Does suPAR Have Diagnostic Importance in Bronchoalveolar Fluid?
Hamdiye Turan1*, Atalay Sahin2, Serif Kurtulus1, Nihayet Bayraktar3 and Hamza Eroglu4
1Department of Chest Diseases, Medical School, Harran University, Sanliurfa, Turkey
2Department of Thoracic Surgery, Medical School, Harran University, Sanliurfa, Turkey
3Department of Biochemistry, Medical School, Harran University, Sanliurfa, Turkey
4Department of, Biostatistics, Medical School, Harran University, Sanliurfa, Turkey
*Corresponding Author:
- Hamdiye Turan
Department of Chest Diseases, Medical School, Harran University, Sanliurfa, Turkey
E-mail: dr_hamdiyeturan@hotmail.com
Received date: September 28, 2023, Manuscript No. ipjl-23-17958; Editor assigned date: October 02, 2023, PreQC No. ipjl-23-17958 (PQ); Reviewed date: October 16, 2023, QC No. ipjl-23-17958; Revised date: January 03, 2025, Manuscript No. ipjl-23-17958 (R); Published date: January 10, 2025, DOI: 10.36648/IPJL.6.1.001
Citation: Turan H, Sahin A, Kurtulus S, Bayraktar N, Eroglu H (2025) Does suPAR Have Diagnostic Importance in Bronchoalveolar Fluid? J Lung Vol:6 No:1
Abstract
suPAR could contribute to the early diagnosis of lung cancer and tuberculosis.
Materials and methods: Bronchoscopy and bronchoalveolar lavage were performed on 66 patients with a pre-diagnosis of lung malignancy and tuberculosis who applied to Harran University Hospital. Simultaneous blood samples were taken. suPAR was studied with the ELISA method. It was compared with 30 healthy individuals as the control group. Informed consent was obtained from the patients for the study.
Results: In the patient group, the median plasma suPAR level was 229.59 ng/L and the BAL fluid level was 45.73 ng/L. The median plasma suPAR level of patients with malignancy was 199.40 ng/L, 246.09 ng/L in TB patients and 234.60 ng/L in other diseases. In BAL fluid, the median plasma suPAR level was found to be 56.78 ng/L in patients with lung cancer, 39.24 ng/L in patients with TB, and 44.00 ng/L in other diseases. When the patient and control groups were compared, a significant difference was found in the median plasma suPAR level.
In the study, it was found that plasma suPAR level decreased with age (plasma levels of suPAR for each year was 1.08 ng/L), while it increased with age in the healthy group. In our study, a significant difference was found between the plasma levels of suPAR in the patient and control groups. The mean plasma levels of suPAR ratios of the patients and healthy individuals were statistically significant and the cutoff level was 136.61 ng/L suPAR, BAL fluid level was not statistically significant.
Conclusion: In our study, plasma level of suPAR in patients with malignancy may be a nonspecific marker in the pre-diagnosis period, and may also be a useful prognostic marker in predicting negative outcomes in the post-diagnosis period. In our study, plasma level of suPAR above the mean in male patients with primary lung adenocarcinoma raise the question of whether there is a difference between cancer subtypes and genders.
Keywords
Bronchoalveolar fluid; suPAR; Lung cancer; Tuberculosis
Introduction
Tuberculosis (TB) is the cause of hundreds of thousands of deaths in the world every year and continues to be an important public health problem [1]. For the diagnosis of pulmonary tuberculosis, bacillus should be shown in the sputum smear. The gold standard in diagnosis is the production of bacillus in culture [2]. TB may radiologically resemble and mimic any lesion [3].
Lung cancer is the most common cause of cancer related deaths in men and women, causing more than one million deaths per year [4]. Most of the cases diagnosed with lung cancer are caught at an advanced stage. Studies on this subject have shown that an average of 35 to 50 days is required to reach the diagnosis and start treatment [5].
The radiological manifestations of tuberculosis may be confused with lung cancer. TB is often misdiagnosed with lung cancer and varying clinical presentations, resulting in delayed treatment initiation. This results in unnecessary diagnostic procedures. This situation delays the diagnosis of possible lung cancer and causes the patient to reach treatment late due to the advanced diagnosis. As a result, it causes increased morbidity and mortality. Like consolidations with irregular edges and thickwalled spaces, radiological features suggestive of malignancy, showing high metabolic activity in Positron Emission Tomography/Computed Tomography (PET/CT) imaging, are also typical for tuberculosis cases seen in the thorax [6].
The normal plasma level of suPAR, an immune-activated protein, is below 3 ng/mL in healthy individuals and above 6 ng/mL in critically ill patients. Low levels indicate a good prognosis and support the patient's decision to treat. High levels indicate the presence, progression and severity of the disease and support greater clinical interest [7].
We aimed to investigate the contribution of suPAR levels in bronchoalveolar lavage fluid and plasma to the diagnosis of TB and Lung cancer in the early period and its usability as a serological marker.
Materials and Methods
Patients who were admitted to the Harran university faculty of medicine, chest diseases and thoracic surgery outpatient clinics with pathology in their radiological imaging and undergone bronchoscopy were included in our study. Approval for our study was obtained from the ethics committee of Harran university, faculty of medicine. Our study was a prospective study. Bronchoscopy was performed on 66 patients with pulmonary tuberculosis, lung cancer and other diagnoses. Plasma samples were taken simultaneously with bronchoalveolar lavage fluid from these patients. The materials were frozen at -80°C. suPAR was studied with the ELISA method. The suPAR levels were determined in the collected BAL and plasma. These patients were compared with the suPAR level studied from plasma samples taken from 30 healthy individuals.
Statistics
Continuous variables were expressed as mean ± standard deviation, and categorical data as numbers and percentages.
Comparisons of categorical data were made with the Chi-square test (Pearson, Fihser's exact test or linear by linear association, as appropriate). Analyzes were performed with IBM SPSS version 26.0 (IBM Corporation, Armonk, NY, USA). Cases where the type 1 error level was below 5% were considered significant.
Results
Bronchoalveolar lavage was performed on 66 patients participating in the study. Of these, 46 (69.69%) were male and 20 (30.31%) were female. As the control group, plasma was taken from 30 healthy individuals. Of these, 17 (56.7%) were male and 13 (43.3%) were female. Considering the difference between the plasma levels of suPAR in the patients and healthy individuals. The median plasma suPAR level concentration of the patients was 229.59 ± 79.03 ng/L. In healthy individuals, the mean was 108.2 ± 2.14 ng/L. This rate was found to be statistically significant (p<0.05). The median plasma suPAR level of the patient group was 121,381 ng/L higher than the control group. Cut off level was found as 136.61 ng/L. The plasma level of suPAR was found to be statistically significant in sick individuals (Table 1).
| Age | TB | CA | Others | Control | Mean |
|---|---|---|---|---|---|
| Gender M/F | 08/3 | 10/3 | 28/13 | 17/13 | |
| Plasma suPAR | 246,09 | 199,4 | 234,59 | 108.2 | 221,38 |
| BAL suPAR | 39,24 | 56,78 | 44,00 | -- | 45,73 |
| Control plasma suPAR | 108,2 |
Table 1: Demographic data of the patients.
In the comparison of plasma suPAR level of the patients between genders, p=0.544, with p=greater than 0.05. The median plasma suPAR level of concentration in men and women was not significantly different in the patient group. When the same comparison was made between healthy individuals, p=0.410 was not found statistically significant. In this case, we can say that there was no significant difference between the genders in the plasma suPAR level of sick and healthy individuals.
When the BAL suPAR level was compared among the patients in terms of gender, p=0.168, was not statistically significant. The median BAL suPAR level of men and women were not significantly different in the patient group.
While the median plasma suPAR level of sick individuals was 229.59 ng/L, this rate was found to be 45.73 ng/L in bronchoalveolar fluid. When the plasma suPAR level of the patients were evaluated among themselves, it was seen that lung cancer, tuberculosis and other diseases were not significant among themselves p>0.05.
Among the BAL suPAR level of the patients, the p-value for all three diagnoses (cancer, tuberculosis, others) was >0.05, and the diagnoses did not have a significant effect on the BAL fluid levels. Since all p values were not greater than 0.05, it was observed that the “fluid” dependent variable did not have a normal distribution in all three diseases (cancer, tuberculosis and others), but the BAL suPAR level were found statistically significant level in the diagnoses themselves (Table 2).
| Age | Patients plasma suPAR (ng/L) | Control plasma suPAR (ng/L) |
|---|---|---|
| mean ± SS | mean ± SS | |
| Less 30 years | 264,81 ± 64,15 | 102,04 ± 11,80 |
| 31-59 years | 243,97 ± 86,78 | 105,06 ± 10,96 |
| More than 60 years | 203,63 ± 65,16 | 114,76 ± 13,33 |
| Test value/p | 3,544/0,034 | 2,912/0,072 |
| Gender | ||
| Female | 240,32 ± 71,53 | 108,22 ± 12,10 |
| Male | 229,07 ± 80,71 | 110,31 ± 14,20 |
| Test value/p | 0,544/0,588 | 0,410/0,674 |
Table 2: Comparison of plasma suPAR levels of the patients according to the groups.
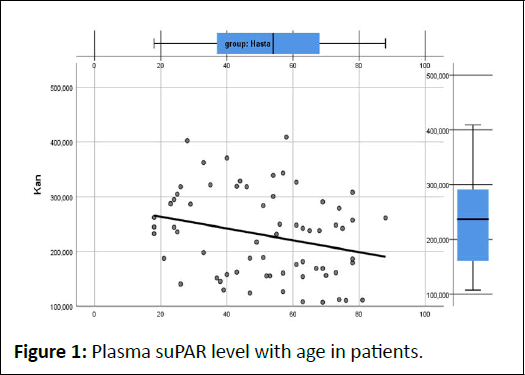
In the patient group, when the age variable increases by 1 year, that is, when the patients' age for 1 year, the levels of the plasma level decreases by 1.08 (ng/L). It was observed that the plasma suPAR level of the patients decreased as their age increased. It was found to be statistically significant as the pvalue= 0.035<0.05. In the bronchoalveolar fluid, it was observed that the suPAR levels did not increase with age (p-value=0.671> 0.05). It was not found statistically significant.
The mean plasma suPAR level of the lung cancer cases was 199.40 ± 77.65 ng/L. This rate was found to be 246 ± 63.76 ng/L in tuberculosis. These rates were not statistically significant when compared to other diseases. The mean suPAR level of patients with lung cancer in the bronchoalveolar fluid was 56.78 ± 21.38 ng/L. This rate was 39.24 ± 24.99 ng/L in cases with tuberculosis. It was not statistically significant when compared to other diseases.
However, when suPAR level in the plasma and bronchoalveolar fluid are examined in terms of diagnoses, the BAL/Plasma suPAR ratio is 0.34, 0.16, 0.21 (lung cancer, tuberculosis and other diseases), respectively, and when the diseases are compared with each other, lung cancer was found to be statistically significant from other lung pathologies (p=0.01).
Discussion
In our study, in which we investigated the use of suPAR levels in bronchoalveolar lavage and plasma samples as an early and serological marker in the diagnosis of TB and lung cancer, a total of 66 patients and 30 healthy individuals were compared. In our study, there were 13 patients with lung cancer and 11 patients with active TB. In our study, it was found that plasma suPAR level decreased with age (patients’ plasma suPAR levels was 1.08 ng/L for each year), while it increased with age in the healthy group. It was found that they were similar in terms of gender and there was no statistically significant difference between the groups for gender and age variables. In our study, a significant difference was found between the plasma suPAR levels of the patient and control groups. While the median plasma suPAR level was 229 ng/L in the patient, this rate was found to be 108 ng/L in healthy individuals. This rate was statistically significant and the cut-off level was 136.61 ng/L. In BAL fluid median suPAR level was found to be 56.78 ng/L in patients with lung cancer, 39.24 ng/L in patients with TB and 44.00 ng/L in other diseases.
suPAR level in BAL fluid was not statistically significant. Lung cancer is the leading cause of death from cancer. Urokinase Plasminogen Activator (uPA) is present in the cell. It has a soluble form in the cell membrane and circulation. This allows both binding and activation of uPA. It thus leads to the conversion of the plasmin gene to plasmin and the resulting proteolytic activity to be associated with plasmin. With this increase, plasmin can affect the spread of cancer in various ways. Through plasmin, cancer cells are either directly promoted to cell migration or disrupted by the extracellular matrix [8].
In lung cancer, suPAR level increases in plasma as a result of shed surface tumor cells and their metastases [9]. Mycobacterium tuberculosis affects the lives of millions of people worldwide, and it is estimated that more than two million people worldwide die from the disease each year. In our study, which is a prospective cohort study, the definitive diagnosis of the patients was a pathological diagnosis of lung cancer and microbiological culture positivity for tuberculosis. Diagnosis of lung cancer (CA) and pulmonary Tuberculosis (TB) requires time. Early diagnosis is difficult. Early diagnosis of both diseases is important in terms of mortality and morbidity, is it possible to contribute to the diagnosis with suPAR? It has been shown in previous studies that suPAR activity, one of the serological markers whose applicability has been investigated in the diagnosis of TB and CA, increases in the fluids, plasma and serum of patients [10]. In our study, the contribution of the results obtained to the differential diagnosis of CA, TB and other (interstitial lung disease, fungal infections, benign radiology) diseases were investigated by comparing the plasma and BAL suPAR levels of CA, TB and other cases with the plasma suPAR level of healthy subjects. The question of whether it can be a rapid, sensitive and specific method for early diagnosis of patients was examined. Increases plasma suPAR levels have been helpful in evaluating the invasion and metastasis process of lung cancer [8]. suPAR, as a promising biomarker, has been shown to be related to prognosis in patients with lung, breast, ovarian and colon cancer patients with increased plasma levels [11].
In our study, it was observed that suPAR increased levels in the plasma lung cancer cases, but it was not significant in the differential diagnosis from other diseases (p>0.005). A comment could not be made because no comparison was made for BAL. Based on the BAL/Plasma suPAR levels in our study, we found it statistically significant compared to other diseases when we used it in lung cancer cases. It can be suggested to us to use the ratio of the BAL and plasma levels of suPAR in the evaluation of cancer risk algorithms. suPAR levels are not only elevated in infections but also in malignancies, infections, or other inflammatory processes such as chronic pleuritis [12].
SUPAR also contributes to the diagnosis of traditional biomarkers (CRP, LDH) [13]. In our study, BAL and plasma suPAR levels were found to be significant between the patient and control groups, but not between diseases. In addition, the ratio of BAL/Plasma suPAR level was significant among the patient groups.
In the studies conducted by Rasmussen et al., it was stated that suPAR increased in the plasma of individuals included in the study with ageing. (0.1 ng/ml per year) (12,13). In our study, we found that the plasma level of suPAR decreased with age (p=0.0035), and it did not increase in the bronchoalveolar fluid (p=0.671). We reached a finding contrary to Ramusse's study. We have considered a heterogeneous group in the form of lung cancer, TB and other lung diseases (Figure 1).
Figure 1: Plasma suPAR level with age in patients.
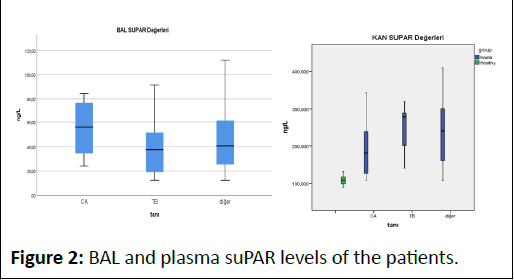
In our study, no significant difference was found in terms of gender and age in suPAR levels in both plasma and BAL fluid. In our study, plasma suPAR level was high in patients with lung cancer and was not specific. In a previous CE study by Bock et al., it was concluded that high suPAR levels are associated with an increased risk of respiratory and other types of cancer, but not with gastrointestinal cancers (Figure 2) [8,10,14,15].
Figure 2: BAL and plasma suPAR levels of the patients.
In our study, the median plasma suPAR level of lung cancer patients was found to be 199.4 ng/dl. When our cancer cases were examined, primary adenocancer cases were five patients and two of them were female patients. The average of their plasma suPAR level was 179.91 ng/dl, which was below the mean value. The median plasma suPAR level of male patients was 301.30 ng/dl, which was higher than the mean value. The average BAL suPAR level of patients diagnosed with adenocancer was 33.53 ng/dl for women and 61.45 ng/dl for men. The BAL suPAR levels of women were found to be lower than the average suPAR levels. This brought to mind the question of whether there was a gender difference in malignancies. Therefore, the difference could not be detected. Otherwise, the majority of gastrointestinal cancers are adenocancers and it raised the question of whether the plasma level was low in the female population.
Conclusion
In our study, while there was a lower plasma suPAR levels of healthy individuals, it was found to be elevated when compared to patients with malignancy or other diseases. While this may be a nonspecific marker in the pre-diagnosis period, it can also be a useful prognostic marker in predicting negative outcomes in the post-diagnosis period.
In addition, our study revealed the necessity of clarifying the answer to the question of whether there is a difference between cancer subtypes and genders, as plasma suPAR levels are above the average in male patients with primary lung adenocarcinoma.
Limitations
Although the plasma suPAR levels were high in male patients with adenocarcinoma in our study, the limited number of patients and the heterogeneity of the diseases were the limitations of the study.
References
- Salari N, Kanjoori AH, Hosseinian-Far A, Hasheminezhad R, Mansouri K, et al (2023) Global prevalence of drug-resistant tuberculosis: A systematic review and meta-analysis. 12:57
- Acharya B, Acharya A, Gautam S, Ghimire SP, Mishra G, et al. (2020) Advances in tuberculosis diagnosis: An update on the molecular diagnosis of Mycobacterium tuberculosis. Mol Biol Rep 47:4065-4075
- Bade BC, Cruz CS (2020) Lung cancer 2020: Epidemiology, etiology, and prevention. Clin Chest Med 41:1-24
[Crossref] [Google Scholar] [PubMed]
- Niyonkuru A, Bakari KH, Lan X (2018) 18F-fluoro-2-deoxy-d-glucose PET/computed tomography evaluation of lung cancer in populations with high prevalence of tuberculosis and other granulomatous disease. PET Clinics 13:19-31
[Crossref] [Google Scholar] [PubMed]
- Siawaya JF, Bapela NB, Ronacher K, Veenstra H, Kidd M, et al. (2008) Immune parameters as markers of tuberculosis extent of disease and early prediction of anti-tuberculosis chemotherapy response. J Infect 56:340-347
[Crossref] [Google Scholar] [PubMed]
- Cobos E, Jumper C, Lox C (2003) Pretreatment determination of the serum urokinase plasminogen activator and its soluble receptor in advanced small-cell lung cancer or non-small-cell lung cancer. Clin Appl Thromb Hemost 9:241-246
[Crossref] [Google Scholar] [PubMed]
- Eugen-Olsen J, Giamarellos-Bourboulis EJ (2015) suPAR: The unspecific marker for disease presence, severity and prognosis. Int J Antimicrob Agents 46:S33-S34
[Crossref] [Google Scholar] [PubMed]
- de Bock CE, Wang Y (2004) Clinical significance of urokinaseâ?ÂÂÂtype Plasminogen Activator Receptor (uPAR) expression in cancer. Med Res Rev 24:13-39
[Crossref] [Google Scholar] [PubMed]
- Olivianto E, Sudarwati S, Nataprawira HM (2019) Soluble urokinase-type plasminogen activator receptor as a biomarker of treatment response in childhood tuberculosis. Int J Microbiol Res 8:262-266
[Crossref] [Google Scholar] [PubMed]
- Rasmussen LJ, Caspi A, Ambler A, Danese A, Elliott M, et al. (2021) Association between elevated suPAR, a new biomarker of inflammation, and accelerated aging. J Gerontol 76:318-327
[Crossref] [Google Scholar] [PubMed]
- Haupt TH, Rasmussen LJ, Kallemose T, Ladelund S, Andersen O, et al. (2019) Healthy lifestyles reduce suPAR and mortality in a Danish general population study. Immun Aging 16:1-2
[Crossref] [Google Scholar] [PubMed]
- Langkilde A, Hansen TW, Ladelund S, Linneberg A, Andersen O, et al. (2011) Increased plasma soluble uPAR level is a risk marker of respiratory cancer in initially cancer-free individuals. Cancer Epidemiol Biomarkers Prev 20:609-618
[Crossref] [Google Scholar] [PubMed]
- Brunner N, Nielsen HJ, Hamers M, Christensen IJ, Thorlaciusâ?ÂÂÂUssing Ol, et al. (1999) The urokinase plasminogen activator receptor in blood from healthy individuals and patients with cancer. APMIS 107:160-167
[Crossref] [Google Scholar] [PubMed]
- Henic E, Borgfeldt C, Christensen IJ, Cassleen B, Hoyer-Hansen G (2008) Cleaved forms of the urokinase plasminogen activator receptor in plasma have diagnostic potential and predict postoperative survival in patients with ovarian cancer. Clin Cancer Res 14:5785-5793
[Crossref] [Google Scholar] [PubMed]
- Rasmussen LJ, Petersen JE, Eugen-Olsen J (2021) Soluble urokinase Plasminogen Activator Receptor (suPAR) as a biomarker of systemic chronic inflammation. Front Immunol 12:780641
[Crossref] [Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences