−9145.
- Ma WL, Subedi B, Kannan K (2014) The occurrence of bisphenol A, phthalates, parabens and other environmental phenolic compounds in house dust: A review. Curr Org Chem 18: 2182−2199.
- Mitro SD, Dodson RE, Singla V, Adamkiewicz G, Angelo FE, et al. (2016) Consumer product chemicals in indoor dust: A quantitative meta-analysis of U.S. studies. Environ Sci Technol 50: 10661-10672.
- Salapasidou M, Samara C, Voutsa D (2011) Endocrine disrupting compounds in the atmosphere of the urban area of Thessaloniki, Greece. Atmos Environ 45: 3720-3729.
- Loganathan SN, Kannan K (2011) Occurrence of bosphenol A in indoor dust from two locations in the Eastern United States and implications for human exposures. Ach Environ Contam Toxicol 61: 68-73.
- Eganhouse RP, Pontolillo J, Gaines RB, Frysinger GS (2009) Isomer-specific determination of 4-nonylphenols using comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. Environ Sci Technol 43: 9306-9313.
- Wu ZY, Zeng ZD, Marriott PJ (2010) Comparative qualitative analysis of nonylphenol isomers by gas chromatography-mass spectrometry combined with chemometric resolution. J Chromatog A 1217: 7759-7766.
- Zhang J, Cooke GM, Curran IHA, Goodyer CG, Cao XL (2011) GC-MS analysis of bisphenol A in human placental and fetal liver samples. J Chromatog B 879: 209-214.
- Luo L, Yang Y, Wang O, Li HP (2017) Determination of 4-n-octylphenol, 4-n-nonylphenol and bisphenol A in fish samples from lake and rivers within Hunan Province, China Microchem J 132: 100-106.
- Fountoulakis M, Drillia P, Pakou C, Kampioti A (2005) Analysis of nonylphenol and nonylphenol ethoxylates in sewage sludge by high performance liquid chromatography following microwave-assisted extraction. J Chromatog A 1089: 45-51.
- Xiao Q, Li Y, Ouyang H, Xu P, Wu D (2006) High-performance liquid chromatographic analysis of bisphenol A and 4-nonylphenol in serum, liver and testis tissues after oral administration to rats and its application to toxicokinetic study. J Chromatog B 83: 322-329.
- Alenazi NA, Manthorpe JM, Lai EPC (2015) Selective extraction of BPA in milk analysis by capillary electrophoresis using a chemically modified molecularly imprinted polymer. Food Control 50: 778-783.
- Sánchez-López E, Montealegre C, Crego AL, Marina ML (2015) Recent contributions of capillary electrophoresis to neuroscience. TrAC Trends Anal Chem 67: 82-99.
- Cunha SC, Fernandes JO (2010) Quantification of free and total bisphenol A and bisphenol B in human urine by dispersive liquid-liquid microextraction (DLLME) and heart-cutting multidimensional gas chromatography-mass spectrometry (MD-GC/MS). Talanta 83: 117-125.
- Becerra V, Odermatt J (2012) Detection and quantification of traces of bisphenol A and bisphenol S in paper samples using analytical pyrolysis-GC/MS. Analyst 137: 2250-2259.
- Belkhamssa N, daCosta JP, Justino COL, Santos PSM, Cardoso S, et al. (2016) Development of an electrochemical biosensor for alkylphenol. Talanta 158: 30-34.
- Ragavan KV, Rastogi NK, Thakur MS (2015) Sensors and biosensors for analysis of bisphenol-A. Trend Analyt Chem 52: 248-260.
- Cecinato A, Balducci B, Mastroianni D, Perilli M (2012) Sampling and analytical methods for assessing the levels of organic pollutants in the atmosphere: PAH, phthalates and psychotropic substances: a short review. Environ Sci Pollut Res 19: 1915-1926.
- NIST (2016) National Institute of Standards and Technology, USA.
- Balducci C, Perilli M, Romagnoli P, Cecinato A (2012) New developments on emerging organic pollutants in the atmosphere. Environ Sci Pollut Res 19: 1875-1884.
- Berkner S, Streck G, Herrmann R (2004) Development and validation of a method for determination of trace levels of alkylphenols ans bisphenol A in atmospheric samples. Chemosphere 54: 575-584.
- Xie Z, Lakaschus S, Ebinghaus R, Caba A, Ruck W (2006) Atmospheric concentrations and air-sea exchanges of octylphenol and nonylphenol monoethoxylate in the North Sea. Environ Pollut 142: 170-180.
- USEPA (2008) National Center for Environmental Assessment, Office of Research and Development. Child specific exposure factors handbook. USA.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences