Impact of Preoperative Oral Acetaminophen Administration on Postoperative Opioid Consumption and Complications in Laparoscopic Sleeve Gastrectomy: A Pilot Study
Emad Alsarraf1*, Sarah Culbreth1, Scott A Shikora2, Darin J Correll3, Kristin L Schreiber3 and Marjan Sadegh1
1Department of Pharmacy, Brigham and Women’s Hospital, 75 Francis St, Boston, MA, USA
2Department of Surgery, Brigham and Women’s Hospital, 75 Francis St, Boston, MA, USA
32Department of Surgery, Brigham and Women’s Hospital, 75 Francis St, Boston, MA, USA
*Corresponding Author:
Emad Alsarraf
Department of Pharmacy
Brigham and Women’s Hospital
75 Francis St, Boston, MA
USA
Tel: +1- (617)-732-7153
E-mail: ealsarraf@partners.org
Received Date: 13 January 2020; Accepted Date: February 06, 2020; Published Date: February 13, 2020
Citation: Alsarraf E, Culbreth S, Shikora SA, Correll DJ, Schreiber KL, et al. (2020) Impact of Preoperative Oral Acetaminophen Administration on Postoperative Opioid Consumption and Complications in Laparoscopic Sleeve Gastrectomy: A Pilot Study. J Pharma Prac Edu Vol.3 No.1. DOI: 10.36648/pharmacy-practice.3.1.21
Copyright: © 2020 Alsarraf E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Opioids are a mainstay for postoperative pain following laparoscopic sleeve gastrectomy (SG). Though effective, opioids are not without risk of adverse events. Preoperative analgesia used to reduce opioid consumption includes intravenous acetaminophen (APAP).
Objectives: This study aimed to evaluate the impact of preoperative oral APAP on postoperative opioid use and complications.
Setting: Educational hospital in the United States of America (USA)
Methods: This was a retrospective study comparing patients who received 975 mg oral APAP preoperatively (APAP group) to a historical control (C group) that did not receive preoperative APAP. The primary outcome was hydromorphone equivalents during the first 24 hours after SG. 50 patients were needed to detect a 20% (0.4 mg) difference in the primary outcome. Secondary outcomes included postoperative pain scores.
Results: A total of 53 patients in the C group and 43 patients in APAP group were evaluated. No significant difference in opioid use was observed between the groups. Similarly, no difference in the pain score was observed. However, a significant time x gender x treatment interaction was observed, such that APAP treated men reported lower pain scores than control in the first 2 hours after surgery.
Conclusion: Although no significant group differences were observed for overall opioid use or pain scores in the first 24 hours after sleeve gastrectomy, secondary analyses suggesting that oral APAP may be more effective in men than women at early postoperative time points may warrant further controlled studies to confirm this finding.
Keywords
Acetaminophen; Opioid analgesics; Bariatric surgery; Postoperative pain
Background
Over the past decade, the utilization of weight-lowering bariatric surgeries in the U.S. has doubled owing to its ability to achieve meaningful and sustainable weight loss and pronounced effects on obesity-related comorbidities [1]. Notably, laparoscopic sleeve gastrectomy (SG) now accounts for approximately 80% of all procedures in the U.S, due to its design simplicity, accelerated rate of hospital recovery, and reduced incidence of postoperative complications [2,3].
Despite years of advances in pain management, opioids are still the mainstay of postoperative pain therapy in most general surgery procedures including Sleeve Gastrectomy [4,5]. There is currently an attempt to utilize strategies to limit postoperative opioid consumption and opioid-related complications. Namely, postoperative opioid consumption has been shown to reduce mobilization, increase the risk of respiratory depression, obstructive sleep apnea, especially, in patients with obesity, and deep vein thrombosis resulting in an increased total cost for the hospital admission [6,7]. Opioid use could also contribute to the high incidence of post-operative nausea and vomiting (PONV), which is one of the most common and problematic complications after bariatric surgery [8].
Multimodal analgesia has emerged as a key element in perioperative pain management as part of enhanced recovery after surgery (ERAS) or “fast-track” protocols [1]. It is believed that optimizing ERAS elements would allow opioid-sparing and reduced opioid-related complications. A common element of ERAS is Acetaminophen (APAP), which is a nonopioid analgesic whose mechanism of action is not fully understood. Its pharmacodynamic effect likely involves central analgesic mechanisms, requiring a sufficient level in the cerebral spinal fluid (CSF) [9]. In addition to its high CNS bioavailability, APAP has a favorable safety profile, making it an attractive analgesic. When used preoperatively in bariatric surgeries, decreased pain scores, lower postoperative opioid use, and reduced incidence of vomiting have been reported with intravenous (IV) APAP [10]. Several multimodal analgesia protocols, at various phases of care, have described enhanced analgesia, reduced opioid use, and reduced opioid complications from greater use of nonopioid analgesics, including APAP, as well as local anesthetic infiltration, in SG [11-13]. Anecdotal evidence of the efficacy of using preoperative oral APAP dose at our own institution suggested a benefit for postoperative analgesia. APAP administration has shown to be bioequivalent to the IV APAP formulation, reaching 89% of levels seen after IV APAP (assuming 1,000 mg dose) [14]. Given this evidence for the relative equivalence of routes of administration, and because IV APAP was not in our institution formulary, preoperative oral APAP was added to our institutional SG protocol. The purpose of this study was to pragmatically and systematically evaluate the impact of the addition of preoperative oral APAP administration on postoperative opioid consumption and pain after SG.
Methods and Materials
Study setting and patient selection
This was a retrospective, single center, cohort study of patients undergoing SG between October 2016 and March 2018 at our institution. Institutional Review Board approval was obtained. The study compared outcomes between patients who underwent SG from October 2016 to April 2017 without preoperative APAP to those who underwent SG from October 2017 to March 2018 and received one dose of 975 mg oral APAP preoperatively. Patients were excluded, if they had a history of severe liver disease (Child-Pugh class B or C), elevated liver enzymes (more than 3 times upper limits of normal), used opioids chronically, or had already had a dose of an analgesic (i.e., APAP, nonsteroidal anti-inflammatory drugs, or COX-2 inhibitors) within 12 hours prior to admission.
Study intervention, surgical technique and standard medications
Patients in the APAP group received 975 mg orally (pharmaceutical associates INC, Greenville SC) either as oral tablets (3 units each containing 325 mg) or oral solution (3 units each containing 325 mg per 5 ml). This study included patients who were diagnosed with morbid obesity and undergoing SG by one of three bariatric surgeons. Full surgical details are outlined elsewhere [15]. Briefly, all procedures were performed laparoscopically, with pneumoperitoneum pressure of 18 mmHg. Staple lines were reinforced with suture or buttressing material and the 15-mm trocar site was closed at the muscle fascia with a 1.0 absorbable suture. All skin incisions were closed with running subcuticular absorbable suture. Of note, port sites were infiltrated with local anesthetic at the conclusion of surgery. The full list of administered preoperative, intraoperative, and postoperative medications is provided in appendix A. In the intraoperative phase, the patient ’ s anesthesiologist determined the type and dose of anesthetic and analgesic agents. In the postoperative phase, the patient’s opioid analgesics were administered as either IV Patient- Controlled Analgesia (PCA) or as intermittent nurseadministered IV boluses (non-PCA) as directed in the physician orders. Antiemetics were also available on a PRN basis.
Data collection and outcomes
Baseline demographics and intraoperative medications were recorded for all patients. Postoperative data were collected throughout the 24-hour period after SG which included opioid analgesics (PCA or non-PCA), non-opioids analgesics, naloxone rescue, antiemetics, pain scores, as well as postoperative recovery unit (PACU) and hospital lengths of stay. The primary outcome of the study was the amount of IV postoperative opioid administration converted to hydromorphone equivalents (HEq in milligrams), collected from the medication administration record during three epochs: 0-8 hours, 8-16 hours, and 18-24 hours after surgery. Secondary outcomes included the average postoperative pain severity scores (numerical rating scale 0 to 10) averaged from multiple scores recorded in the medical record during the first 2-hour post-surgery, from 2-hour to midnight on the day of surgery, and on the 1st postoperative day, naloxone rescue use (%), and total length of stay in hospital and PACU (in hours).
Statistical analysis
Sample size was calculated based on the amount of 24 hours post op opioid consumption in a sample group of patients undergoing this procedure (mean 2.0 mg, SD 0.6) in hydromorphone equivalents (HEq in milligrams). We considered that a 20% decrease in opioid use during this period would be clinically meaningful (decrease by 0.4 mg). Setting power at 0.9 and alpha at 0.05, approximately 45 patients per group were estimated to allow observation of this difference between groups. Groups were compared using Student’s t-test or Mann- Whitney U-test, as appropriate, for continuous data, and Chisquare (X2) for categorical data. Spearman and Pearson correlations were calculated for associations between age and outcome variables. Repeated measures ANOVAs were used to evaluate the effect of treatment and gender on opioid use and pain, with preoperative pain included as a covariate. SPSS (IBM Corp, version 25.0) was used for all analyses and p<0.05 was considered statistically significant.
Results
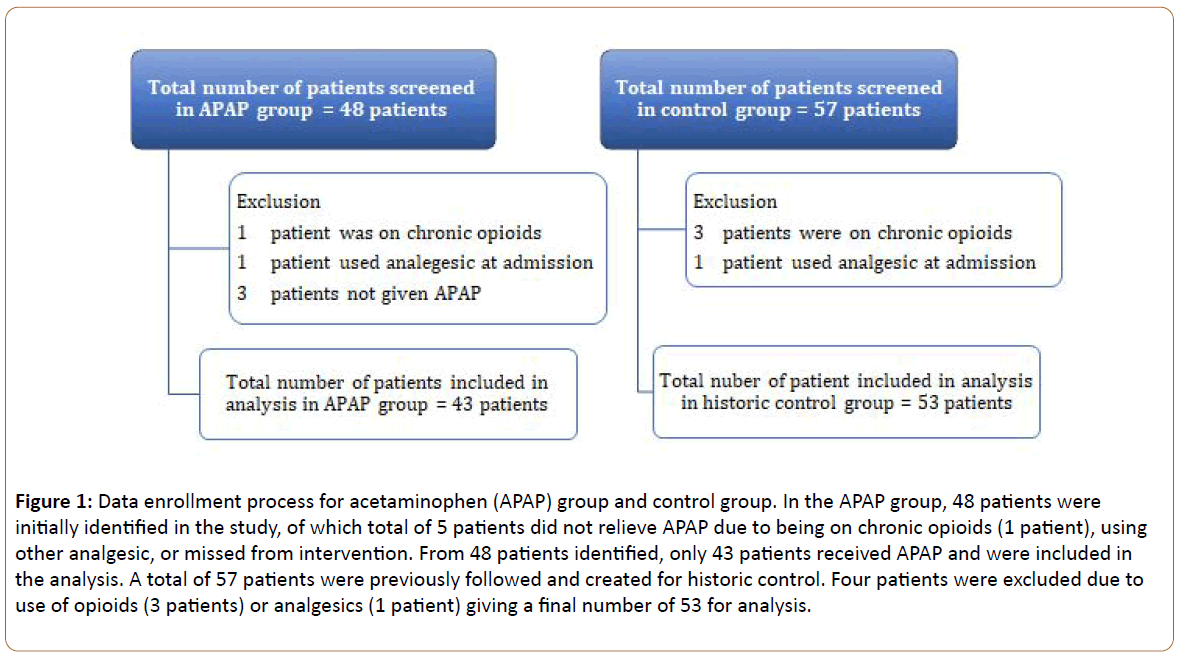
We screened 57 patients for the control group and 48 for the APAP group. After exclusion, a total of 53 patients in the control group and 43 patients were included in the APAP group (Figure 1). Table 1 shows the baseline demographics and intraoperative data. No differences were observed in baseline demographics, with the exception of higher baseline pain score in the APAP group. In the APAP group, 32% received acetaminophen oral solution and the remainder received tablets. The median administration time was 61 minutes (IQR 42-83) prior to the procedure. Only 4 (9%) patients received their dose later than 30 minutes before the procedure and none received their dose later than 15 minutes before the procedure, thus allowing for adequate absorption of drug.
| Baseline demographics | APAP (N=43) | Control (N=53) | p |
|---|---|---|---|
| Age, mean years (±SD) | 46 (10) | 43 (12) | 0.25 |
| Males, n (%) | 12 (28) | 7 (13) | 0.12 |
| BMI, mean kg/m2 (±SD) | 46 (10) | 45 (9) | 0.37 |
| Weight, mean kg (±SD) | 132 (33) | 124 (27) | 0.43 |
| Surgeries by surgeon 1, n (%) | 31 (72) | 43 (81) | 0.23 |
| Surgeries by surgeon 2, n (%) | 6 (14) | 3 (6) | 0.59 |
| Surgeries by all other surgeons, n (%) | 5 (12) | 7 (13) | 0.83 |
| Intraoperative data | |||
| Operation time, mean minutes (±SD) | 74 (28.5) | 68.8 (22) | 0.35 |
| HEq, median mg (IQR) | 2.5 (1.5-3.0) | 2.3 (1.9-2.5) | 0.72 |
| Ketorolac, n (%) | 27 (63) | 33 (62) | 0.45 |
| Midazolam, n (%) | 31 (72) | 42 (79) | 0.25 |
| Dexmedetomidine, n (%) | 40 (93) | 44 (83) | <0.01 |
| Ondansetron, n (%) | 40 (93) | 45 (85) | 0.22 |
| Dexamethasone, n (%) | 35 (81) | 40 (75) | 0.43 |
HEq: Hydromorphone Equivalent, N: group number, n: number/incidence, SD: Standard Deviation, IQR: Interquartile range
Table 1: Analysis is represented in the table.
Figure 1: Data enrollment process for acetaminophen (APAP) group and control group. In the APAP group, 48 patients were initially identified in the study, of which total of 5 patients did not relieve APAP due to being on chronic opioids (1 patient), using other analgesic, or missed from intervention. From 48 patients identified, only 43 patients received APAP and were included in the analysis. A total of 57 patients were previously followed and created for historic control. Four patients were excluded due to use of opioids (3 patients) or analgesics (1 patient) giving a final number of 53 for analysis.
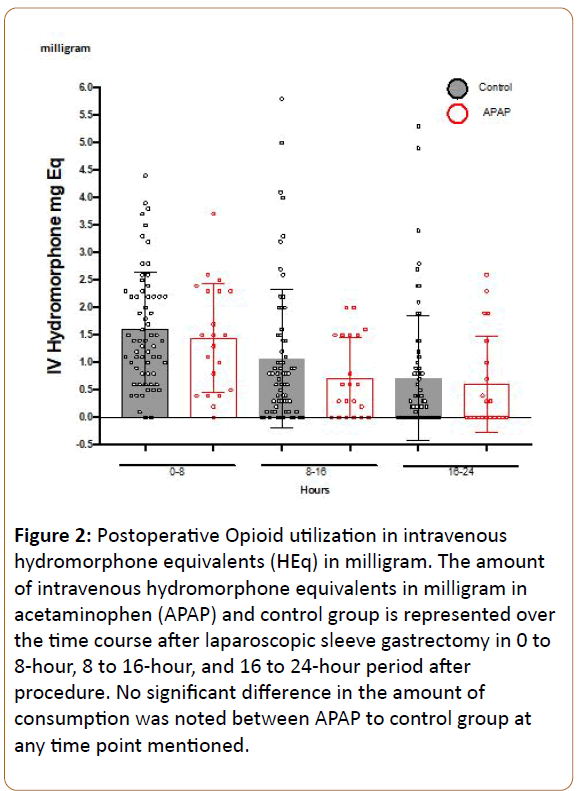
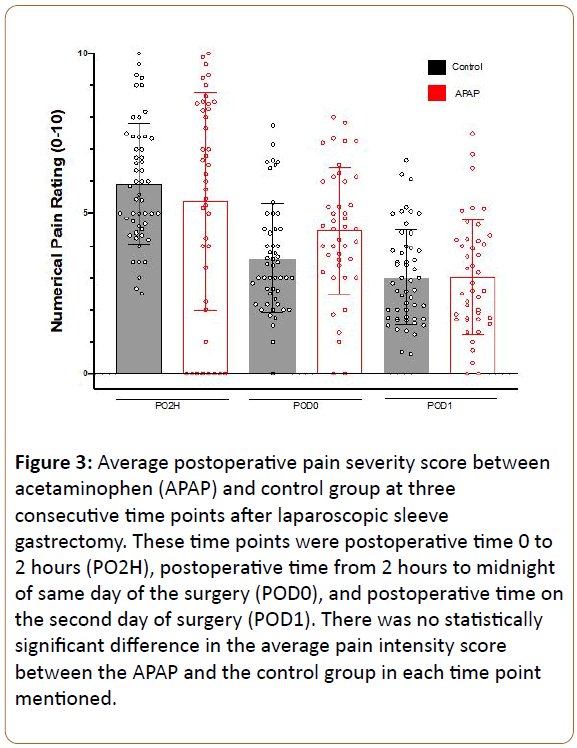
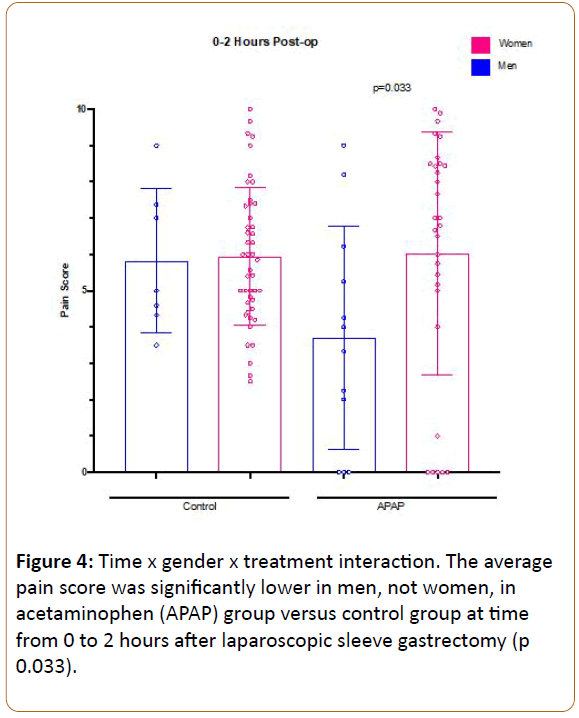
In order to assess for group differences, we employed repeated measures ANOVA, with preoperative pain as a covariate, in order to control for the small differences seen between groups. We investigated opioid use from 0-8, 8-16, and 16-24 hours as the outcome, revealing a significant main effect of time, with opioid use decreasing over time (F:4.85, df(2), p 0.01), but no difference was observed between patients receiving APAP vs. control overall (F:2.09, df(1), p 0.155), even when including preoperative pain score as a covariate (Figure 2). We also investigated average reported pain scores during PACU stay (period 0-2 hours), POD 0 (period 2 hours after surgerymidnight), and POD1 (period midnight-midnight on day after surgery). Repeated measures ANOVA revealed a significant main effect of time, with pain decreasing over time (F:24.8, df(2), p<0.001), but no main effect of group was seen across the timepoints (F:0.84, df(1), p 0.773) (Figure 3), and no main effect of gender of pain was observed (F:0.97, df(1), p 0.33). However, a significant time by gender by treatment interaction was observed (F:5.3, df(2), p 0.007), such that lower reported pain scores were observed amongst male patients receiving APAP compared to control males early postoperative period (0-2 hours after surgery), with no such differences observed between female groups (Figure 4). This may suggest that APAP was effective at reducing pain scores in men, but not women, during this early time period. Post hoc Mann-Whitney U-test comparing men and women receiving APAP confirmed a significantly lower pain score among men (p 0.033).
Figure 2: Postoperative Opioid utilization in intravenous hydromorphone equivalents (HEq) in milligram. The amount of intravenous hydromorphone equivalents in milligram in acetaminophen (APAP) and control group is represented over the time course after laparoscopic sleeve gastrectomy in 0 to 8-hour, 8 to 16-hour, and 16 to 24-hour period after procedure. No significant difference in the amount of consumption was noted between APAP to control group at any time point mentioned.
Figure 3: Average postoperative pain severity score between acetaminophen (APAP) and control group at three consecutive time points after laparoscopic sleeve gastrectomy. These time points were postoperative time 0 to 2 hours (PO2H), postoperative time from 2 hours to midnight of same day of the surgery (POD0), and postoperative time on the second day of surgery (POD1). There was no statistically significant difference in the average pain intensity score between the APAP and the control group in each time point mentioned.
Figure 4: Time x gender x treatment interaction. The average pain score was significantly lower in men, not women, in acetaminophen (APAP) group versus control group at time from 0 to 2 hours after laparoscopic sleeve gastrectomy (p 0.033).
Patient age was inversely correlated with pain scores (Pearson Rho: -0.233, p 0.023) and opioid use (Pearson Rho: -0.329, p 0.022) across groups, similar to previous studies showing that younger patients report more severe pain and consume more opioids postoperatively. More patients in the APAP group also received postoperative APAP (about 90%) than in the control group (60%, p<0.01). Postoperative ketorolac use was similar between groups (84% in APAP group vs. 81% in the control group (p 0.23). Comparison of PACU and hospital lengths of stay indicated that the APAP group had slightly longer PACU length of stay (8.9 hours IQR 7.3-10.0 vs. 7.9 hours IQR 6.3-9.0; p 0.02), but shorter overall hospital stay (52.6 hours IQR 36.2-55.7 vs. 54.4 IQR 51.3-57.3; p 0.03). None of the patients in either group received naloxone rescue.
Discussion
This pilot study was performed to make use of a step-wise addition of a non-opioid analgesic (oral APAP) that occurred as part of a larger plan for comprehensive implementation of a bariatric ERAS protocol for SG at our institution. To our knowledge, this is the first pragmatic clinical study to assess the impact of a dose of preoperative oral APAP on postoperative pain after SG, despite the fact that oral APAP is commonly and increasingly used as an inexpensive piece of a multimodal analgesic strategy. Although no significant differences were observed overall for opioid use or reported pain scores in the first 24-hour period after surgery, secondary analyses investigating differences in effect by gender and time period suggested that oral APAP may be effective in men, but not women, in the very early postoperative period (0-2 hours). This early postoperative effect is consistent with previously reported time to reach maximum concertation (Tmax) of APAP in CSF at a median of 4 hours following oral administration [14]. Further controlled studies are needed to confirm this finding and clarify the difference in response between genders.
Adding APAP as a preoperative analgesic is theorized to reduce opioid use and associated complications. ERAS protocols include optimization of standardized nonopioid analgesics and anesthetics, counselling, nutrition, and early mobilization [11,12]. Multimodal preemptive analgesia has been utilized for a variety of surgical procedures. Based on a strong incentive amongst bariatric surgeons to decrease postoperative opioid use, due to the relatively higher risk of opioid side effects in this population, we examined effectiveness of preemptive APAP on post SG opioid use and pain reduction. We chose to implement the use of oral as opposed to IV formulation of APAP, given its availability, cost, and optimal bioequivalent profile. Since the onset of oral APAP is within 30 minutes, and over 95% of patients in this study received the dose of APAP within the absorption window, we may assume that optimal therapeutic equivalency was achieved. Although there was a trend toward lower opioid use during all observed time periods (Figure 2) in the APAP group, a significant difference was only observed amongst men at the early timepoint.
A meta-analysis by Doleman and colleagues evaluated the role of a single preoperative dose of IV APAP for management of postoperative pain in 544 patients who underwent general elective surgeries [10]. Although this analysis demonstrated significant reduction in morphine use, the clinical effect size was relatively modest. This meta-analysis also found a significant reduction in pain scores one hour after surgery. Our study did not find a significant group difference in pain severity scores overall. However, when factoring in gender, there appeared to be efficacy amongst male but not female patients during the earliest time period (PO2H) (Figure 4). Doleman and colleagues investigated many different types of surgeries but lacked data for body mass index (BMI), making it somewhat unclear to what extent their findings can be extrapolated to the SG population. We did observe a small but statistically significant correlation between BMI and average pain scores (Spearman Rho 0.25, p 0.015), perhaps indicating that preemptive APAP effects in SG patients could potentially benefit from weight-based adjustment.
APAP is capable of rapidly diffusing to the CNS by through an intact blood-brain-barrier (BBB) due to negligible protein binding and reasonable lipid solubility. However, this process requires sufficiently high plasma-to-CSF concentration gradient. A study of plasma APAP levels given orally 40 minutes prior to the procedure demonstrated that APAP plasma levels of 10 to 20 mcg/ml are essential to achieve effective pain relief, with established effective concentration for 50% effect (EC50) of 15.2 mcg/ml [14]. Another recent pharmacokinetic study in severely obese adolescents has shown that current recommendations of APAP to a maximum dose of 1,000 mg resulted in serum concentrations below detection limits within 2 hours after administration [16]. It was concluded that dosing APAP in obese patients is better predicted using total body mass with allometric scaling. Importantly, no significant difference was seen in total weight or BMI between sexes in this study.
Previously, El Chaar et al. conducted a randomized controlled trial of IV APAP in patients undergoing laparoscopic Roux-en-Y gastric bypass and SG [17] investigating the economic impact of a single dose given 30 minutes before surgery. Similar to the current findings, no significant difference in opioid consumption or length of stay was observed, thus providing no evidence for inpatient cost reduction. It did, however, show a notable difference in cost savings, based on reduced ED visits postdischarge. El Chaar et al. notably also differs from the current study in that patients received 4 doses of IV APAP over 24 hours prior to the procedure, and that only 20% of the procedures performed were SG.
Our analysis of PACU and hospital lengths of stay indicated that the APAP group had statistically longer PACU length of stay (8.9 hours IQR 7.3-10.0 vs. 7.9 hours IQR 6.3-9.0; p 0.02) but shorter hospital stay (52.6 hours IQR 36.2-55.7 vs. 54.4 IQR 51.3-57.3; p 0.03), although the numerical differences were not clinically meaningful. Bed availability on the floor is the main factor impacting length of PACU stay at our hospital. Other possible reasons of inconclusive outcome are the contribution of glycemic and blood pressure control which are known to impact on these two outcomes in patients undergoing SG [18].
There are a few limitations to this study. First, all patients had surgery at a single academic hospital by one of three bariatric surgeons, with involvement of surgical and anesthesia trainees, potentially making it less generalizable to other settings of care. Second, patients were not randomly assigned to APAP or control, and groups occurred in sequence, potentially leading to systematic bias that has not been adequately captured in this analysis. Third, pain scores were extracted from the medical record, and thus may be prone to variance in reporting by different nurses [19]. However, a similar degree of variability was seen across groups, and, given that many pain scores were recorded for each time epoch and averaged, these scores likely represent a reasonable estimation of pain over time, compared to a single timepoint. Fourth, optimal analgesia utilizes multiple modalities, combining different analgesics with varying mechanisms, resulting in additive or synergistic analgesia with less adverse effects from the sole administration of an individual analgesic. APAP may have a role in the multimodal approach, but interactions of APAP with other analgesic modalities were beyond the scope of the study.
Conclusion
In summary, these findings suggest that the addition of preoperative oral APAP to a standard analgesia protocol in SG did not substantially reduce postoperative opioid consumption or pain scores across the first 24 hours after surgery. Secondary analysis detected a modest analgesic effect in men, but not women, in the time period early after surgery (up to 2 hours), and well as higher pain scores amongst younger patients and those with higher BMI. A larger randomized controlled trial would be needed to confirm differential efficacy across sexes, as well as explore appropriate dosing of oral APAP on postoperative analgesia and opioid use following SG.
References
- Barajas Gamboa MD, Gonzalez Nuñez MD. Pain Management in Weight Loss Surgery: Aiming for Multimodal Approach. Adv Obes Weight Manag Control 2016;5(2):00125.
- English WJ, Demaria EJ, Brethauer SA, Mattar SG, Rosenthal RJ, et al. (2018) American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis 14:259-263.
- Thorell A, MacCormick AD, Awad S, Reynolds N, Roulin D, et al. (2016) Guidelines for perioperative care in bariatric surgery: enhanced recovery after surgery (ERAS) society recommendations. World Journal of Surgery 40:2065-2083.
- Weingarten TN, Sprung J, Flores A, Baena AM, Schroeder DR, et al. (2011) Opioid requirements after laparoscopic bariatric surgery. Obes Surg 21:1407-1412.
- Care Pathway Development for Laparoscopic Sleeve Gastrectomy (internet). American Society for Metabolic and Bariatric Surgery.
- Benyamin R, Trescot AM, Datta S (2008) Opioid complications and side effects. Pain Physician 11:105-120.
- Ahmad S, Nagle A, Mccarthy RJ, Fitzgerald PC, Sullivan JT, et al. (2008) Postoperative hypoxemia in morbidly obese patients with and without obstructive sleep apnea undergoing laparoscopic bariatric surgery. Anesth Analg 107:138-143.
- Halliday TA, Sundqvist J, Hultin M, Walldén J (2017) Post-operative nausea and vomiting in bariatric surgery patients: An observational study. Acta Anaesthesiologica Scandinavica 61:471-479.
- Singla NK, Parulan C, Samson R, Hutchinson J, Bushnell R, et al. (2012) Plasma and cerebrospinal fluid pharmacokinetic parameters after single-dose administration of intravenous, oral, or rectal acetaminophen. Pain Practice 12:523-532.
- Doleman B, Read D, Lund JN, Williams JP (2015) Preventive Acetaminophen Reduces Postoperative Opioid Consumption, Vomiting, and Pain Scores After Surgery. Reg Anesth Pain Med 40:706-712.
- Lam KK, Mui WL (2016) Multimodal analgesia model to achieve low postoperative opioid requirement following bariatric surgery. Hong Kong Med J 22:428-434.
- Malczak P, Pisarska M, Piotr M, Wysocki M, Budzynski A, at al. Enhanced Recovery after Bariatric Surgery. Obes Surg 27:226-235.
- Ng JJ, Leong WQ, Tan CS, Poon KH, Lomanto D, et al. (2017) A multimodal analgesic protocol reduces opioid-related adverse events and improves patient outcomes in laparoscopic sleeve gastrectomy. Obesity Surgery 27:3075-3081.
- Jibril F, Sharaby S, Mohamed A, Wilby KJ (2015) Intravenous versus oral acetaminophen for pain: systematic review of current evidence to support clinical decision-making. Can J Hosp Pharm 68:238.
- Samakar K, McKenzie TJ, Tavakkoli A, Vernon AH, Robinson MK, et al. (2016) The Effect of laparoscopic sleeve gastrectomy with concomitant hiatal hernia repair on gastroesophageal reflux disease in the morbidly obese. Obes Surg 26:61-66.
- Hakim M, Anderson BJ, Walia H, Tumin D, Michalsky MP, et al. (2019) Acetaminophen pharmacokinetics in severely obese adolescents and young adults. Paediatr Anaesth 29:20-26.
- El Chaar M, Stoltzfus J, Claros L, Wasylik T (2016) IV acetaminophen results in lower hospital costs and emergency room visits following bariatric surgery: A double-blind, prospective, randomized trial in a single accredited bariatric center. J Gastrointest Surg 20:715-724.
- Weingarten TN, Hawkins NM, Beam WB, Brandt HA, Koepp DJ, et al. (2015) Factors associated with prolonged anesthesia recovery following laparoscopic bariatric surgery: A retrospective analysis. Obesity Surgery 25:1024-1030.
- Apfel CC, Turan A, Souza K, Pergolizzi J, Hornuss C (2013) Intravenous acetaminophen reduces postoperative nausea and vomiting: A systematic review and meta-analysis. Pain 154:677-689.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences