Exploring Genes Associated with the SexDependent Variations in Lung Cancer
Kshitish K Acharya*
1Department of Applied Biotechnology, Institute of Bioinformatics and Applied Biotechnology, Bangalore, India
- *Corresponding Author:
- Acharya KK, Department of Applied Biotechnology, Institute of Bioinformatics and Applied Biotechnology, Bangalore, India E-mail: juneindia@gmail.com
Received Date: August 12, 2021; Accepted Date: August 26, 2021; Published Date: Sepftember 6, 2021
Citation: Acharya KK (2021) Exploring genes Associated with the Sex-Dependent Variations in Lung Cancer. J Lung Vol.2 No.4: 06.
Abstract
Genome-wide variations in men vs women seem to exist beyond reproductive tissues. Many genomic variations across the genders may be related to the cancer cause, prognosis, and therapeutic response. Since the prevalence, progression and outcome of lung cancer subtypes vary across genders, there was a need to explore molecular level variations potentially associated with gender bias among lung cancer patients. Genes involved in DNA repair and drug/toxicity-related metabolism and DNA adducts have been frequently studied in this context. Several other genes associated with the gender disparities in lung cancer have also been identified based on differences in mutations, copy numbers, epigenetic aspects including methylation and non-coding RNAs, and gene expression. I collated several such recent studies and tried to list key genes highlighted in such reports. But the list of genes is likely to be huge as mass-scale studies have identified hundreds of genes with potential association with gender disparities among lung cancer patients. I summarise the overall current trend in research towards identifying genes associated with gender bias in lung cancer. The review indicated the need for continued large-scale screening of genes and genomic regions. This stress on omics approach is particularly important to systematically prioritise the genes with the gender-biased association for further studies in the context of smoking and other compounding variables in each subtype of lung cancer.
Introduction
ALung cancer is one of the most common types of cancer globally. It contributes to approximately 13% of cancer occurrences in each gender. There are two main types of lung cancer: Non- Small Cell Lung Cancer (NSCLC) which contributes about 80% of lung cancer, and Small Cell Lung Cancer (SCLC). NSCLC has been further identified mainly into adenocarcinoma (LUAD), Squamous Cell Carcinoma (SCC), and large cell carcinoma.
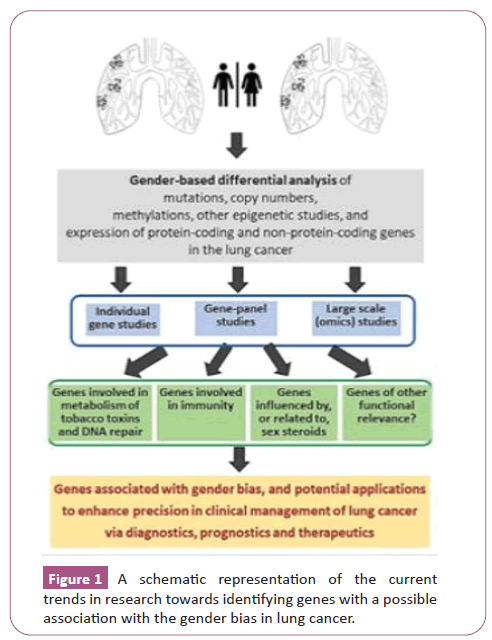
Gender variations, probably stemming from a combination of physiological and genetic differences, can potentially influence different aspects of lung cancer initiation, its development, clinical management, and outcome. A better understanding of such influences, especially at the molecular level, can help the clinical management of each subtype of lung cancer across the genders [1,2]. Studies so far have indicated the role of different molecular aspects of gender biases that may affect lung cancer. The molecular aspects of focus in such studies include mutations in some genes, copy number variations, epigenetics, expression of genes, and a combination of such factors. There seems to be a significant role in the interplay of such genetic factors with behavioral aspects such as smoking, infections and other environmental exposures. I used a combination of PubMed and other search engines, based on the recommendations for literature search made by Bajpai et al. [3], compiled relevant publications from recent years, and reviewed the key findings concerning the genes involved in sex-dependent differences in lung cancer. I aimed to capture the overall current trend of research in this direction (Figure 1) and list some of the genes highlighted in such studies as examples.
Gender bias in lung cancer prevalence, development and survival is a well-known phenomenon: Sex-dependent variations in various aspects of cancer, including the susceptibility, diagnostics, prognostics, therapeutic response and toxicity in cancer patients, could be significant. For example, studies have indicated a higher cancer incidence and related mortality in men than women, and a possible later stage diagnosis in smoking women [4-8]. Risks associated with lung cancer across genders have been reported differently by various group [9]. Studies on chemotherapeutic treatments for lung cancer patients have shown more pronounced toxicity and response rate and enhanced post-treatment survival of women than men [10,11]. Gender of the LUAD and SCC patients has been suggested as a prognostic factor [12]. A recent analysis of 4-year data from multiple countries on a total of 236,114 NSCLC and 43,167 SCC cases found that net survival is up to 4% higher among women than men [13].
Explorations of molecular genetic aspects of lung cancer have increased this decade; researchers have identified several generelated variations across the genders in lung cancer patients: Genome-wide variations in men vs women seem to exist beyond reproductive tissues. The examples include changes in the DNA such as mutations, variations in the copy number and epigenetic aspects, and tissue-level expression variations in the protein-coding and non-coding RNAs. Some of these changes may be related to the cancer cause, prognosis, and therapeutic response. Many studies used multiple-gene panels to explore the involvement of specific genes in sex-dependent variations in different types of cancers. Holistic or omics approaches have also been common among recent studies. The smoking status of female patients has particularly received significant attention when exploring the molecular aspects underlying lung cancer risks, progression and outcome. Genes involved in DNA repair and drug/toxicity related metabolism and DNA adducts have been frequently studied in the context of gender bias in lung cancer. Examples of such studies are summarized below.
Many studies focus on smoking habits and associated gender bias among lung cancer patients. For example, DNA adducts, mutations in TP53 and KRAS genes, and the expression of the CYP1A1 gene seem to be higher in the lungs of smoking women patients. In contrast, DNA repair may be less efficient in their lungs [7,9]. Gender and age-related differences have also been shown in the amount of drug-metabolizing enzymes in mouse tissues [14]. Higher levels of DNA adducts among smoking women have been indicated [15,16]. Sex-based differences are reported in the differential expression of genes among smoking and nonsmoking lung cancer patients [17].
Mutations, expressions and copy numbers of many specific genes are indicated to be gender-specific markers for cancer prognosis [18,19]. reported a possible association of single nucleotide polymorphisms in the MTHFR gene with lung cancer risk in women but not in men. Vaissiere et al. [20] found higher methylation levels in men than women in one (RASSF1A) of the five genes studied. A significantly higher proportion (>50%) of a set of cancer-drug-target genes were also found to have a gender bias in their mutations, copy numbers, methylations or expression levels 7.
The application of omics approaches has generated large scale molecular data, and meta-analysis of such datasets can help holistic screening of molecules associated with various aspects of lung cancer: Recently a comprehensive meta-analysis was performed on gene expression data from 1100 samples corresponding to 13 microarray studies from the Gene Expression Omnibus (GEO) and RNA-sequencing data from The Cancer Gene Atlas (TCGA) database17. This transcriptomic analysis identified 213 and 187 genes up and down-regulated, respectively, in cancerous lung tissues of women who smoked, while 230 and 244 genes were up- and down-regulated, respectively, in similar tissues of male patients who smoked. The genes SNX20, GIMAP6, MTMR2, FAM171B, IDH1, MOBP, FBXO17, LPXN and WIPF1 stood out as they were differentially expressed in the cancer-affected lungs of female smokers in at least 4 microarray studies, as well as in TCGA-RNA-Seq data. Among these genes, SNX20, MTMR2, LPXN and WIPF1 were more consistently differentially expressed. This study also identified 352 and 442 genes to be differentially expressed across the genders in adenocarcinoma.
Another transcriptomic analysis of LUAD by Li et al. [21] used TCGA and two microarray datasets. They suggested an association of 34 and 15 metabolic genes lung cancer susceptibility in men and women, respectively. A Next-Generation Sequencing (NGS) analysis of 68 genes on DNA from 52 pre-processed and fixed tissues of non-adenocarcinoma non-small-cell lung cancer subtypes suggested that frequency of mutations in EGFR, ALK, MET, ERBB2, RET and DDR2 is higher in Chinese women, among squamous cell carcinoma never-smoker patients [22].
System-Level Functional Significance of Gender-Biased Genes
AA significantly (FDR p<0.1) high number of genes up-regulated in cancerous lungs of smoking female patients were found to be involved in ‘immune responses’. These immune-related functions included leukocyte and lymphocyte activation and aggregation, cell-adhesion, protein-phosphorylation, proteolysis and signal transduction17. Such up-regulated genes also showed enrichment for pathways related to immune response. These pathways included Class I MHC mediated antigen processing and presentation, and signalling pathways such as the NGF, PI3-Akt, DAP12, EGFR and Insulin receptor pathways. On the contrary, genes up-regulated in the lungs of smoking male lung cancer patients did not show such immune-related functional association; they were mainly involved in ‘positive regulation of gene expression’, and other signalling pathways, including RAS, VEGF, insulin-receptor signalling, and ‘cell cycle’. Perez- Diez et al. [23] also performed a different type of meta-analysis recently. Their meta-analysis of GO terms and pathways indicated increased immune responses, purinergic signalling, and lipidrelated processes in women with lung adenocarcinoma patients than in men. It is interesting to see variations in the results of transcriptomic analyses by multiple approaches, including meta AA significantly (FDR p<0.1) high number of genes up-regulated in cancerous lungs of smoking female patients were found to be involved in ‘immune responses’. These immune-related functions included leukocyte and lymphocyte activation and aggregation, cell-adhesion, protein-phosphorylation, proteolysis and signal transduction17. Such up-regulated genes also showed enrichment for pathways related to immune response. These pathways included Class I MHC mediated antigen processing and presentation, and signalling pathways such as the NGF, PI3-Akt, DAP12, EGFR and Insulin receptor pathways. On the contrary, genes up-regulated in the lungs of smoking male lung cancer patients did not show such immune-related functional association; they were mainly involved in ‘positive regulation of gene expression’, and other signalling pathways, including RAS, VEGF, insulin-receptor signalling, and ‘cell cycle’. Perez- Diez et al. [23] also performed a different type of meta-analysis recently. Their meta-analysis of GO terms and pathways indicated increased immune responses, purinergic signalling, and lipidrelated processes in women with lung adenocarcinoma patients than in men. It is interesting to see variations in the results of transcriptomic analyses by multiple approaches, including metaanalysis. There are some common trends of observations across different studies, but there are also variations. Nevertheless, more such analyses can help short-listing key functional aspects and a set of reliable genes for further exploration. Integrated analysis of genomics and transcriptomics/proteomics is also needed in the context of gender bias in each subtype.
Thus, substantial information exists on the molecular basis of gender disparities in lung cancer characteristics. Some efforts are being made to use such information towards applications in diagnostics, prognostics and therapeutics of lung cancer of specific subtypes. However, more extensive screening of candidate genes and intergenic regions is needed before systematically analysing them individually or in smaller groups within them.
Conclusion
The list of some of the commonly studied genes in the context of gender bias in lung cancer presented here is far from being comprehensive. The observations emphasis two key aspects: a) Hundreds of genes may have a significant gender bias in their expression among smoking lung cancer patients. Similarly, there seem to be several genes with gender bias among nonsmoking lung cancer patients and people without the disorder. b) For each such gene, the consistency of expression-based gene association varies across patients/studies. A maximum of 5 of the 13 microarray studies indicated gender bias for any gene’s expression-based gender bias among smoking female patients. More holistic studies and a more comprehensive list of key such gender-bias genes, followed by a meta-analysis of various types, may help us get enough information to prioritise the genes for further exploration individually or in combination. During such meta-analysis, it may be essential to consider other parameters, such as smoking, ethnicity, diet, infections, which may influence lung cancer and gender disparities. Such analysis has the potential to enhance our precision in the clinical management of thoracic malignancies.
Acknowledgement
This work was partially supported by the Department of Electronics, IT, BT and S&T of the Government of Karnataka, and BdataA, Bengaluru and Shodhaka Life Sciences Pvt. Ltd., Bengaluru.
Conflict of Interest
The author is a scientific advisor for Shodhaka Life Sciences Pvt. Ltd., Bengaluru
References
- Tanoue LT (2021) Women and Lung Cancer. Clinics in Chest Medicine. 42: 467-482.
- Baiu I, Titan AL, Martin LW, Wolf A, Backhus L (2021) The role of gender in non-small cell lung cancer: a narrative review. J Thorac Dis. 13: 3816-3826.
- Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, et al. (2009) Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 18: 1174-82.
- Bajpai A, Davuluri S, Haridas H, Kasliwal G, Deepti H, et al. (2011) In search of the right literature search engine(s). Nature Precedings. 2101: 3.
- Oberaigner W, Siebert U (2011) Do women with cancer have better survival as compared to men after adjusting for staging distribution? Eur J Public Health. 21: 387-91.
- Marosi C (2006) Gender aspects of treatment and drug related toxicity in medical oncology. Wien Med Wochenschr. 156: 534-40.
- Lopes-Ramos CM, Quackenbush J, DeMeo DL (2020) Genome-wide sex and gender differences in cancer. Front Oncol. 10: 597788.
- Tolwin Y, Gillis R, Peled N (2020) Gender and lung cancer-SEER-based analysis. Ann Epidemiol. 46: 14-19
- Stapelfeld C, Dammann C, Maser E (2020) Sex-specificity in lung cancer risk. Int J Cancer. 146: 2376-2382
- Singh S, Parulekar W, Murray N, Feld R, Evans WK, et al. (2005) Influence of sex on toxicity and treatment outcome in small-cell lung cancer. J Clin Oncol. 23: 850-6
- Wakelee HA, Wang W, Schiller JH, Langer CJ, Sandler AB, et al. (2006) Survival differences by sex for patients with advanced non-small cell lung cancer on Eastern Cooperative Oncology Group trial 1594. J Thorac Oncol. 1: 441-6.
- Yuan Y, Liu L, Chen H, Wang Y, Xu Y, et al. (2016) Comprehensive Characterization of Molecular Differences in Cancer between Male and Female Patients. Cancer Cell. 29: 711-722.
- Araghi M, Fidler-Benaoudia M, Arnold M, Rutherford M, Bardot A, et al. (2021) International differences in lung cancer survival by sex, histological type and stage at diagnosis: an ICBP SURVMARK-2 Study. Thorax. 2020-216555.
- Li X, Lu Y, Ou X, Zeng S, Wang Y, et al. (2020) Changes and sex- and age-related differences in the expression of drug metabolizing enzymes in a KRAS-mutant mouse model of lung cancer. PeerJ. 8: e10182.
- Ryberg D, Hewer A, Phillips DH, Haugen A (1994) Different susceptibility to smoking-induced DNA damage among male and female lung cancer patients. Cancer Res. 54: 5801-3.
- Mollerup S, Ryberg D, Hewer A, Phillips DH, Haugen A (1999) Sex differences in lung CYP1A1 expression and DNA adduct levels among lung cancer patients. Cancer Res. 59: 3317-20.
- Davuluri S, Bajpai AK, Thirumurugan K, Acharya KK (2021) The molecular basis of gender disparities in smoking lung cancer patients. Life Sci. 267: 118927.
- Li CH, Haider S, Shiah YJ, Thai K, Boutros PC (2018) Sex Differences in Cancer Driver Genes and Biomarkers. Cancer Res. 78: 5527-5537.
- Shi Q, Zhang Z, Li G, Pillow PC, Hernandez LM, et al. (2005) Sex differences in risk of lung cancer associated with methylene-tetrahydrofolate reductase polymorphisms. Cancer Epidemiol Biomarkers Prev. 14: 1477-84.
- Vaissière T, Hung RJ, Zaridze D, Moukeria A, Cuenin C, et al. (2009) Quantitative analysis of DNA methylation profiles in lung cancer identifies aberrant DNA methylation of specific genes and its association with gender and cancer risk factors. Cancer Res. 69: 243-52.
- Li Y, He CL, Li WX, Zhang RX, Duan Y (2020) Transcriptome analysis reveals gender-specific differences in overall metabolic response of male and female patients in lung adenocarcinoma. PLoS One. 15: e0230796.
- Zhao Y, Dong Y, Zhao R, Zhang B, Wang S, et al. (2020) Expression Profiling of Driver Genes in Female Never-smokers With Non-adenocarcinoma Non-small-cell Lung Cancer in China. Clin Lung Cancer. 21: e355-e362
- Pérez-DÃez I, Hidalgo MR, Malmierca-Merlo P, Andreu Z, Romera-Giner S, et al. (2021) Functional signatures in non-small-cell lung cancer: A systematic review and meta-analysis of sex-based differences in transcriptomic studies. Cancers (Basel). 13: 143.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences