ISSN : 2574-0431
Synthesis and Catalysis: Open Access
Highly Selective Catalytic Reduction of Nitroarenes over Heterogeneous Transition Metal Catalysts: Nano- Catalysts- the New Challenges
DICATECh, Politecnico di Bari, Bari, Italy
- *Corresponding Author:
- Matilda Mali
DICATECh, Politecnico di Bari, via Orabona
4 I-70125 Bari, Italy
Tel: +39 080 5963666
E-mail: matilda.mali@poliba.it
Received Date: March 27, 2017; Accepted Date: April 25, 2017; Published Date: May 04, 2017
Citation: Mali M. Highly Selective Catalytic Reduction of Nitroarenes over Heterogeneous Transition Metal Catalysts: Nano-Catalysts–the New Challenges. Synth Catal. 2017, 2:2.
Abstract
A brief review of nano-catalysts application in the reduction of nitro-compounds into corresponding anilines was reported. The influence of the metal nature, catalyst's supports and catalysts particle size in the reaction' selectivity was highlighted. Nano-structure of catalyst advantages was reported evincing future challenges.
Keywords
Catalytic reduction; Nitro aromatics; Nano-catalysts; Reuse/recyclable; Green catalytic conditions
Introduction
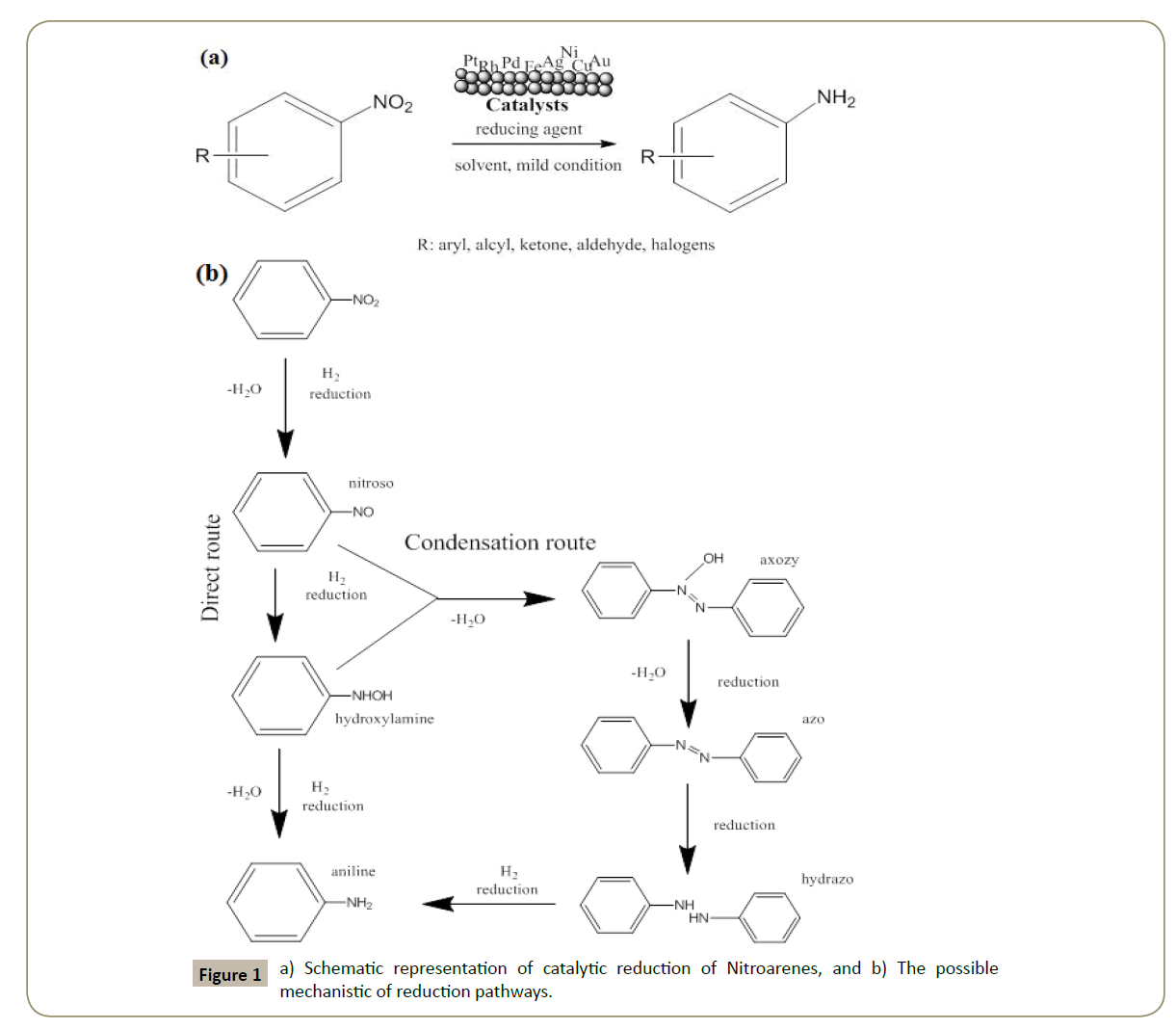
Catalytic reduction of Nitroarenes is a key reaction for two important reasons: First of all because it is one of the most utilized remediation application for the removal of nitro compounds from the environment and secondly because hydrogenation of nitro aromatics is considered as the most effective way to produce corresponding amino aromatics and has been widely used in production scale.
It is well-known that nitro compounds are important contaminants for the environment and living organism as well [1,2]. Their toxic and carcinogenic nature due to the presence of nitro groups in their structure is widely demonstrated [3,4]. Several remediation methods are proposed in literature such as photocatalytic degradation [5], or electrochemical methods [6]. Nevertheless, the reduction of nitro groups into amino compounds through catalytic hydrogenation is one of the most utilized [7]. In the Figure 1 is reported the schematic reaction of catalytic reduction of Nitroarenes (Scheme 1).
On the other side, amino-compounds are very important intermediates in the chemistry and industry of dyes, pigments, agrochemicals and herbicides, pharmaceuticals, rubber manufacturing, chelating agents, textile industry, and photographic chemicals as well [8,9].
Many effective protocols have been developed to improve the selectivity of the hydrogenation process aiming at reducing the presence of byproducts deriving mainly from hydrogenolysis of other carbon-heteroatom's bonds present in the Nitroarenes structure that affect the selectivity and restrains the hydrogenation rates leading to difficulty in separating the final pure products [10-13]. In addition, the presence of other sensitive functional groups such as ketones, halogens, alkenes, nitrile groups render often difficult the selectivity of the hydrogenations of nitro-compound, because these sensitive functionalities are reduced faster by hydrogenation than the nitro groups [14]. All these undesirable reactions cannot be avoided completely over conventional catalyses.
Different factors can be stressed in order to enhance the selectivity and to reduce the amounts of the by-products. We can divide them into three action classes:
• Working on the nature of catalyst (metal, support and metal particle size).
• Modifying the reaction conditions (solvent, temperature and pressure) etc.
• Using additives such as promoter, inhibitor and poison factors.
Modification on catalysts structure and use of noble metal nanoclusters on organic or inorganic supports constitutes one of the main applications widely exploited. The value of Nanoparticles (NPs) is one of the most important discoveries that have recently been developed in catalysis [15-20]. Metal nanoclusters, as building blocks for preparing heterogeneous catalysts, offer new possibilities of universal significance for designing and constructing structure controllable catalysts [21- 24].
In this short editorial, we evidenced on one side, the great efforts and remarkable contributions of different authors for finding solutions to the existing drawbacks in the hydrogenation of nitro compounds and more generally in the catalytic reduction reactions and on the other side to persuade on finding answers exploiting the progress of nanoscience, of which catalysis is an important field of application.
Stress is placed on development of metal nanostructures by using innovative supports such as polyelectrolyte brushes, polyionic liquids, micelles, dendrimers that can provide superior selectivity as well as create premises for implementation of highly reuse/ recyclable catalysts according to green chemistry principles.
Nano-structured Catalysts
Selectivity-dependence on metal particle size and condition reactions
The addition of metal complexes to the catalytic system can considerably modulate both the activity and the selectivity of the hydrogenation process. Many catalysts have been utilized for the reduction of nitro compounds [18-20,25,26], such as metal supported catalysts, polymer-protected metal nanoclusters, organometallic complexes or inorganic metal compounds. Nevertheless, the selectivity of the hydrogenation process resulted to be metal type dependent and structure-sensitive. Different metals and changes in catalyst's particle size drive to different selectivity rates and different by-products.
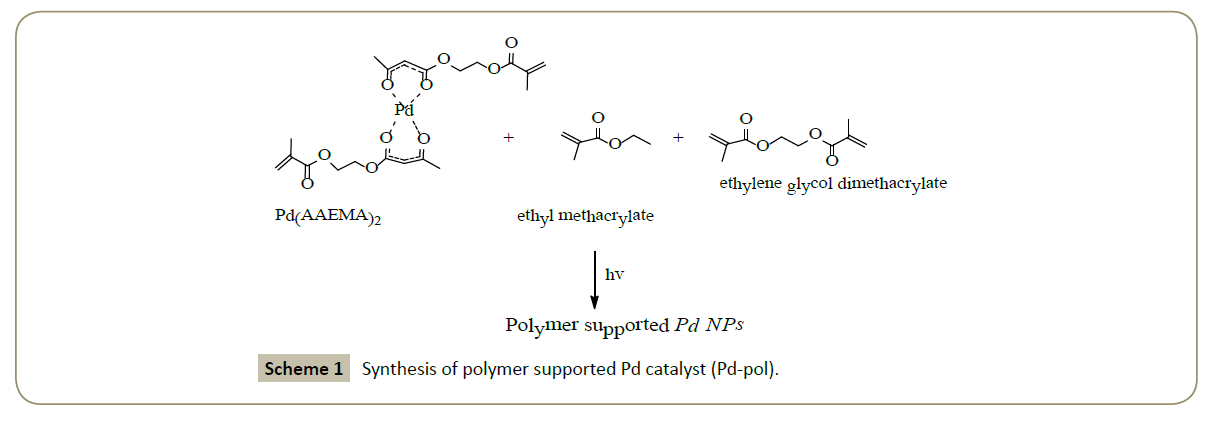
Palladium is widely applied in the catalytic hydrogenation at low hydrogen pressure and room temperature [27]; Cerveny [28] provided a wide review on use of palladium as catalyst for hydrogenation reactions conducting an investigation on the kinetics of catalytic hydrogenations in the liquid state. The exceptional properties of palladium, which in some places are confronted with those of platinum, are reported in detail such as the existence of many forms of palladium on support available, each with its own selectivity or its capacity to give hydrogenation reaction in mild condition or its high versatility for hydrogenation of many types of bonds. A polymer supported palladium catalyst, obtained by copolymerization of Pd(AAEMA)2 [AAEMA- =deprotonated form of 2-(acetoacetoxy)ethyl methacrylate] with ethyl methacrylate (co-monomer) and ethylene glycol dimethacrylate (cross-linker), was proposed by Dell’Anna et al. [18] as catalyst with an excellent activity and selectivity in the hydrogenation reactions of substrates such as olefins, aromatic and unsaturated aldehydes, unsaturated ketones and nitro compounds.
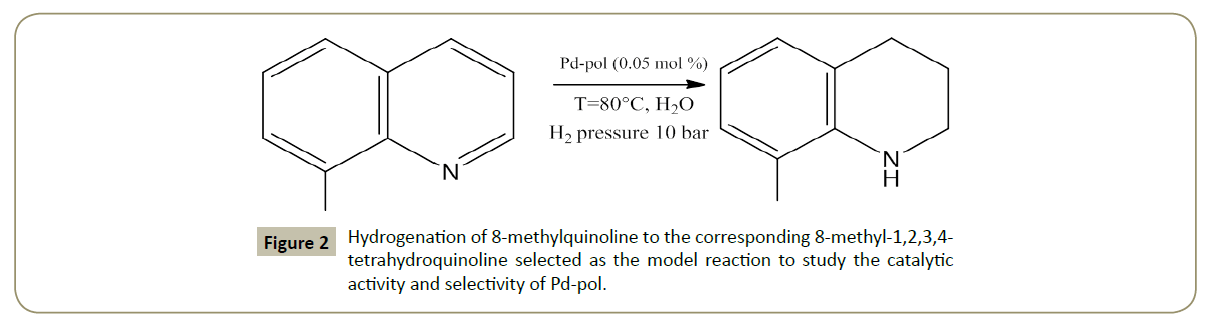
Application of this polymer supported Pd catalysts was exploited successfully [20] for hydrogenation in aqueous medium of quinolines to 1,2,3,4-tetrahydroquinolines under mild temperature (80°C) and H2 pressure (10 bar) (Figure 2).
Figure 2: Hydrogenation of 8-methylquinoline to the corresponding 8-methyl-1,2,3,4- tetrahydroquinoline selected as the model reaction to study the catalytic activity and selectivity of Pd-pol.
Both studies demonstrated that the active species promoting reduction process resulted to be nano-particles (NPs) in situ formed during the catalytic reactions. Analyses of the mechanism of nanoparticles active species in different catalytic reduction were deeply reported in Mastrorilli et al. [24].
Previous studies [29] demonstrated the dependence of hydrodechlorination of chlorobenzene from the size of Pd particles supported on alumina, while in Carturan et al. [30] it was supposed that the hydrogenation rate of nitrobenzene decreased upon increasing the metal dispersion of supported palladium catalysts.
In a very recent overview [31] the catalytic properties for monometallic and bimetallic Pd catalysts was also reported. The paper highlighted on one side the high performance of Pd catalyst synthetized in nano-size particles to reduce hydrogenolysis and on the other, the drawbacks related with the aggregation process that lead to losses effectiveness in the conversion rates of the hydrogenation reactions. Previous studies [32] demonstrated that the ease of dehalogenation depends on, besides the active metal, to the halogen type identifying an order among halogens (I>Br>Cl>F).
Furthermore, the overall structure and the reaction conditions need to be stressed in order to avoid undesirable by-products and to inhibit aggregation of palladium nano-particles. Further investigations in this direction are still needed.
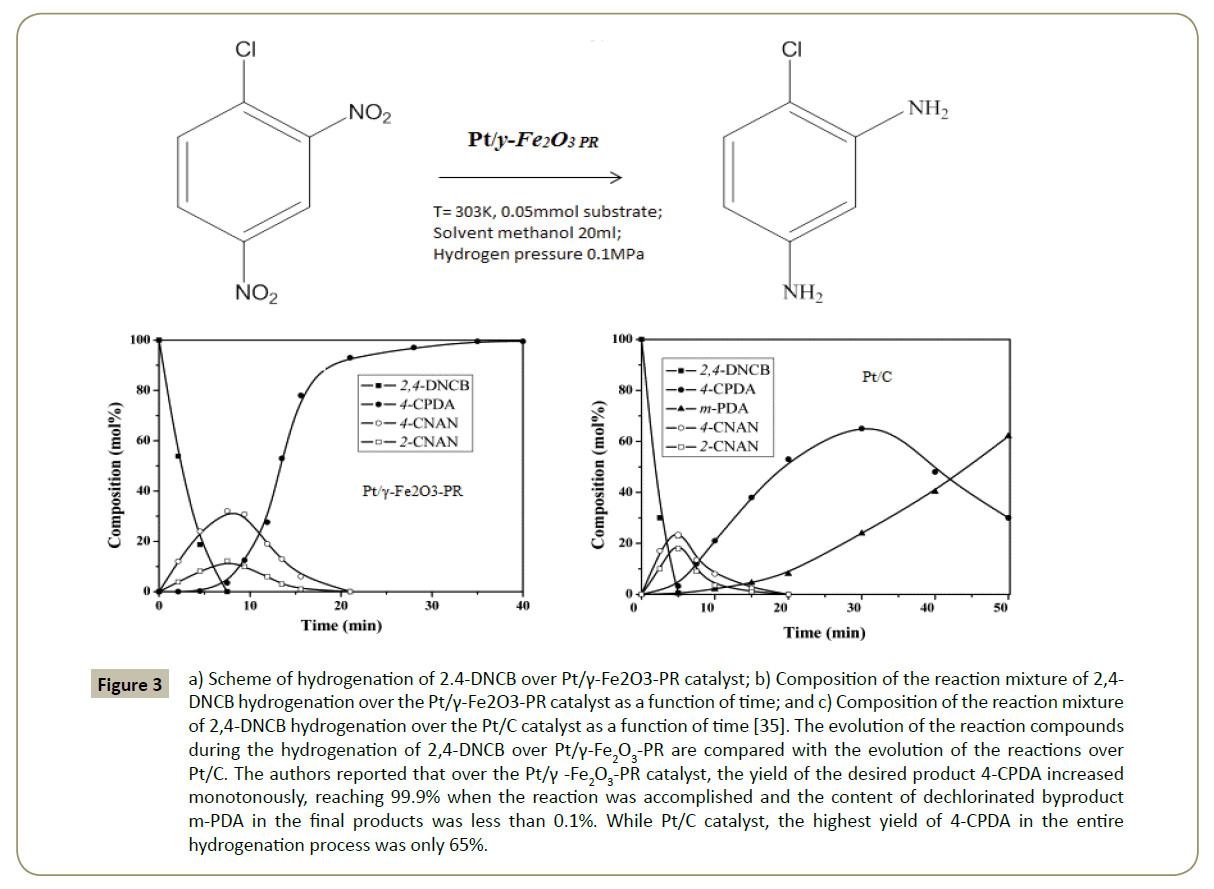
Platinum-based nano-catalysts are particularly attractive for minimizing dehalogenation combined with a fast rate of reduction of the nitro group [33]. Nevertheless, they are highly expensive due to high cost of its salts. Similarly to palladium, Hoechst [34] reported the catalyst’s particle size effect the hydrogenation of halonitroaromatics over Pt catalysts as it. Coq et al. [12] found that the relative adsorption strength between cloronitrobenzene and hydrogen increased when the Pt particle size decreased. Nevertheless, in order to avoid losses of catalysts conversion rates, modification of supports in which nano-particle' catalysts are anchored is considered key factors affecting the selectivity. Liang et al. 2008 [35], shown that a partially reduced Pt/γ-Fe2O3 magnetic nanocomposite catalyst (Pt/γ -Fe2O3-PR) exhibited excellent catalytic properties in the selective hydrogenation of 2,4-dinitrochlorobenzene and iodonitrobenzenes (Figure 3). This kind of supports (Pt/γ-Fe2O3) developed for the first time; enhance the suppression of hydrodehalogenation process. The authors found that CO chemisorption on the Pt nanoparticles deposited in this kind of structures was very weak, implying thus a weak tendency of the electronic back-donation from the Pt nanoparticles to the π* antibonding orbitals of the adsorbed molecules: and this was considered the main cause of the superior selectivity to the haloanilines in the hydrogenation reactions.
Figure 3: a) Scheme of hydrogenation of 2.4-DNCB over Pt/γ-Fe2O3-PR catalyst; b) Composition of the reaction mixture of 2,4- DNCB hydrogenation over the Pt/γ-Fe2O3-PR catalyst as a function of time; and c) Composition of the reaction mixture of 2,4-DNCB hydrogenation over the Pt/C catalyst as a function of time [35]. The evolution of the reaction compounds during the hydrogenation of 2,4-DNCB over Pt/γ-Fe2O3-PR are compared with the evolution of the reactions over Pt/C. The authors reported that over the Pt/γ -Fe2O3-PR catalyst, the yield of the desired product 4-CPDA increased monotonously, reaching 99.9% when the reaction was accomplished and the content of dechlorinated byproduct m-PDA in the final products was less than 0.1%. While Pt/C catalyst, the highest yield of 4-CPDA in the entire hydrogenation process was only 65%.
The modification of catalyst structure by different additives is also considered advantageous of combining high selectivity without losing too much activity. Several supports and additives are reported in literature. Platinum nanoparticles stabilized by an ionic-liquid-like-copolymer (IP) immobilized in various ionic liquids (ILs) was developed by Yuan et al. [36] that found a substantial increase in the efficiency of catalytic process for the selective hydrogenation of aromatic chloronitro compounds to aromatic chloroamines. This reaction, that have a considerable commercial significance due to its important industrial applications, allowed to establish an universal catalyst– ionic liquid system for the conversion of aromatic chloronitro compounds to aromatic chloroamines. The use of ionic liquid system for a best recyclability is demonstrated in the manuscript that, in addition, underlined the considerable turnover number (750 fold much more than Raney nickel catalyst). A comparative analyses for using ionic liquid as excellent media for the heterogeneously catalyzed hydrogenation of halonitrobenzenes to corresponding haloanilines is studied also by Xu et al. [33]. The authors selected three type catalysts of Raney nickel, carbon-supported platinum and palladium and concluded that Platinum is the best one. The paper revalidated also different methods for solving the dehalogenation problem, over the modification of the nano-structure of catalysts.
As member of Platinum group, Rhodium was also exploited in different catalytic reduction reactions [37-39]. Supported rhodium catalyzed hydrogenation reactions under mild conditions was reported by Dell’Anna et al. [40]. The authors investigated the catalytic activity of a cross-linked polymer obtained by reaction of Rh(cod)(AAEMA) [AAEMA‑deprotonated form of 2-(acetoacetoxy)ethyl methacrylate] and suitable acrylamides as comonomers, a structure similar with those proposed for Palladium. The hydrogenation reactions of different substrates, despite nitrobenzene’s, such as olefins, unsaturated aldehydes and ketones were investigated by authors and the results obtaining resulted satisfactory. Polymer protected rhodium catalysts was synthetized by also by Madgadelene et al. [41]. Polybenzimidazole-supported rhodium catalyst synthetized was found to be effective for the reduction of nitrophenols, nitrobenzoic acids and nitroanilines in methanol at room temperature and at 1 atm hydrogen. The authors demonstrate, in addition, the important influence of temperature, concentrations of the catalyst, substrate and nature of the solvent on the reaction rates. Nevertheless, the study on rhodium nano-system catalyst are still scarcely investigated therefore further studies are needed.
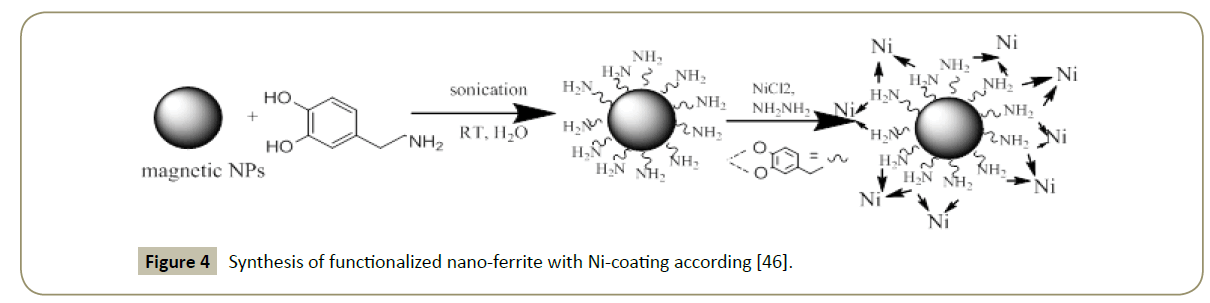
Nickel is also another metal largely utilized in the catalytic hydrogenation due to its low costs. It is mostly utilized in its Raney form. Ni use in the hydrogenation reactions date back to 1897, when Paul Sabatier, a French Chemist discovered that the introduction of a trace of nickel metal enabled the addition of hydrogen to molecules of hydrocarbon compounds [42]. Nevertheless, their magnetic capacities, that can make it very interesting catalysts, are still narrowly utilized [43-45]. The modification of metal size and the selection of appropriate supports can enhance a lot Nickel-catalysts performance. An efficient synthesis of Ni catalyst supported on nano-ferrite and magnetically recyclable was utilized in the hydrogenation and transfer hydrogenation reactions by Polshettiwar et al. [46]. Despite the potential inherent stability and activity of this metal, no enough examples of a nanoparticle-supported Ni catalyst had been reported in literature. In the Figure 4 the schematic synthesis and functionalization of magnetic nanoparticles according Polshettivar is reported.
Figure 4: Synthesis of functionalized nano-ferrite with Ni-coating according [46].
Usman et al. [47] reported a very interesting support for nano-size Nickel particles. Highly ordered mesoporous silica (KIT-6) was used as support by the wet impregnation method, and the performance of Ni-catalyst was evaluated in the hydrogenation of edible vegetable oil, comparing with that of Ni/Activated carbon prepared using the same method as well as with unsupported Nickel. The results confirmed the improvement of conversion rate with an increase in mass of supported Nickel on KIT-6. The same supporting structure should be tested also in nitroarene reduction reactions in further researches.
Other metals, such as gold or silver nanoparticles have also both economic benefits respect to Pt and Pd in catalytic hydrogenation [48]. Copper resulted excellent catalyst for many reactions, either hydrogenation of organic compounds, alcohol decomposition or dehydrogenation of alcohols and ester hydrogenolysis [49]. Nevertheless, it requires elevated temperatures and pressures (250-300°C/250-300 atm. Ruthenium (Ru) is utilized at nano-structures level. It resulted active at mild temperature and pressures 70-80°C/ 60-70 atm. It is very resistant to poisoning. Nonetheless, Ruthenium remains scarcely studied due to less stability and high cost of its salts [50-52].
Remaining Drawbacks and Future Challenges
The list of noble and transition metals that are modified at nano-structure and subsequently employed in the catalytic hydrogenation is really long (i.e., Ag [53], Co [54], and Zn [55]; bimetallic nanoparticles like alloys of Fe-Pd [56], Pd–Pt [8], and Pt/Au and Pd/Cu [57,58]. Nano-structures in fact offer surely several intrinsic advantages mainly connected with: i) Energy-efficient conversion processes; ii) Mild reaction condition; iii) Low-cost catalysts and recyclability of precious metals.
The use of the ever increasing number of tailor-made metal catalytic species is right associated with the different still existing problems such as the solubility of different components, catalyst instability, metal leaching during the recycling procedure, losses in conversion rates due to aggregation process of nano particles, all factors that limit the utility of some metals-catalysts. Indeed, since single solution problems does not exist, an urgent need to develop advanced technologies able address the persisting challenges in the reduction reactions leave enough space for future researches.
Despite the great efforts already done, unsolved aspects should be considered such as: development of less expensive and easily available protocols, use of non-precious metal catalyst able to reduce the unavoidable formation of harmful derivate, implementation of protocols that lead to higher conversion yields in final pure products; strategies regarding modulation of metal particle size; introducing of support effects that avoid losses of catalytic activity due to metal aggregation effects.
Some issues are already tackled in literature: i.e., the use of stabilization catalysts support systems with different materials like gum, graphene, polymers, zeolites, perlite, carbon, metal oxides surfactants, and ionic liquids have already been addressed and other stabilizing systems based on responsive materials like microgels, micelles, dendrimers, yolk–shell, core–shell structures, and core satellite have been developed for controlling the catalytic activity of nanoparticle catalysts [59]. Begum at al. [7] reported recently a robust review that summarizing some important achievements and advanced methods applied so far in terms of metal nanoparticles stabilized by biological substances [60] or in micellar systems [61]. Interesting aspects regard the use of microgels as support for metal-nano particles as reported recently in literature [4].
A Nano catalyst stabilized on the surface of polymer particles with magnetic core is another items already addressed but need further application. Using of nano-catalytic system easily magnetically separable is tackled by Usman at al., as previously reported but a recent important research can be mentioned: implemented an interesting catalyst, named Fe3O4@P (EGDMA-co-MAA), a core-shell microspheres with magnetite core and polymeric shell made of ethylene glycol dimethacrylate (EGDMA) and methacrylic acid (MAA) and used this core–shell system as a template for the fabrication of Au NPs on the surface of polymeric shells. Other supports, such as dendrimers, carbon and silicate stabilized metal nanoparticles have to be considered as advance catalysts-protecting systems since they inhibit the release of nanoclusters from supports acting as branched protecting groups [47].
Conclusion
Different catalytic systems used for the degradation of nitroaromatic compounds based on the literature are described and main factors affecting the selectivity and performance of the catalytic reduction of Nitroarenes are underlined. The advantage of using nano-structure catalysts are evidenced posing the attention on the still remaining unsolved problems that can be summarized as follow:
• Losses of catalysts efficiency due to aggregation process of metal particles.
• Low purity of final products and the complexity of product separations methods.
• Occurring of undesirable intermediate products, sometime harmful, during the catalytic conversion.
The paper aimed to persuade the implementation of researches for advanced and green methods to be utilized in the synthesis and stabilization of metal nanoparticles used as catalysts for the reduction of Nitroarenes and other organic pollutants and encourages exploration of nano-science progress for individuation of no expensive and easily available protocols able to address existing drawbacks.
References
- Kovacic P, Somanathan R (2014) Nitroaromatic compounds: Environmental toxicity, carcinogenicity, mutagenicity, therapy and mechanism. J Appl Toxicol 34: 810-824.
- Mali M, Dell’Anna MM, Mastrorilli P, Damiani L, Fdez-Ortiz De Vallejuelo S, et al. (2016) Identification of hot spots through a new cumulative hazard index. Case study: port of Bari, Italy. Ecol Indicators 60: 548-556.
- Peres CM, Agathos SN (2010) Biodegradation of nitroaromatic pollutants: from pathways to remediation. Biotechnol Ann Rev 6: 197-220.
- Xiao-Qiong W, Xing-Wen W, Qing H, Jiang-Shan S, Hong-Wu Z (2015) In situ synthesized gold nanoparticles in hydrogels for catalytic reduction of nitroaromatic compounds. Appl Surf Sci 331: 210-218.
- Nezamzadeh-Ejhieh A, Khorsandi S (2013) Photocatalytic degradation of 4-nitrophenol with ZnO supported nano-clinoptilolite zeolite. J Ind Eng Chem.
- Jiang P, Zhou J, Zhang A, Zhong Y (2010) Electrochemical degradation of p-nitrophenol with different processes. J Environ Sci 22: 500-506.
- Begum R, Rehan R, Farooqi HZ, Butt Z, Ashraf S (2016) Physical chemistry of catalytic reduction of nitroarenes using various nanocatalytic systems: past, present, and future. J Nanopart Res 18: 231-255.
- Eunsuk K, Han S, Moon BK (2013) Efficient chemoselective reduction of nitro compounds and olefins using Pd-Pt bimetallic nanoparticles on functionalized multi-wall-carbon nanotubes. Catal Commun 45: 25-29.
- Gupta VK, Atar N, Yola ML, Ustundag Z, Uzun L (2014) A novel magnetic Fe@Au core-shell nanoparticles anchored graphene oxide recyclable nanocatalyst for the reduction of nitrophenol compounds. Water Res 48: 210-217.
- Greenfield H, Dovell FS, (1967) Metal sulfide catalysts for hydrogenation of halonitrobenzenes to haloanilines. J Org Chem 32: 3670-3671.
- Kosak JR (1980) Hydrogenation of haloaromatic nitro compounds. In: Catalysis in Organic Syntheses; Jones, W.H. Ed, Academic Press: NY, USA pp: 107-117.
- Coq B, Tijani A, Figueras F (1991) Particle size effect on the kinetics of p-chloronitrobenzene hydrogenation over platinum/alumina catalysts. J Mol Catal 68: 331-338.
- Coq B, Tijani A, Figueras F (1992) Influence of alloying platinum for the hydrogenation of p-chloronitrobenzene over PtM/Al2O3 catalysts with M=Sn, Pb, Ge, Al, Zn. J Mol Catal 71: 317-333.
- Xiao C, Wang X, Lian C, Liu H, Liang M, et al. (2012) Selective Hydrogenation of Halonitrobenzenes. Current Organic Chemistry 16: 280-296.
- Astruc D, Lu F, Aranzaes JR (2005) Nanoparticles as recyclable catalysts: the frontier between homogeneous and heterogeneous catalysis. Angew Chem Int Ed 44: 7852-7872.
- Yan N, Xiao C, Kou Y (2010) Transition metal nanoparticle catalysis in green solvents. Coord Chem Rev 254: 1179-1218.
- Sarkar S, Guibal E, Quignard F, SenGupta AK (2012) Polymer-supported metals and metal oxide nanoparticles: synthesis, characterization, and applications. J Nanopart Res 14: 1-24.
- Dell’Anna MM, Intini S, Romanazzi G, Rizzuti A, Leonelli C, et al. (2014) Polymer supported palladium nanocrystals as efficient and recyclable catalyst for the reduction of nitroarenes to anilines under mild conditions in water. J Mol Cat A: Chemical 395: 307-314.
- Dell’Anna MM, Mali M, Mastrorilli P, Cotugno P, Monopoli A (2014) Oxidation of benzyl alcohols to aldehydes and ketones under air in water using a polymer supported palladium catalyst. J Mol Cat A: Chem 386: 114-119.
- Dell’Anna MM, Mali M, Manno D, Cotugno P, Monopoli A, et al. (2014). Highly selective hydrogenation of quinolines promoted by recyclable polymer supported palladium nanoparticles under mild conditions in aqueous medium. Appl Catal A Gen 481: 89-95.
- Kónya Z, Puntes VF, Kiricsi I, Zhu J, Alivisatos P, et al. (2002) Novel Two-Step Synthesis of Controlled Size and Shape Platinum Nanoparticles Encapsulated in Mesoporous Silica. Catal Lett 81: 137-144.
- Zhu J, Kónya Z, Puntes VF, Kiricsi I, Miao CX, et al. (2003) Encapsulation of Metal (Au, Ag, Pt) Nanoparticles into the Mesoporous SBA-15 Structure. Langmuir 19: 4396-4402.
- Yonezawa T, Matsune H, Kimizuka N (2003) Formation of Isolated Spherical Three-dimensional Nanoparticle Assembly as Stable Submicron-sized Units by Using an Inorganic Wrapping Technique. Adv Mater 15: 499-509.
- Mastrorilli P, Dell’Anna MM, Mali M, Rizzuti A, Zapparoli M, et al. (2015) Resin-Immobilized Palladium Nanoparticle Catalysts for Organic Reactions in Aqueous Media: Morphological Aspects. Molecules 20: 18661-18684.
- Nishimura S (2001) Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis. John Wiley & Sons, Inc., NY, USA.
- Jagadeesh RV, Surkus AE, Junge H, Pohl MM, Radnik J, et al. (2013) Nanoscale Fe2O-based catalysts for selective hydrogenation of nitroarenes to anilines. Science 342: 1073-1076.
- Rylander PN (1979) Catalytic hydrogenation. In Catalytic Hydrogenation in Organic Synthesis; Academic Press: NY, USA pp: 235-248.
- Cerveny L (1988) Palladium Catalysts in hydrogenation reactions.
- Ferrat G, Coq B, Figueras F (1986) Conversion of chlorobenzene over palladium and rhodium catalysts of widely varying dispersion. J Catal 101: 434-441.
- Carturan G, Facchin G, Cocco G, Navazio G, Cubitosa G (1983) Hydrogenation of nitro compounds with supported palladium catalysts: influence of metal dispersion and nitro compound nature. J Catal 82: 56-61.
- McCue AJ, Anderson JA, (2015) Recent advances in selective acetylene hydrogenation using palladium containing catalysts. Front Chem Sci Eng 9: 142-153.
- Kratky V, Kralik M, Mecarova M, Stolcova M, Zalibera L, et al. (2002) Effect of catalyst and substituents on the hydrogenation of chloronitrobenzenes. Appl Catal A 235: 225-233.
- Xu DQ, Hu ZY, Li WW, Luo SP, Xu ZY (2005) Hydrogenation in ionic liquids: An alternative methodology toward highly selective catalysis of halonitrobenzenes to corresponding haloaniline. J Mol Cat A: Chemical 235: 137-142.
- Hoechst AG (1972) Heterocyclic compounds carrying ethylenic-double bonds, as fluorescent optical brighteners. Fr Pat 2127092.
- Liang M, Wang X, Liu H, Wang Y (2008) Excellent catalytic properties over nanocomposite catalysts for selective hydrogenation of halonitrobenzenes. J Catalysis 255: 335-342.
- Yuan X, Yan N, Xiao C, Li C, Fei Z, et al (2010) Highly selective hydrogenation of aromatic chloronitrocompounds to aromatic chloroamines with ionic-liquid-like copolymer stabilized platinum nanocatalysts in ionic liquids. Green Chem 12: 228-233.
- Miao D, Liu ZM, Han BX, Huang J, Sun ZY et al. (2006) Ru nanoparticles immobilized on montmorilionite by ionic liquids: A highly efficient heterogeneous catalyst for the hydrogenation of benzene. Angew Chem Int 45: 266-269.
- Yan N, Zhao C, Luo C, Dyson PJ, Liu HC, et al. (2006) One-step conversion of cellobiose to C6-alcohols using a ruthenium nanocluster catalyst.
- Wang J, Feng J, Qin R, Fu H, Yuan M, et al. (2017) Supported Rhodium Catalysts for Ammonia-Borane Hydrolysis: Dependence of the Catalytic Activity on the Highest Occupied State of the Single Rhodium Atoms Tetrahedron: Asymmetry 18: 1643-1652.
- Dell’Anna MM, Mastrorilli P, Rizzuti A, Suranna GP, Nobile CF (2000) Synthesis and copolymerization of rhodium(I) and palladium(II) complexes with the deprotonated form of 2(acetoacetoxy)ethyl methacrylate. Inorg Chim Acta 304: 21-25.
- Madgadelene RM, Leelamani EG, Nanje Gowda NM (2004) Hydrogenation of nitroarenes using polybenzimidazole-supported rhodium catalyst. J Mol Cat A: Chem 223: 17-20.
- Sabatier P, Senderens JB (1905) New methane synthesis. Annales de Chimie et de Physique. 4: 319-323.
- Du Y, Chen H, Chen R, Xu N (2004) Synthesis of paminophenol from p nitrophenol over nanosized nickel catalysts. Appl Catal A 277: 259-264.
- Rizhi C, Yan D, Weihong X, Nanping X (2006) The effect of titania structure on Ni/TiO2 catalysts for p-nitrophenol hydrogenation. Chin J Chem Eng 14: 665-669.
- Rizhi C, Yan D, Weihong X, Nanping X (2007) Effect of Alumina Particle Size on Ni/Al2O3 Catalysts for p-Nitrophenol Hydrogenation. Chin J Chem Eng 15: 884-888.
- Polshettiwar V, Barawati B, Varma SR (2009) Nanoparticle-supported and magnetically recoverable nickel catalyst: a robust and economic hydrogenation and transfer hydrogenation protocol. Green Chem 11: 127-131.
- Usman MA, Alaje TO, Ekweme VI, Awe EA (2012) Investigation of the Catalytic Performance of a Novel Nickel/Kit-6 Nanocatalyst for the Hydrogenation of Vegetable Oil. SRN Chem Engin V.
- Corma A, Serna P (2006) Chemoselective hydrogenation of nitro compounds with supported gold catalysts. Science J 313: 332-334.
- Rupinder K, Bonamali P (2015) Cu nanostructures of various shapes and sizes as superior catalysts for nitro-aromatic reduction and co-catalyst for Cu/TiO2 photocatalysis. Appl Catal A Gen 491: 28-36.
- Fonseca GS, Umpierre AP, Fichtner FPF, Teixeira SR, Dupont J (2003) The Use of Imidazolium Ionic Liquids for the Formation and Stabilization of Ir0 and Rh0 Nanoparticles: Efficient Catalysts for the Hydrogenation of Arenes Chem Eur J 9: 3263-3271.
- Prechtl MHG, Scariot M, Scholten JD, Machado G, Teixeira SR, et al. (2008) Nanoscale Ru (0) particles: Arene hydrogenation catalysts in imidazolium ionic liquids. Inorg Chem 47: 8995-8999.
- Nakamula I, Yamanoi Y, Yonezawa T, Imaoka T, Yamamoto K, et al. (2008) Nanocage catalysts—rhodium nanoclusters encapsulated with dendrimers as accessible and stable catalysts for olefin and nitroarene hydrogenations. Chem Commun 44.
- Ajmal M, Farooqi ZH, Siddiq M (2013) Silver nanoparticles containing hybrid polymer microgels with tunable surface plasmon resonance and catalytic activity. Korean J Chem Eng 30: 2030-2036.
- Demirci S, Sahiner N (2015) The Use of Metal Nanoparticle-Embedded Poly(ethyleneimine) Composite Microgel in the Reduction of Nitrophenols. Water Air Soil Pollut 226: 1-13.
- Mahdavi H, Tamami B, (2005) Reduction of Nitro‐Aryl Compounds with Zinc in the presence of Poly[N‐(2‐aminoethyl)acrylamido]trimethylammonium chloride as a Phase‐Transfer Catalyst. Synthet Commun 35: 125-31.
- Dong T, Luo H, Wang Y, Hu B, Chen H (2011) Stabilization of Fe-Pd bimetallic nanoparticles with sodium carboxymethyl cellulose for catalytic reduction of para-nitrochlorobenzene in water. Desalination 271: 11-19.
- Pozun ZD, Rodenbusch SE, Keller E, Tran K, Tang W, et al. (2013) A Systematic Investigation of p-Nitrophenol Reduction by Bimetallic Dendrimer Encapsulated Nanoparticles. J Phys Chem C Nanomater Interfaces 117: 7598-7604.
- Redel E, Kramer J, Thomann R, Janiak C (2009) Synthesis of Co, Rh and Ir nanoparticles from metal carbonyls in ionic liquids and their use as biphasic liquid-liquid hydrogenation nanocatalysts for cyclohexene. J Organomet Chem 694: 1069-1075.
- Pengxiang Z, Xingwen F, Deshun H, Guiying Y, Didier A (2015) Basic concepts and recent advances in nitrophenol reduction by gold and other transition metal nanoparticles. Coord Chem Rev 287: 114-136.
- Nasrollahzadeh M, Sajadi SM, Rostami-Vartooni A, Bagherzadeh M, Safari R (2015) Immobilization of copper nanoparticles on perlite: green synthesis, characterization and catalytic activity on aqueous reduction of 4-nitrophenol. J Mol Catal A: Chem 400: 22-30.
- Yu Y (2014) Ionic liquid-Pluronic P123 mixed micelle stabilized water-soluble Ni nanoparticles for catalytic hydrogenation. J Colloid Interface Sci 415: 117-126.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences