Hematuria and Fever in a Transplant Patient with Polycystic Kidney Disease and Past Borderline Cellular Rejection: A Case of Late-Onset Adenovirus Infection
Rachel Séguin-Tremblay1, Lauriane Vittoz1, and Sarah Higgins1,2*
1Department of Medicine, University of Sherbrooke, Quebec, Canada
2Department of Nephrology, University of Sherbrooke, Quebec, Canada
- *Corresponding Author:
- Sarah Higgins
Department of Nephrology,
University of Sherbrooke, Quebec,
Canada,
E-mail: sarah.higgins@usherbrooke.ca
Received date: October 14, 2022, Manuscript No. IPNT-22-14785; Editor assigned date: October 17, 2022, PreQC No. IPNT-22-14785 (PQ); Reviewed date: October 26, 2022, QC No. IPNT-22-14785; Revised date: November 08, 2022, Manuscript No. IPNT-22-14785 (R); Published date: November 15, 2022, DOI: 10.36648/ipnt.6.7.1
Citation: Tremblay RS, Vittoz L, Higgins S (2022) Hematuria and Fever in a Transplant Patient with Polycystic Kidney Disease and Past Borderline Cellular Rejection: A Case of Late-Onset Adenovirus Infection. J Nephrol Transplant Vol. 6 No.7:1.
Abstract
Adenovirus (ADV) is a viral pathogen causing graft dysfunction in renal transplant recipients with an occurrence of 4% in case series. It usually presents within 6 months of transplant, and co-occurrence with Poly-Cystic Kidney Disease (PCKD) is rare. Diagnosis is a challenge in PCKD patients who haven’t undergone nephrectomy since hematuria, fever, and Acute Kidney Injury (AKI) are the typical clinical features at presentation. Moreover, it is difficult to distinguish adenovirus from acute cellular rejection on renal biopsy. Herein, we discuss an exceptional case of late-onset ADV infection in a PCKD transplant patient with past borderline cellular rejection to detail our team’s approach to the diagnosis and treatment.
The patient was known for a past medical history of borderline T Cell-Mediated Rejection (TCMR) at 16 weeks post-transplant. He presented after 28 months of transplant with a rise in his creatinine to 172 μmol/L (baseline 133 μmol/L) accompanied by high-grade fever, hematuria, and diarrhea. During hospitalization, quantitative PCR for ADV in urine was positive for more than 950 million copies. A graft biopsy revealed tubuleinterstitial lesions with mononuclear infiltrates and exudative necrosis suggestive of viral infection. He received a full course of antibiotics, and all other investigations were negative.
Final immunohistochemical staining for ADV was negative but our working diagnosis remained adenovirus interstitial nephritis. We lowered mycophenolate mofetil to 500 mg PO bid, increased prednisone to 20 mg PO die, and completed two treatments of IV immunoglobulins. The patient’s fever resolved and creatinine decreased to 160 μmol/L from a maximum of 250 μmol/L per hospitalization.
This case report demonstrates the importance of lowering our clinical index of suspicion for ADV infection even past one year of renal transplant. It is an example of ADV interstitial nephritis despite negative immunohistochemical staining. In a scenario where TCMR rejection and ADV infection couldn’t be fully distinguished on renal biopsy, the conservative treatment led to positive outcomes.
Keywords
Adenovirus; Kidney transplant; PCKD; Cellular rejection; Hematuria
Background
Much like polyomavirus and cytomegalovirus, Adenovirus (ADV) is now well recognized as a viral pathogen causing graft dysfunction in renal transplant recipients [1]. Literature describing diagnosis and treatment options for invasive ADV infections has grown exponentially in the last 20 years, giving rise to the 2019 American Society of Transplantation Infectious Diseases Community of Practice guidelines [2]. Nevertheless, there is, to our knowledge, no literature exploring the interaction of primary kidney disease with ADV infection. Also, guidelines do not address the treatment of patients who have undergone graft rejection in the past.
Despite the apparent ubiquity of the virus, pathogenicity in renal transplant remains rare, with an occurrence as low as 4% in most case series [3]. Its classic clinical presentation in kidney recipients is fever, hematuria, and Acute Kidney Injury (AKI) presenting within one year of transplant [1, 4, 5]. This causes certain diagnostic challenges in patients with Poly-Cystic Kidney Disease (PCKD) who have not undergone nephrectomy. These difficulties are amplified in late presentations, where ADV does not strike as a likely culprit [6].
The interpretation of the kidney biopsy in ADV infection also presents certain diagnostic hurdles: The cytopathogenic effects of the virus have a lot in common with those found in T Cell- Mediated Rejection (TCMR) [7]. Such ambiguity entails a supplementary challenge when it comes to treating patients who have had a borderline cellular rejection diagnosis in the past.
This case report details our team’s approach to the diagnosis and treatment of late-onset ADV infection in a PCKD transplant patient with past borderline cellular rejection.
The Case
Clinical presentation
A 58-year-old man with PCKD underwent a deceased kidney transplant in our nephrology unit in 2018. This patient’s medical history was notable for a left kidney nephrectomy in the context of recurring hemorrhagic cysts requiring multiple arterial embolizations. He had undergone peritoneal dialysis between 2015 and 2016, switching to hemodialysis via a right tunnelled jugular catheter in late 2017. This change was motivated by recurring peritonitis and an incisional hernia.
The patient was not highly immunized (cPRA 0%) and the flow-cytometry cross-matching was negative. The 66-year-old transplant donor was EBV and CMV negative, whereas the patient had positive serology for EBV. CMV serology remained negative at the time of transplant. As per our usual transplant protocol, Basiliximab 20 mg IV, Solumedrol 8 mg/kg, Tacrolimus 0.1 mg/kg, and mycophenolate mofetil 1 g PO were used for induction. TMP-SMX 1 co DS PO die was used for prophylaxis. As CMV was negative in both donor and recipient, no antiviral prophylaxis was used. The immediate postoperative period was uneventful and the patient left the hospital after 8 days with a creatinine of 164 μmol/L (DFGe = 40 ml/minute). His diuresis was noted as normal at the time of discharge, and no hematuria was noted after the removal of the urinary catheter. As per protocol in our medical center, a graft Doppler echography and scintigraphy were conducted. The former revealed normal vascular flow and structure and the latter described acute tubular necrosis with a good prognosis. The patient was left with Prednisone 1 mg/kg on a 6-week tapper, down to 5 mg PO die. He was also prescribed Tacrolimus 5 mg PO bid and Cellcept 1 g PO bid. Leaving the hospital, his tacrolimus blood levels were at 8.7 ug/L.
First Acute Kidney Injury: Borderline Cellular Rejection
Little more than 16 weeks after the transplant, the patient’s creatinine rose to 172 μmol/L, from a nadir creatinine of 133 μmol/L. His tacrolimus levels had always been above the recommended 10 ug/L after leaving the hospital and he reported being compliant with immunosuppressive medication, which at that point was Tacrolimus 2.5 mg PO bid, Mycophenolate mofetil 1 g PO bid and prednisone 5 mg PO die. A kidney graft biopsy was undertaken and reported isolated T2 tubulitis. The SV40 and C4D markers were negative and no vasculitis was reported. Vascular fibrosis compatible with normal aging was also present. The patient was treated for a borderline cellular rejection with Methylprednisone 500 mg IV/d x 3. Prednisone was increased to 7.5 mg PO die at discharge. His creatinine returned to baseline within a month.
Second Acute Kidney Injury: Fever and Hematuria
Twenty-eight months after the transplant, the patient presented to the hospital with 10 days of fatigue and macroscopic hematuria and diarrhea. A high-grade fever of 40°C was noted on the physical exam. Acute kidney injury with a creatinine of 296 μmol/L and leukocytosis were evident in blood work. At that time, the patient was still on the same immunosuppressant regimen. Tacrolimus blood levels were within range (6–8 ug/L).
Investigations
The patient was hospitalized and investigated for more than 14 days. First-line ultrasound imaging did not reveal the source of fever or bleeding, as no hemorrhagic or infected cysts were apparent in the native right kidney and the kidney graft was normal.
Repeated hemocultures and serologies for fastidious bacteria, mycosis and viruses were completed and were all negative, i.e. CMV, EBV, Bartonella, Mycobacterias, Blastomyces, Polyomavirus, COVID-19, Q-fever, Coccidiomycès, Cryptococcus, and parvovirus.
Since AKI limited the use of contrast, a PET scan was ordered. Increased radiotracer uptake was seen on kidney graft images. In this context, ultrasound was repeated but was still negative. Labelled white blood cell scintigraphy was ordered and showed, as did the PET scan, increased nonspecific metabolic activity around the kidney graft.
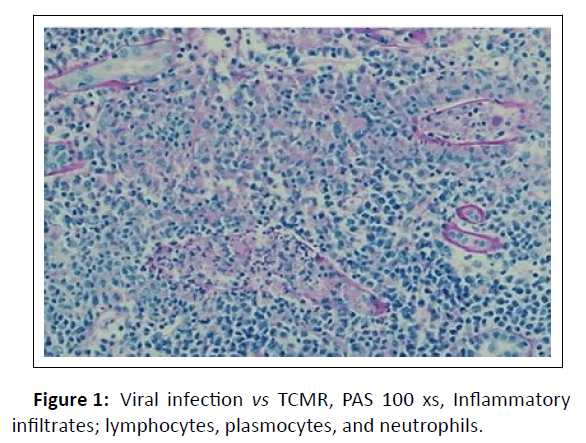
A graft biopsy was completed 16 days after admission based on the persistence of symptoms and the imaging being suggestive of kidney graft inflammation. The renal allograft biopsy showed severe tubulointerstitial nephritis with lymphocytes, plasma cells and neutrophils. There was tubular epithelial cell necrosis, and cell debris filled the tubular lumen. Some of the tubular basement membranes were ruptured and granulomas were identified. The glomeruli and blood vessels were spared and immunostaining for C4d was negative. Immunohistochemical stains for CMV and BKV were negative. Unfortunately, immunohistochemical staining for adenovirus was also negative. Nevertheless, ADV remained a potential culprit for AKI. However, acute TCMR could not be ruled out (Figure 1).
Figure 1: Viral infection vs TCMR, PAS 100 xs, Inflammatory infiltrates; lymphocytes, plasmocytes, and neutrophils.
Finally, quantitative PCR for ADV in urine was revealed positive for more than 950 million copies. No serum PCR or serology was completed.
Treatment
The patient was heavily hydrated. He received 14 days of empiric antibiotics. When, 4 days after completing antibiotic treatment, the patient did not defervesce, doxycycline was started, and a trial of 10 days was completed.
Upon confirmation of ADV infection via urine PCR and kidney biopsy results, mycophenolate mofetil was lowered to 500 mg PO bid and prednisone was increased to 20 mg PO die. Two treatments of IV immunoglobulins were also given.
Evolution
After lowering mycophenolate mofetil and IV immunoglobulins, the patient’s fever resolved and creatinine dropped back to 160 μmol/L. It had hovered around 250 during hospitalization, after initial rehydration. At the follow-up after discharge, his creatinine was 174 for a GFR of 34 ml/min/1.732. Hematuria and fever had resolved.
Discussion
This case exposes the clinical challenges associated with diagnosing ADV graft infection in a PCKD patient presenting with fever, hematuria, diarrhea and AKI. When interpreting kidney graft biopsy, distinguishing adenovirus interstitial nephritis from acute cellular rejection can become problematic especially when immunohistochemical staining for ADV is negative.
Our review of the literature revealed other cases of later onset ADV in graft kidney [5,6]. However, only 3 case reports present co-occurrence of PCKD and ADV graft kidney infection [8,9]. One of these cases occurred more than a year after transplant and presented with diarrhea, as did our patient [9]. The other two cases presented within one year and had more typical symptoms of fever with hematuria and urinary frequency [8,9]. The scarcity of literature describing concomitant ADV graft infection and PCKD precludes any discussion concerning the link between primary kidney pathology and viral infection. On the other hand, the possibility of hemorrhagic or infected cysts in the non-nephrectomized PCKD transplant population certainly poses diagnostic challenges and distracts from the possibility of a viral cause for infection.
The 2019 American Society of Transplantation Infectious Diseases Community of Practice guidelines describe histopathologic analysis as the gold standard for diagnosing ADV infection in symptomatic graft recipients. Rather than being a condition for diagnosis, immunohistochemical staining for adenovirus is mentioned as a useful tool to distinguish infection from rejection. Additionally, PCR-based testing is recommended when pathology is not available, and results should always be correlated to clinical symptoms [10]. In the case reviewed in this article, although immunohistochemistry for adenovirus was negative, the very high urinary viral load and the patient’s clinical presentation strongly suggest adenovirus interstitial nephritis rather than acute TCMR rejection.
The vast spectrum of severity associated with ADV infection in the kidney graft recipient commands a personalized approach to treatment. In our literature review, infection was associated with both life-threatening conditions and self-limited diseases. Kidney injuries menacing graft survival were also reported, much like in the case detailed above [11].
As with most viral infections in solid organ transplants, one of the cornerstones of treatment for ADV infection is the reduction of immunosuppression [12]. There is debate regarding which agent should be lowered first [13]. It is our practice to reduce or even stop mycophenolate when kidney graft recipients are diagnosed with an invasive viral infection or reactivation i.e. CMV, EBV, and BKV. Hence, we chose to reduce mycophenolate as soon as ADV graft infection was suspected in our patient. Prednisone was increased to reduce inflammation caused by viral infection. This approach was also used to empirically treat TCMR since it couldn’t be completely ruled out based on the patient's history of past borderline cellular rejection and findings on histopathologic analysis [14-16].
The second therapeutic tool used for the treatment of suspected kidney graft ADV infection was IV immunoglobulin. This therapy has biological plausibility, and its use is widely reported [17]. However, no association with virological response has been demonstrated, so use is only recommended in selected patients with hypogammaglobulinemia [18-20]. As in the case described above, dosing immunoglobulins to identify patients who are most likely to benefit from this therapy is not part of our practice and transplant patients affected with invasive viral infections are treated empirically.
Lastly, cidofovir is now recommended for the treatment of “severe, progressive or disseminated ADV infection in solid organ transplant recipients” [21]. This antiviral medication is a nucleotide analogue of cytosine that inhibits the viral DNA polymerase. Notably, its use is associated with AKI and Fanconi syndrome [22-25]. In the specific case of a kidney transplant, there are, in our opinion, few arguments to support the use of this nephrotoxic drug. Our team would consider prescribing the drug if the patient's life was at stake since it otherwise seems counterproductive in terms of graft survival.
Conclusion
In conclusion, based on our experience through the management of this case and after our literature review, some changes in practice will be implemented in our center: 1) We will lower our clinical index of suspicion for ADV infection after one year of transplant. 2) Along with urinary ADV PCR measurement, serum PCR measurement in symptomatic patients will be measured to help solidify our diagnosis. 3) We will continue our conservative approach to the treatment of ADV graft infection. Indeed, lowering mycophenolate, increasing prednisone and administering immunoglobulin will remain the main components of the therapy [26-29]. Considering its associated kidney toxicity, we will refrain from using cidofovir unless it is prescribed as a life-saving therapy.
Acknowledgments
None
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicting interests
The Authors declare they have no conflict of interest.
Ethical approval
Ethical approval to report this case was obtained from our local ethic committee (CRCHUS: 2021-4169).
Abbreviations
Poly-Cystic Kidney Disease (PCKD); Acute Kidney Disease (AKD); Adenovirus (ADV); T-Cell Mediated Rejection (TCMR); Epstein-Barr Virus (EBV); Cytomegalovirus (CMV).
References
- Florescu MC, Miles CD, Florescu DF (2013) What do we know about adenovirus in renal transplantation. Nephrol Dial Transplant 28: 2003-2010.
[Crossref], [Google Scholar], [Indexed]
- Florescu DF, Schaenman JM, Practice ASTIDCo (2019) Adenovirus in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 33: e13527.
[Crossref], [Google Scholar], [Indexed]
- Watcharananan SP, Avery R, Ingsathit A, Malathum K, Chantratita W, et al. (2011) Adenovirus disease after kidney transplantation: course of infection and outcome in relation to blood viral load and immune recovery. Am J Transplant 11: 1308–1314.
[Crossref], [Google Scholar], [Indexed]
- Hensley JL, Sifri CD, Cathro HP, Lobo P, Sawyer RG, et al. (2009) Adenoviral graft-nephritis: case report and review of the literature. Transpl Int 22: 672-677.
[Crossref], [Google Scholar], [Indexed]
- Kolankiewicz LM, Pullman J, Raffeld M, Kopp JB, Glicklich D (2010) Adenovirus nephritis and obstructive uropathy in a renal transplant recipient: Case report and literature review. NDT Plus 3: 388-392.
[Crossref], [Google Scholar], [Indexed]
- Klein J, Kuperman M, Haley C, Barri Y, Chandrakantan A, et al. (2015) Late presentation of adenovirus-induced hemorrhagic cystitis and ureteral obstruction in a kidney-pancreas transplant recipient. Proc (Bayl Univ Med Cent) 28: 488-491.
[Crossref], [Google Scholar], [Indexed]
- Mehta V, Chou PC, Picken MM (2015) Adenovirus disease in six small bowel, kidney and heart transplant recipients; pathology and clinical outcome. Virchows Arch 467: 603-608.
[Crossref], [Google Scholar], [Indexed]
- Buchanan W, Bowman JS, Jaffers G (1990) Adenoviral acute hemorrhagic cystitis following renal transplantation. Am J Nephrol 10: 350-351.
[Crossref], [Google Scholar], [Indexed]
- Dawood US, Nelson A, Wu D, Otto S, Russ GR (2014) Disseminated adenovirus infection in kidney transplant recipient. Nephrology (Carlton) 19: 10-13.
[Crossref], [Google Scholar], [Indexed]
- Alsaad KO, Tobar A, Belanger E, Ahmad M, Cattran DC, et al. (2007) Late-onset acute haemorrhagic necrotizing granulomatous adenovirus tubulointerstitial nephritis in a renal allograft. Nephrol Dial Transplant 22: 1257-1260.
[Crossref], [Google Scholar], [Indexed]
- Asim M, Chong-Lopez A, Nickeleit V (2003) Adenovirus infection of a renal allograft. Am J Kidney Dis 41: 696-701.
[Crossref], [Google Scholar], [Indexed]
- Barraclough K, Oliver K, Playford EG, Preston J, Campbell S, et al. (2009) Life-threatening adenovirus infection in a kidney transplant recipient. NDT Plus 2: 250-253.
[Crossref], [Google Scholar], [Indexed]
- Ferreira GF, Oliveira RA, Lucon M, de Paula FJ, Lucon AM, et al. (2009) Hemorrhagic cystitis secondary to adenovirus or herpes simplex virus infection following renal transplantation: four case reports. Transplant Proc 41: 4416-4419.
[Crossref], [Google Scholar], [Indexed]
- Hofland CA, Eron LJ, Washecka RM (2004) Hemorrhagic adenovirus cystitis after renal transplantation. Transplant Proc 36: 3025-3027.
[Crossref], [Google Scholar], [Indexed]
- Kozlowski T, Nickeleit V, Andreoni K (2011) Donor-transmitted adenovirus infection causing kidney allograft nephritis and graft loss. Transpl Infect Dis 13: 168-173.
[Crossref], [Google Scholar], [Indexed]
- Lachiewicz AM, Cianciolo R, Miller MB, Derebail VK (2014) Adenovirus causing fever, upper respiratory infection, and allograft nephritis complicated by persistent asymptomatic viremia. Transpl Infect Dis 16: 648-652.
[Crossref], [Google Scholar], [Indexed]
- Park UJ, Hyun SK, Kim HT, Cho WH, Han SY (2015) Successful treatment of disseminated adenovirus infection with ribavirin and intravenous immunoglobulin in an adult renal transplant recipient: A case report. Transplant Proc 47: 791-793.
[Crossref], [Google Scholar], [Indexed]
- Rady K, Walters G, Brown M, Talaulikar G (2014) Allograft adenovirus nephritis. Clin Kidney J 7: 289-292.
[Crossref], [Google Scholar], [Indexed]
- Ramirez J, Bostock IC, Martin-Onraet A, Calleja S, Sanchez-Cedillo A, et al. (2013) Fever, haematuria, and acute graft dysfunction in renal transplant recipients secondary to adenovirus infection: Two case reports. Case Rep Nephrol 2013: 195753.
[Crossref], [Google Scholar], [Indexed]
- Rosario RF, Kimbrough RC, Van Buren DH, Laski ME (2006) Fatal adenovirus serotype-5 in a deceased-donor renal transplant recipient. Transpl Infect Dis 8: 54-57.
[Crossref], [Google Scholar], [Indexed]
- Saliba M, Kfoury Assouf H, Abbas S, Hanna AP, Kamel G, et al. (2019) Adenovirus Infection as a Cause of Fever of Unknown Origin and Allograft Dysfunction in a Kidney Transplant Recipient. Exp Clin Transplant 17: 411-413.
[Crossref], [Google Scholar], [Indexed]
- Seralathan G, Kurien AA (2018) Adenovirus Interstitial Nephritis: An Unusual Cause for Early Graft Dysfunction. Indian J Nephrol 28: 385-388.
[Crossref], [Google Scholar], [Indexed]
- Stalder H, Hierholzer JC, Oxman MN (1977) New human adenovirus (candidate adenovirus type 35) causing fatal disseminated infection in a renal transplant recipient. J Clin Microbiol 6: 257-265.
[Crossref], [Google Scholar], [Indexed]
- Storsley L, Gibson IW (2011) Adenovirus interstitial nephritis and rejection in an allograft. J Am Soc Nephrol 22: 1423-1427.
[Crossref], [Google Scholar], [Indexed]
- Sujeet K, Vasudev B, Desai P, Bellizzi J, Takara LN, et al. (2011) Acute kidney injury requiring dialysis secondary to adenovirus nephritis in renal transplant recipient. Transpl Infect Dis 13: 174-177.
[Crossref], [Google Scholar], [Indexed]
- Umekawa T, Kurita T (1996) Acute hemorrhagic cystitis by adenovirus type 11 with and without type 37 after kidney transplantation. Urol Int 56: 114-116.
[Crossref], [Google Scholar], [Indexed]
- Veer M, Abdulmassih R, Como J, Min Z, Bhanot N (2017) Adenoviral nephritis in a renal transplant recipient: Case report and literature review. Transpl Infect Dis 19: 4.
[Crossref], [Google Scholar], [Indexed]
- Xu J, Patel KV, Dsouza M, Almendral J, Mody K, et al. (2018) Disseminated adenovirus infection in heart and kidney transplant. Turk Kardiyol Dern Ars 46: 231-233.
[Crossref], [Google Scholar], [Indexed]
- Yagisawa T, Nakada T, Takahashi K, Toma H, Ota K, et al. (1995) Acute hemorrhagic cystitis caused by adenovirus after kidney transplantation. Urol Int 54: 142-146.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences