Evolving Concept of Brown Adipose Tissue in Humans
Miwako Nishio1*and Kumiko Saeki1,2
1 Department of Laboratory Molecular Genetics of Hematology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo 113-8510, Japan
2 Department of Regenerative Medicine, Research Institute, National Center for Global Health and Medicine, Tokyo 162-8655, Japan
- *Corresponding Author:
- Miwako Nishio
Department of Laboratory Molecular Genetics of Haematology,
Graduate School of Medical and Dental Sciences,
Tokyo Medical and Dental University,
Tokyo,
Japan,
Tel: +81-358035882
E-mail: mnishio.lmg@tmd.ac.jp
Received Date: December 22, 2020;Accepted Date: January 05, 2021;Published Date: January 12, 2021
Citation: Nishio M, Kumiko S (2021) Evolving Concept of Brown Adipose Tissue in Humans. Endocrinol Metab Vol. 5 No.1: 155.
Description
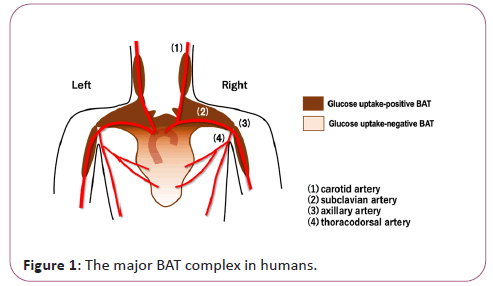
Brown Adipose Tissue (BAT) was first recognized as a heat- producing fat in small hibernating animals. BATs contribute not only to robust thermogenesis after hibernation but also to the maintenance of body temperature under acute cold exposure. They are distributed in specific sites of the body such as cervical, supraclavicular, axillary, paravertebral, periaortic and suprarenal regions. The existence of BAT in humans was described as early as in 1908 in autopsy reports [1], where the interscapular BAT was termed as “interscapular gland” that has cervical, clavicular and scapular processes. Its distribution sites correspond with those of the major arteries in the upper torso (Figure 1).
After more than one hundred years, human BAT was rediscovered, being driven by the fact that healthy individuals show unexpected radioactive signals in cervical, supraclavicular and axillary regions in 18F-FDG-PET studies [2-5]. A large volume of evidence has shown that the existence of BATs correlates with healthy metabolic status (example: non-obese, insulin-sensitive). The largest BAT in mice is located in interscapular regions and termed as interscapular BAT (iBAT). Since 18F-FDG signals in interscapular regions were detected only in infants in humans, the energy source of iBAT may be shifted from glucose to lipid in adult humans. Thermogenic ability of human BATs is activated by beta3-adrenergic stimuli [6] and Atrial Natriuretic Peptide (ANP) signals [7] under physiological conditions. Whether there are still other molecules that activate thermogenesis by BATs remains elusive. Since BATs are hyperactivated under cancer cachexia, certain cancer-related substances may possible serve as BAT activators [8]. Although BATs are distributed separately in the body, they create functional coalition via secreting factors [8]. BATs improve metabolism not only by enhancing energy expenditure via heat production but also by producing metabolism-improving secreting factors. BATs produce various bioactive substances, which are collectively called as BATokines. Among the proteins reported to serve as BATokines, only NGR4 and ANGPTL8 shows BAT-specific gene expression profiles [8]. Therefore, there may be still additional BATokines to be discovered. For example, there may be BAT-derived leptin sensitizer(s). It is known that BAT-ablated mice via genetic modification showed severe leptin resistance and, as a result, suffered from morbid obesity [9]. By contrast, UCP-1-deficient mice showed only mild leptin resistance without obesity [10]. Therefore, BATs guarantee leptin sensitivity independent of their thermogenic activities and energy expenditure. Besides soluble proteins, BATs produce low molecular weight substances and exosomes, which are shown to contribute to the improvement of metabolism [8]. Furthermore, BATs produce larger sized Extracellular Vesicles (EVs), in which are detected mitochondria [11]. The involvements of those EVs in metabolic improvement in humans are now awaiting to be explored (Figure 2).
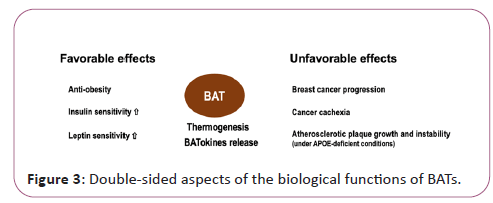
Although BATs are generally recognized as preferable tissues with anti-obesity and metabolism-improving functions, they may also be involved in promoting unhealthy conditions such as cancer cachexia, breast cancer progression and atherosclerotic plaque growth and instability as shown in APOE-deficient situations [8]. In this sense, BATs may be a double-edged sword (Figure 3). In clinically applying BATokines to health promotion, the dose as well as the mode of the usage should be carefully considered.
BATs are distributed in cervical, supraclavicular and axillary regions, which are adjacent to the main branches of the aortic artery. Not only widely recognized BATokines such as NGR4, ANGPTL8, FGF21, IL6, GDF15, BAT produces various substances including lipids, exosomes and larger extracellular vesicles [11]. BATs exert both favorable and unfavorable effects depending on the situations.
References
- Bonnot E (1908) The Interscapular gland. J Anat Physiol 43: 43-58.
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, et al. (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509-1517.
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R et al. (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360: 1518-1525.
- Van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, et al. (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500-1508.
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, et al. (2009) High incidence of metabolically active brown adipose tissue in healthy adult humans: Effects of cold exposure and adiposity. Diabetes 58: 1526-1531.
- Cypess AM, Weiner LS, Roberts-Toler C, Elia EF, Kessler SH et al. (2015) Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 21: 33-38.
- Carper D, Coue M, Nascimento EBM, Barquissau V, Lagarde D et al. (2020) Atrial natriuretic peptide orchestrates a coordinated physiological response to fuel non-shivering thermogenesis. Cell Rep 32: 108075.
- Nishio M, Saeki K (2020) The Remaining mysteries about brown adipose tissues. Cells 9: 2449.
- Mantzoros CS, Frederich RC, Qu D, Lowell BB, Maratos-Flier E et al. (1998) Severe leptin resistance in brown fat-deficient uncoupling protein promoter- driven diphtheria toxin A mice despite suppression of hypothalamic neuropeptide Y and circulating corticosterone concentrations. Diabetes 47: 230-238.
- Okamatsu-Ogura Y, Nio-Kobayashi J, Iwanaga T, Terao A, Kimura K et al. (2011) Possible involvement of uncoupling protein 1 in appetite control by leptin. Exp Biol Med 236: 1274-1281.
- Oka M, Kobayashi N, Matsumura K, Nishio M, Nakano K et al. (2020) New role for growth/differentiation factor 15 in the survival of transplanted brown adipose tissues in cooperation with Interleukin-6. Cells 9: 1365.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences