Differential Frequencies and Functions of NK Cells and CD127 (+) ILC1s in the Terminal Ileum of Crohn’s Disease Patients
Sarah C Glover1*, Andria L Doty1, Ying Tang1, Dalton Berrie1, Atif Iqbal2, Sanda A Tan2 and Jian Li1
1Division of Gastroenterology, Hepatology and Nutrition, Department of Medicine, University of Florida, Gainesville, FL, USA
2Department of Surgery, College of Medicine, University of Florida,Gainesville, FL,USA
- *Corresponding Author:
- Glover SC
Department of Medicine, Division of Gastroenterology, Hepatology
and Nutrition, University of Florida, Gainesville, FL, USA
Tel: +352-265-8971
E-mail: Sarah.glover@medicine.ufl.edu
Received date: October 12, 2017; Accepted date: October 25, 2017; Published date: November 1, 2017
Citation: Sarah C Glover, Andria L Doty, Ying Tang, Dalton Berrie, Atif Iqbal, et al. (2017) Role of Immunologists in the Development of Health Care System. J Immuno Immunother. Vol. 1 No. 1:3
Copyright: © 2017 Glover SC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Crohn’s disease (CD) is a chronic immunemediated disorder of the human gut with an unknown etiology. Traditionally, adaptive immunity has been believed to play a major role in this process. However, emerging evidence also suggests the importance of innate immunity for the pathogenesis of CD.
Methods: We collected surgical terminal ileum samples from 15 CD patients and biopsy terminal ileum samples of an-IL10RA-deficiency-caused-CD patient at different time points. Then we isolated lamina propria mononuclear cells (LPMC) from harvested tissues through enzyme digestion and mechanical dissociation. Finally, we compared the alteration of frequencies and functions of NK cells and CD127(+) ILC1s between inflamed ileums and unaffected ileums of our CD patient cohort by multicolor flow cytometry.
Results: The frequency of NK cells within CD3(-) CD14(-) CD19(-) CD20(-) CD45 (+) CRTH2 (-) LPMCs was significantly increased in the inflamed ileums (51.3%, n=10) compared to unaffected tissues (29.4%, n=5). Then, the frequency of IFN- γ (+) NK cells was also upregulated in the inflamed tissues (34.0%, n=10) compared to control (9.2%, n=5). Further, we observed that the NK cell frequencies were gradually reduced and accompanied by the restoration of normal mucosal architecture in the terminal ileum of our IL10RAdeficiency- caused-CD patient after successful immunosuppressive treatment. In addition, we noticed that the frequency of CD127(+) ILC1s within CD3(-) CD14(-) CD19(-) CD20(-) CD45(+) CRTH2(-) LPMCs was augmented as well in the inflamed tissues (2.2%, n=10) compared to unaffected areas (0.76%, n=5). They were also potent IFN-γ producers.
Conclusions: NK cells and CD127(+) ILC1s displayed differential frequencies and functions in the inflamed terminal ileum of our CD patient cohort. Better understanding of the development, differentiation, phenotypic and functional regulation of these two subsets in the human gut may shed new light on approaches for the modulation of immunity in the future.
Keywords
Crohn’s disease; NK cells; CD127(+) ILC1s; Intestinal inflammation; Innate immunity
Introduction
Crohn’s disease (CD) is one type of inflammatory conditions of the human gut. This chronic debilitating disease has a deep impact on patients' life quality. Half of them will have to receive surgery within 10 years after diagnosis because of the development of intestinal complications [1]. Unfortunately, the exact mechanism for the pathogenesis of CD is not completely understood yet and much of the focus in CD research in the past has been on adaptive immunity. Recently, a large body of literature has emerged evaluating the role of innate immunity in the pathogenesis of CD [2]. Newly identified Innate Lymphoid Cells (ILCs) are important components of the innate immune system. As the first line of defense, they have critical effector and regulatory functions in host response, maintenance of mucosal homeostasis and tissue repair, especially along the surfaces of intestinal mucosa [3-6]. ILCs functionally mirror effector T cells but lack antigen specific receptors [7]. According to their specific transcription factor and cytokine production features, ILCs are divided into cytotoxic NK cells, T-bet (+) ILC1s, Gata3 (+) ILC2s and RORγt (+) ILC3s [8,9]. Accumulating evidence suggests the involvement of ILCs in the pathogenesis of CD, especially the IFN-γ-producing ILC subsets, since IFN-γ is an important mediator of the intestinal inflammation [10-15]. Takayama et al showed that IFN-γ-producing-NKp46 (+) NKp44 (-) NK cells were predominant in the inflamed colon of CD patients [16]. Bernink et al. reported the expansion of IFN-γ- producing CD127 (+) ILC1s in the inflamed ileum of CD patients (10). Fuchs et al discovered the accumulation of CD103(+) IFN-γ- producing ILC1 subset in the intraepithelial compartment of the intestinal mucosa of CD patients [12]. However, Simoni et al failed to identify the CD127 (+) ILC1 subset across healthy and inflamed human tissues, including the gut through their mass cytometry (CyTOF) approach [17]. Due to these various findings, more research is needed to characterize the phenotype and functions of ILCs in the gut of CD patients.
In this study, we focused on the cytotoxic NK cells and CD127(+)ILC1s in the ileal lamina propria of CD patients. Here, NK were defined as CD3(-) CD14(-) CD19(-) CD20(-) CRTH2(-) CD127(-) CD45(+) CD56(+) cells and CD127(+)ILC1s were defined as CD3(-) CD14(-) CD19(-) CD20(-) CRTH2(-) CD45(+) NKp44(-) CD117(-) CD127(+)cells. We aimed to compare the frequency and function changes of NK cells and CD127(+) ILC1s between the inflamed surgical ileum samples and unaffected tissues of CD patients. Also, we investigated the dynamic frequency alterations of NK cells and CD127(+) ILC1s during the mucosal healing process of terminal ileum in an-IL10RA-deficiencycaused- CD patient after anakinra (an IL1 receptor antagonist) treatment. More knowledge of the NK cells and CD127(+) ILC1s biology in CD patients could offer potential therapeutic opportunities for better outcomes.
Materials and Methods
Subjects
Fresh surgical terminal ileum samples were collected from 15 CD patients and terminal ileum biopsy samples were collected from an-IL10RA-deficiency-caused-CD patient at the UF Health Shands Hospital (Gainesville, FL) after obtaining written informed consent from each patient. All subjects gave written informed consent in accordance with the Declaration of Helsinki. All experiments were performed according to University of Florida Institutional Review Board approved protocol.
Lamina propria mononuclear cells (LPMC) isolation from human intestine
After surgery, pathologists from UF Health Shands Hospital performed gross examination of the surgical samples, helped us to differentiate the macroscopically unaffected tissues or inflamed tissues and cut it for us. Fresh tissues were then transferred to the lab on ice within one hour after the surgery. LPMCs were isolated essentially by mechanically disassociation and enzyme digestion as previously described [15]. Briefly, fresh surgical tissue was cut into little strips, washed twice with Mg2+ and Ca2+-free HBSS media containing 0.5 mM DTT at 37°C in the shaker for 10 min at the speed of 175 rpm. The supernatant was discarded. Remaining tissue was further minced and digested in 25 ml DMEM supplemented with 75U/ml Collagenase type XI, 20μg/ml Dispase neutral protease II, 500 U/ml DNase, 0.5 mM DTT and 1% FBS at 37°C in the shaker for 15 min, filtered through a 70 μm cell strainer. The supernatant was collected and centrifuged at 1200 rpm for 8 min. All cells were slowly cryopreserved in cell freezing medium and transferred to liquid nitrogen for future experiments.
Cell culture
K562 cells (ATCCR -CCL-243TM) were cultured in RPMI 1640 Medium (Cat#72400-047, Gibco), supplemented with 2mM Lglutamine (Cat#25030-08, Life Technologies), 100 U/ml penicillin, 100μg/ml streptomycin (Cat#15140-122, Life Technologies), and 10% fetal bovine serum (Cat#26140-079, Gibco).
Fluorescence activated cell sorting (FACS) of primary human NK cells
Antibodies used for flow cytometric analyses are described in the supporting information. After thawing the frozen human LPMC from liquid nitrogen, we added pre-warmed complete RPMI-1640 media dropwise, and the cells were then spun down at 800 rpm for 10 min. The resulting cell pellet was washed twice with 1xPBS and the cell density was adjusted to 1 × 106 cells/ml. Next, 1 μl/ml reconstituted LIVE/DEAD™ Fixable Yellow dye (Cat# L34959, Life technologies) was added and incubated on ice for 30min, protected from light. The cells were then washed twice and incubated with conjugated monoclonal antibodies including anti-human Lineage Cocktail 3(CD3, CD14, CD19 and CD20), anti-CD45, anti-CD127, and anti- CD56 at room temperature for 15min in the dark. The cells were washed and sorted with a FACSAriaTM II instrument (BD, Bioscience, San Jose, CA) in the Cytometry core at University of Florida.
Flow cytometry surface and intracellular staining
For the surface staining, cells were stained with live/dead fixable yellow dye and anti-Human lineage/CRTH2/CD45/CD127/ CD56/CD117/NKp44 antibodies as described above. Next, 100 μl of fixation medium (Cat#Gas004, Life technologies) was added and incubated for an additional 15 min in the dark at room temperature. The cells were then washed and suspended with stain buffer (Cat#554656, BD, Biosciences).
For intracellular cytokine staining, 200 ng/ml Phorbol 12- Myristate 13-Acetate (Cat#P1585, Sigma-Aldrich, St. Louis, MO), 1μg/ml Ionomycin (Cat#I3909, Sigma-Aldrich, St. Louis, MO) and 1 μl/ml Monensin (Cat#420701, Biolegend, San Diego, CA) were added for the last 4.5 hours of cell culture. After fixing with BD fixation medium (Cat#Gas004), cells were incubated with antihuman IFN-γ and anti-human IL-17A or IL-22 in the 100 μl BD permeabilization medium (Cat#Gas004), for 60 min in the dark at room temperature, washed, and suspended with stain buffer. For AHR staining, cells were fixed and permeabilized and stained with anti-AHR for 1 h. Flow cytometry was performed with the LSR II instrument (BD, Bioscience, San Jose, CA). Data were analyzed using FlowJo software (Version 9.8.1, Tree Star Inc., Ashland, Or).
Degranulation assay
Evaluation of NK cell degranulation was accomplished by CD107α surface expression as previously described [18]. 1 × 104 sorted primary human NK cells were co-cultured with K562 cells in the 96 well round-bottom plate at a 1:2 effector-to-target ratio per well, and incubated at 37°C in a 5% CO2 incubator for 2 hours. The cells were then harvested and evaluated for CD56 and CD107α surface expression by flow cytometry. The experiments were performed in triplicate.
Histology
Fresh human gut tissues were fixed in 10% neutral-buffered formalin for 20 hours, embedded in paraffin, sectioned and stained with hematoxylin and eosin. For immunofluorescence, human CD56 staining was performed using monoclonal mouse anti-human NCAM antibody (ab9272, Abcam, Cambridge, MA) 1:25 overnight at 4°C, followed by Alexa 555-conjugated goatanti- mouse antibody 1:500 for 60mins. Human CD3 staining was performed using monoclonal rabbit anti-human CD3 antibody (ab16669, Abcam, Cambridge, MA) 1:25 overnight at 4°C, followed by Alexa 488-conjugated goat-anti-rabbit antibody 1:500 for 60 mins. Nuclear counterstaining was performed with DAPI. After staining, slides were examined with Zeiss microscope (Zeiss). Images were taken with Axiovision.
Statistical analyses
Statistical significance was determined using Mann-Whitney U test which was performed using GraphPad Prism 6 software (GraphPad Software La Jolla,CA). The asterisk (*) indicates a significant difference (p value<0.05).
Results
Differential frequencies of NK cells and CD127 (+) ILC1s in the ileum of CD patients
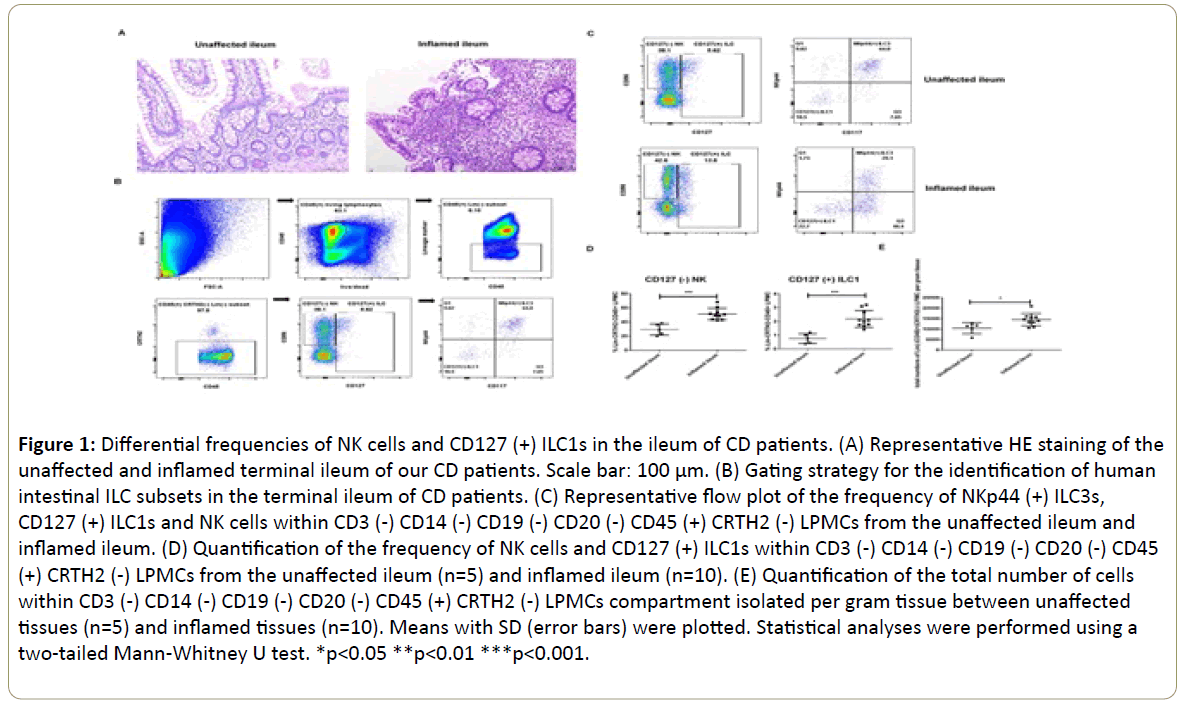
We collected 15 surgical terminal ileum samples from adult CD patients who failed medication treatment, developed intestinal complications and received surgical therapy. Their clinical characteristics are listed in Table 1. The HE staining of the inflamed tissues revealed disrupted architecture of the mucosa and increased density of lymphocyte infiltration compared to the unaffected areas which had relatively intact structure (Figure 1A). Then we analyzed the frequencies of NK cells and CD127(+) ILC1s in the lamina propria of our cohort. Gating strategy was shown in Figure 1B. Also, as mentioned earlier, NK cells were defined as CD3(-) CD14(-) CD19(-) CD20(-) CRTH2(-) CD127(-) CD45(+) CD56(+) cells and CD127(+) ILC1s were defined as CD3(-) CD14(-) CD19(-) CD20(-) CRTH2(-) CD45(+) NKp44(-) CD117(-) CD127(+) cells in our study. The frequency of NK cells within CD3(-) CD14(-) CD19(-) CD20(-) CD45(+) CRTH2(-) LPMCs was significantly increased in the inflamed ileums (51.3%, n=10) compared to unaffected tissues (29.4%, n=5) (Figure 1C and 1D). Meantime, the frequency of CD127(+) ILC1s within CD3(-) CD14(-) CD19(-) CD20(-) CD45(+) CRTH2(-) LPMCs was increased as well in the inflamed tissues (2.2%, n=10) compared to the unaffected areas (0.76%, n=5) (Figure 1C and 1D). The total cell number of CD3(-) CD14(-) CD19(-) CD20(-) CD45(+) CRTH2(-) LPMCs we isolated per gram tissue went up significantly in the inflamed ileums (Figure 1E). Consistent with other studies, the NKp44(+) ILC3s was abundant in the unaffected ileums whose frequency decreased under the inflamed condition (Figure 1C). Finally, it is important to note that we only included CD3, CD14, CD19 and CD20 in our lineage antibody cocktail and other cells could contaminate our ILC subsets.
| CD patients | Gender | Age | Complication | Medication |
|---|---|---|---|---|
| Patient 1 | F | 26 | Extensive submucosal fibrosis and luminal narrowing | Anti-IL-12/23 p40 Ustekinumab |
| Patient 2 | F | 31 | Appendiceal goblet cell carcinoid and adenocarcinoma | Anti-α4β7 Vedolizumab |
| Patient 3 | F | 46 | Small Bowel Obstruction and terminal ileum narrowing | Adalimumab and Azathioprine |
| Patient 4 | F | 60 | Ileitis, mural fibrosis and serosal fibrous adhesions | Anti-α4β7 Vedolizumab |
| Patient 5 | M | 23 | Ileitis, anal fissure, erythema nodosum | Anti-IL-12/23 p40 Ustekinumab |
| Patient 6 | F | 26 | Perianal fistula and perianal skin tags | Anti-IL-12/23 p40 Ustekinumab |
| Patient 7 | F | 57 | Submucosal edema in the terminal ileum | Anti-α4β7 Vedolizumab |
| Patient 8 | M | 42 | Small bowel fistulae | Anti-TNFa Infliximab |
| Patient 9 | F | 35 | Severe stricture at the descending colon | Anti-IL-12/23 p40 Ustekinumab |
| Patient 10 | M | 37 | Small intestine stricture and fistulas that involve both small bowel and colon | Anti-IL-12/23 p40 Ustekinumab |
| Patient 11 | F | 36 | Enteroenteric fistula, pelvic abscess, strictures of the sigmoid, rectum, and ileocecal valve | Anti-IL-12/23 p40 Ustekinumab |
| Patient 12 | F | 20 | Nonspecific bowel wall thickening and edema extending from the transverse colon to the rectum | Anti-α4 Natalizumab |
| Patient 13 | F | 40 | Small bowel perforation and terminal ileum stricture | Anti-TNFa Infliximab |
| Patient 14 | F | 35 | Stricture at the ileocecal valve | Vedolizumab and Mercaptopurine |
| Patient 15 | M | 65 | Small bowel perforation and enterocutaneous fistula with intramuscular abscess | Infliximab and Azathioprine |
| Patient 16(biopsy) | M | 28 | Small bowel and colon fistulae | Anakinra IL-1receptor antagonist |
Table 1: The clinical characteristics of CD patients.
Figure 1: Differential frequencies of NK cells and CD127 (+) ILC1s in the ileum of CD patients. (A) Representative HE staining of the unaffected and inflamed terminal ileum of our CD patients. Scale bar: 100 μm. (B) Gating strategy for the identification of human intestinal ILC subsets in the terminal ileum of CD patients. (C) Representative flow plot of the frequency of NKp44 (+) ILC3s, CD127 (+) ILC1s and NK cells within CD3 (-) CD14 (-) CD19 (-) CD20 (-) CD45 (+) CRTH2 (-) LPMCs from the unaffected ileum and inflamed ileum. (D) Quantification of the frequency of NK cells and CD127 (+) ILC1s within CD3 (-) CD14 (-) CD19 (-) CD20 (-) CD45 (+) CRTH2 (-) LPMCs from the unaffected ileum (n=5) and inflamed ileum (n=10). (E) Quantification of the total number of cells within CD3 (-) CD14 (-) CD19 (-) CD20 (-) CD45 (+) CRTH2 (-) LPMCs compartment isolated per gram tissue between unaffected tissues (n=5) and inflamed tissues (n=10). Means with SD (error bars) were plotted. Statistical analyses were performed using a two-tailed Mann-Whitney U test. *p<0.05 **p<0.01 ***p<0.001.
Differential functions of NK cells and CD127 (+) ILC1s in the ileum of CD patients
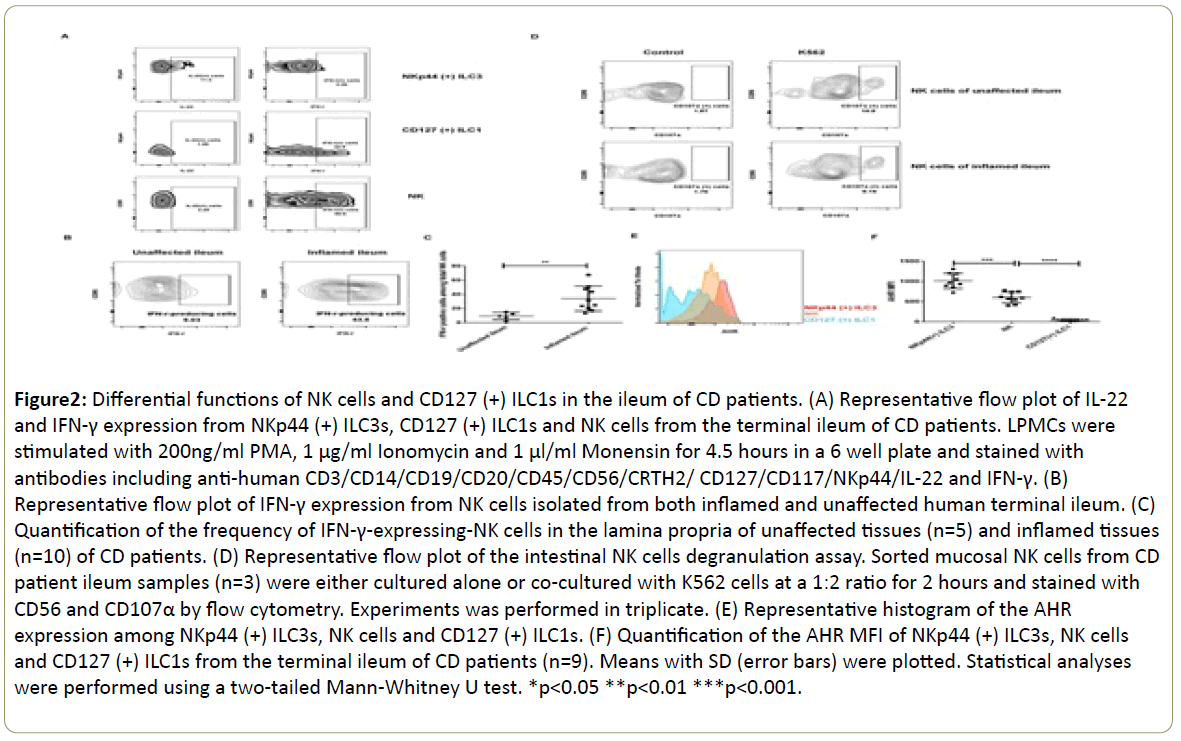
Subsequently, we analyzed the cytokine production pattern of our ILC subsets and focused on IFN-γ, IL-22 and IL-17A since they were key products of ILC family and crucial mediators of intestinal inflammation [19]. NKp44(+) ILC3s displayed more IL-22-producing potential instead of IFN-γ. On the contrary, CD127(+) ILC1s and NK cells tended to produce more IFN-γ instead of IL-22 (Figure 2A). However, we were unable to detect IL-17A expression among our ILC subsets (data not shown). Previously, we reported that the frequency of IFN-γ-producing CD127 (+) ILC1s was upregulated in the inflamed tissues of this CD cohort (15). Here, we noticed that the frequency of IFN-γ- producing-NK cells was also significantly increased in the inflamed ileums (34.0%, n=10) compared to control (9.2%, n=5) (Figure 2B and 2C). Moreover, we sorted NK cells and performed degranulation assay by measuring CD107α expression after coculture with target K562 cells. This population displayed certain levels of cytotoxicity (Figure 2D). For the CD127(+) ILC1s, we were unable to collect enough cells to perform this assay. In order to further characterize our ILC subsets, we measured their expression of AHR, a transcription factor which is essential for the regulation of ILC phenotype and function in the gut [20,21]. NKp44(+) ILC3s had the highest AHR expression which possibly correlated with their IL-22-production potential. NK cells displayed intermediate AHR expression level while CD127(+) ILC1s in our study did not express AHR (n=9) (Figure 2E and 2F).
Figure 2: Differential functions of NK cells and CD127 (+) ILC1s in the ileum of CD patients. (A) Representative flow plot of IL-22 and IFN-γ expression from NKp44 (+) ILC3s, CD127 (+) ILC1s and NK cells from the terminal ileum of CD patients. LPMCs were stimulated with 200ng/ml PMA, 1 μg/ml Ionomycin and 1 μl/ml Monensin for 4.5 hours in a 6 well plate and stained with antibodies including anti-human CD3/CD14/CD19/CD20/CD45/CD56/CRTH2/ CD127/CD117/NKp44/IL-22 and IFN-γ. (B) Representative flow plot of IFN-γ expression from NK cells isolated from both inflamed and unaffected human terminal ileum. (C) Quantification of the frequency of IFN-γ-expressing-NK cells in the lamina propria of unaffected tissues (n=5) and inflamed tissues (n=10) of CD patients. (D) Representative flow plot of the intestinal NK cells degranulation assay. Sorted mucosal NK cells from CD patient ileum samples (n=3) were either cultured alone or co-cultured with K562 cells at a 1:2 ratio for 2 hours and stained with CD56 and CD107α by flow cytometry. Experiments was performed in triplicate. (E) Representative histogram of the AHR expression among NKp44 (+) ILC3s, NK cells and CD127 (+) ILC1s. (F) Quantification of the AHR MFI of NKp44 (+) ILC3s, NK cells and CD127 (+) ILC1s from the terminal ileum of CD patients (n=9). Means with SD (error bars) were plotted. Statistical analyses were performed using a two-tailed Mann-Whitney U test. *p<0.05 **p<0.01 ***p<0.001.
Improved intestinal inflammation accompanied by reduction of mucosal NK cell frequency
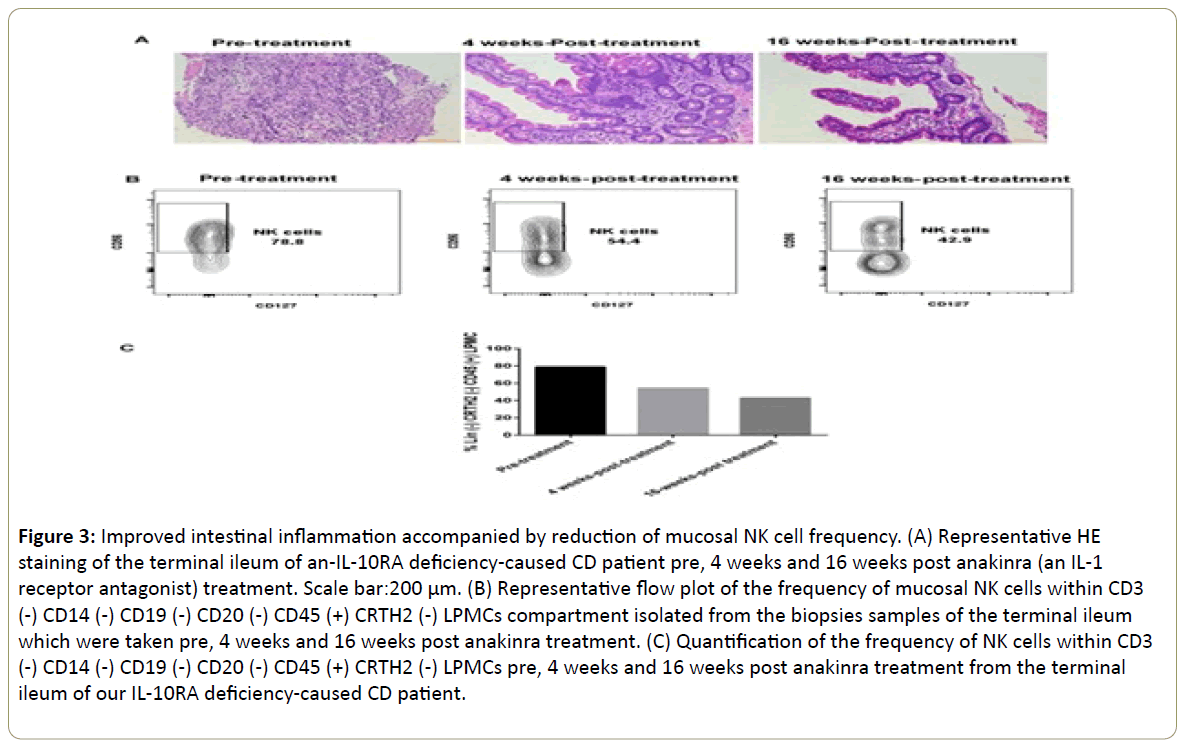
Previously, we presented a severe CD case due to an IL10RA mutation who demonstrated a dramatic response to anakinra (an IL-1R antagonist) therapy. This patient suffered from an IL-10RA deficiency-caused intestinal inflammation (Figure 3A) and the anakinra treatment led to marked clinical, endoscopic, and histological improvement accompanied by reduced levels of pro-inflammatory cytokines in the gut, including IFN-γ [22,23]. During the recovery of intestinal inflammation (Figure 3A), the percentage of NK cells within CD3(-) CD14(-) CD19(-) CD20(-) CD45(+) CRTH2(-) LPMCs compartment dropped from 78.8% pre-treatment to 54.4% post 4 weeks of anakinra treatment. The number further decreased to 42.9% after 16 weeks of anakinra treatment (Figure 3B and 3C). However, we were unable to determine the frequency changes of CD127(+) ILC1s during this process. It might be because of the relative small size of biopsy samples. The reduction of NK cells, along with the process of mucosal healing and tissue restitution, likely suggested that NK cells promoted the tissue damage process in the terminal ileum of this patient. The blocking of IL-1 signaling pathway might contribute to the reduction of NK cells in the gut of this CD patient.
Figure 3: Improved intestinal inflammation accompanied by reduction of mucosal NK cell frequency. (A) Representative HE staining of the terminal ileum of an-IL-10RA deficiency-caused CD patient pre, 4 weeks and 16 weeks post anakinra (an IL-1 receptor antagonist) treatment. Scale bar:200 μm. (B) Representative flow plot of the frequency of mucosal NK cells within CD3 (-) CD14 (-) CD19 (-) CD20 (-) CD45 (+) CRTH2 (-) LPMCs compartment isolated from the biopsies samples of the terminal ileum which were taken pre, 4 weeks and 16 weeks post anakinra treatment. (C) Quantification of the frequency of NK cells within CD3 (-) CD14 (-) CD19 (-) CD20 (-) CD45 (+) CRTH2 (-) LPMCs pre, 4 weeks and 16 weeks post anakinra treatment from the terminal ileum of our IL-10RA deficiency-caused CD patient.
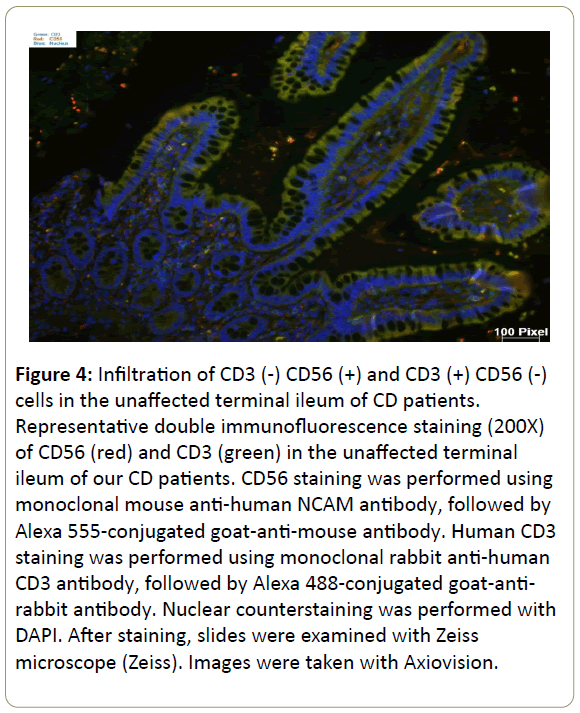
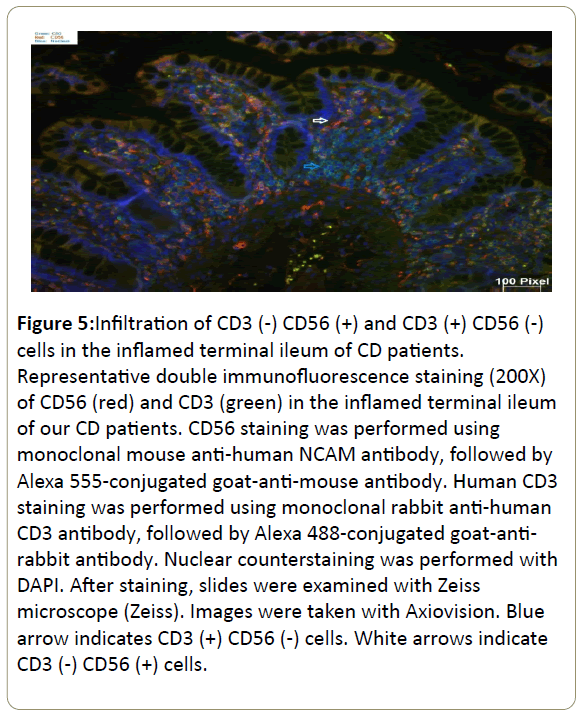
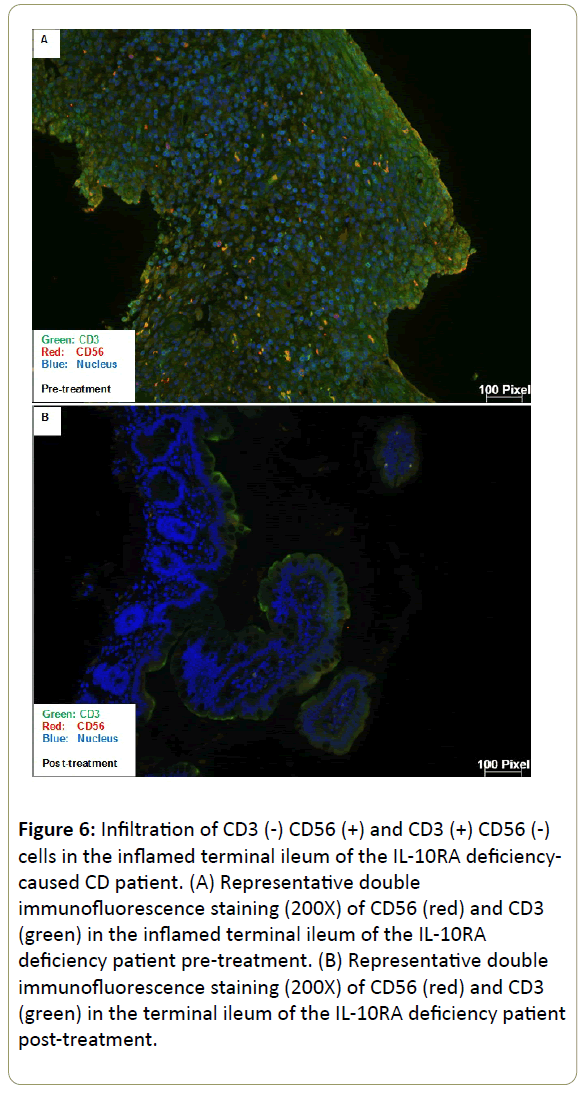
Infiltration of CD3(-) CD56(+) and CD3(+) CD56(-) cells in the inflamed ileum of CD patients. We then performed double staining for CD56 and CD3 by immunofluorescence of our CD patients gut tissue sections. The unaffected ileum displayed normal villous mucosal architecture with fewer immune cells infiltration in the lamina propria (Figure 4). On the contrary, there was villous atrophy (shortening and flattening) and increased infiltration of immune cells in the lamina propria of inflamed tissues (Figure 5). Specifically, there was more CD3(+) CD56(-) (blue arrow) cells and CD3(-) CD56(+) (white arrow) cells in the lamina propria of inflamed areas (Figure 5). In addition, CD3(+) CD56(-) cells and CD3(-) CD56(+) cells accumulated in the lamina propria of pre-treatment terminal ileum of the IL10RAdeficiency patient as well compared to post-treatment (Figure 6).
Figure 4: Infiltration of CD3 (-) CD56 (+) and CD3 (+) CD56 (-) cells in the unaffected terminal ileum of CD patients. Representative double immunofluorescence staining (200X) of CD56 (red) and CD3 (green) in the unaffected terminal ileum of our CD patients. CD56 staining was performed using monoclonal mouse anti-human NCAM antibody, followed by Alexa 555-conjugated goat-anti-mouse antibody. Human CD3 staining was performed using monoclonal rabbit anti-human CD3 antibody, followed by Alexa 488-conjugated goat-antirabbit antibody. Nuclear counterstaining was performed with DAPI. After staining, slides were examined with Zeiss microscope (Zeiss). Images were taken with Axiovision.
Figure 5: Infiltration of CD3 (-) CD56 (+) and CD3 (+) CD56 (-) cells in the inflamed terminal ileum of CD patients. Representative double immunofluorescence staining (200X) of CD56 (red) and CD3 (green) in the inflamed terminal ileum of our CD patients. CD56 staining was performed using monoclonal mouse anti-human NCAM antibody, followed by Alexa 555-conjugated goat-anti-mouse antibody. Human CD3 staining was performed using monoclonal rabbit anti-human CD3 antibody, followed by Alexa 488-conjugated goat-antirabbit antibody. Nuclear counterstaining was performed with DAPI. After staining, slides were examined with Zeiss microscope (Zeiss). Images were taken with Axiovision. Blue arrow indicates CD3 (+) CD56 (-) cells. White arrows indicate CD3 (-) CD56 (+) cells.
Figure 6: Infiltration of CD3 (-) CD56 (+) and CD3 (+) CD56 (-) cells in the inflamed terminal ileum of the IL-10RA deficiencycaused CD patient. (A) Representative double immunofluorescence staining (200X) of CD56 (red) and CD3 (green) in the inflamed terminal ileum of the IL-10RA deficiency patient pre-treatment. (B) Representative double immunofluorescence staining (200X) of CD56 (red) and CD3 (green) in the terminal ileum of the IL-10RA deficiency patient post-treatment.
Discussion
In the present study, we showed that NK cells and CD127(+) ILC1s accumulated in the inflamed terminal ileum of our CD patient cohort. They were IFN-γ-producers and had differential AHR expression. In addition, we demonstrated the recovery of intestinal inflammation in the terminal ileum of an-IL10RAdeficiency- caused-CD patient accompanied by the decrease of NK cell frequency. The dramatic alteration of IFN-γ-producing ILC subsets under the inflamed conditions of our CD patient cohort reveals their non-redundant role in the context of human intestinal inflammation.
NK cells are the prototypes of the ILC family and are important innate effector lymphocytes that play a crucial role in early host defense against different pathogens as well as malignant transformation [24]. Their major function is cytotoxic killing, but they are also an important source of IFN-γ, especially along the mucosa. They provide an early source of IFN-γ which promotes the maturation of DC which, in turn, leads to enhanced production of IL-12 and drives the expansion of Th1 cells [25]. In addition, NK cell produced-IFN-γ can directly act on naïve T cells and induce their differentiation into IFN-γ- producing Th1 cells, which are one of the main effector T cells infiltrating the inflamed tissue among various types of autoimmune diseases [26,27]. Many studies have suggested the involvement of NK cell-sourced-IFN-γ in the pathogenesis of autoimmunity [28]. Hervier et al. reported that NK cells from patients with active systemic lupus erythematosus had the capacity to produce large amounts of IFN-γ [29]. Cristina and her colleagues demonstrated that IFN-γ-producing NK cells accumulated in the synovial fluid of chronic arthritis patients [30]. Dungan et al. showed that NK cell-derived IFN-γ promoted the development of experimental autoimmune encephalomyelitis [31]. Takayama et al. showed that the balance of NKp44 (+)/NKp46 (+) NK cells was disrupted in the inflamed colon of CD patients, and concluded that NKp46 (+) NK cells mediated the pathogenesis of CD by producing IFN-γ [16]. Consistent with this study, we showed the enrichment of IFN-γ- producing NK cells in the inflamed terminal ileum of our CD cohort. Additionally, we observed a gradual reduction of the frequency of NK cells in the terminal ileum of one of our patients after anakinra treatment. Overall, our data suggested a pathogenic role of IFN-γ-producing NK cells in the inflamed terminal ileum of CD patients and appropriate regulation of NK cell cytokine production is of paramount significance. It is important to note that our patients are not therapy-naïve and have been under medication which might also contribute to the increased frequency of NK cells.
In 2009, Cella et al reported that a human NK cell subset provided an innate source of IL-22 for mucosal immunity [32]. This is the first publication that identified human NKp44 (+) ILC3 subset. In 2013, Bernink et al showed that 60-75% of the total non-cytotoxic ILCs in the lamina propria of human ileum are NKp44 (+) ILC3s at steady state. The CD127(+) ILC1s expanded and became dominant ILC subset in the inflamed ileum of CD patients [10,33]. They further proposed that IL-22-producing- NKp44 (+) ILC3s converted into IFN-γ-producing-CD127 (+) ILC1s in the gut of CD patients [10,33]. However, in 2016, Simoni et al used mass cytometry including a broad range of surface markers and transcription factors to profile human ILCs across healthy and inflamed tissues. They found clear phenotypic delineation of ILC2s and ILC3s. Surprisingly, they failed to detect CD127(+) ILC1s in any of the tissues assessed [17]. In our study, we observed CD127(+) ILC1 subset in the intestinal lamina propria, especially in the inflamed tissues. They had the capacity to produce IFN-γ and did not express AHR. Our limitation is that our lineage marker just included CD3, CD14, CD19 and CD20. Also we did not stain T-bet. Therefore, we could not rule out the possibility that there is contamination of other cells within our CD127(+) ILC1s in this study. Actually, the current definition of ILC1s is problematic. The well-known characteristic of ILC1s is their capacity to produce IFN-γ. But unlike ILC2s and ILC3s which are well defined by a bunch of markers, the specific markers for the ILC1 identity are still missing [34]. Future studies are needed to identify unique collection of markers for ILC1s. In addition, the heterogeneity within ILC1s makes it even more challenging to evaluate the specific contributions of each ILC1 subset in the processes of protective host defense, chronic inflammation as well as restoration of tissue integrity. Fuchs et al identified the CD103(+) ILC1s which are also CD56(+) in the intraepithelial compartment of human gut [12]. Roan et al. showed that the frequency of CD4(+) ILC1s increased in the peripheral blood of individuals with systemic sclerosis [35,36]. In the mouse, Klose et al. reported the liver resident NKp46 (+) NK1.1 (+) T-bet (+) Eomes (-) ILC1 subset (36). Further studies will be required to differentiate the relationship of the various ILC1 subsets in different locations.
In conclusion, cytotoxic NK cells and CD127(+) ILC1s displayed differential frequencies and functions as well as transcription factor AHR expression statuses in the terminal ileum lamina propria of our CD patient cohort. The dramatic changes of human intestinal ILC phenotype and function under the chronic inflammatory conditions make it an attractive therapeutic target. Selectively targeting different ILC subset probably could ensure outcomes that are more promising for CD patients.
Acknowledgements
This study is supported by NIH RO1 CA113975-A2.This work was also supported by the Gatorade Trust through funds distributed by the University of Florida, Department of Medicine. We would also thank Molecular Pathology Core of UF for the immunofluorescence staining. JL, ALD and SCG conceived and designed experiments. JL, YT and DB performed the experiments. AI and SAT provided surgical samples from CD patients and their clinical feature analysis. JL and SCG analyzed the data and drafted the manuscript. ALD, AI and SAT provided intellectual expertise in flow cytometry, innate immunity and clinical immunology. All authors reviewed and edited the manuscript.
Conflict of Interest
The authors declared no conflicts of interest in this study. Sarah Glover, DO is a consultant for AbbVie, Janssen and Takeda. Sarah Glover, DO has received grant support from AbbVie, Bristol Myers Squibb, Celgene, Gilead, Janssen, Genentech, Millennium, Pfizer, Receptos, Takeda and UCB.
References
- Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L (2017) Crohn's disease. Lancet 389(10080): 1741-1755.
- Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A (2014) Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 13 : 3-10.
- Ebbo M, Crinier A, Vely F, Vivier E (2017) Innate lymphoid cells: major players in inflammatory diseases Nat Rev Immunol.
- Hepworth MR, Fung TC, Masur SH, Kelsen JR, McConnell FM, et al. (2015) Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4(+) T cells. Science 348: 1031-1035.
- Hepworth MR, Monticelli LA, Fung TC, Ziegler CG, Grunberg S, et al. (2013) Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 498 : 113-117.
- Klose CS, Artis D (2016) Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol 17: 765-774.
- Walker JA, Barlow JL, McKenzie AN (2013) Innate lymphoid cells--how did we miss them? Nat Rev Immunol 13: 75-87.
- Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, et al. (2013) Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol 13: 145-149.
- Serafini N, Vosshenrich CA, Di Santo JP (2015) Transcriptional regulation of innate lymphoid cell fate. Nat Rev Immunol 15: 415-428.
- Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, et al. (2013) Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol 14: 221-229.
- Forkel M, Mjosberg J (2016) Dysregulation of Group 3 Innate Lymphoid Cells in the Pathogenesis of Inflammatory Bowel Disease. Curr Allergy Asthma Rep 16: 73.
- Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, et al. (2013) Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity 38: 769-781.
- Geremia A, Arancibia-Carcamo CV, Fleming MP, Rust N, Singh B, et al. (2011) IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med 208: 1127-1133.
- Li J, Doty AL, Iqbal A, Glover SC (2016) The differential frequency of Lineage(-)CRTH2(-)CD45(+)NKp44(-)CD117(-)CD127(+)ILC subset in the inflamed terminal ileum of patients with Crohn's disease. Cell Immunol 304-305: 63-68.
- Li J, Doty AL, Tang Y, Berrie D, Iqbal A, et al. (2017) Enrichment of IL-17A+ IFN-gamma+ and IL-22+ IFN-gamma+ T cell subsets is associated with reduction of NKp44+ ILC3s in the terminal ileum of Crohn's disease patients. Clin Exp Immunol 190: 143-153.
- Takayama T, Kamada N, Chinen H, Okamoto S, Kitazume MT, et al. (2010) Imbalance of NKp44(+)NKp46(-) and NKp44(-)NKp46(+) natural killer cells in the intestinal mucosa of patients with Crohn's disease. Gastroenterology 139: 882-892.
- Simoni Y, Fehlings M, Kloverpris HN, McGovern N, Koo SL, et al. (2017) Human Innate Lymphoid Cell Subsets Possess Tissue-Type Based Heterogeneity in Phenotype and Frequency. Immunity 46: 148-161.
- Alter G, Malenfant JM, Altfeld M (2004) CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 294: 15-22.
- Artis D, Spits H (2015) The biology of innate lymphoid cells. Nature 517: 293-301.
- Li J, Doty A, Glover SC (2016) Aryl hydrocarbon receptor signaling involves in the human intestinal ILC3/ILC1 conversion in the inflamed terminal ileum of Crohn's disease patients. Inflamm Cell Signal 3.
- Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, et al. (2012) The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36: 92-104.
- Li J, Shouval DS, Doty AL, Snapper SB, Glover SC (2017) Increased Mucosal IL-22 Production of an IL-10RA Mutation Patient Following Anakinra Treatment Suggests Further Mechanism for Mucosal Healing. J Clin Immunol 37: 104-107.
- Shouval DS, Biswas A, Kang YH, Griffith AE, Konnikova L, et al. (2016) Interleukin 1beta Mediates Intestinal Inflammation in Mice and Patients With Interleukin 10 Receptor Deficiency. Gastroenterology 151: 1100-1104.
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S (2008) Functions of natural killer cells. Nat Immunol 9: 503-510.
- Crouse J, Xu HC, Lang PA, Oxenius A (2015) NK cells regulating T cell responses: mechanisms and outcome. Trends Immunol 36: 49-58.
- Laouar Y, Sutterwala FS, Gorelik L, Flavell RA (2005) Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol 6 : 600-607.
- Pallmer K, Oxenius A (2016) Recognition and Regulation of T Cells by NK Cells. Front Immunol 7: 251.
- Popko K, Gorska E (2015) The role of natural killer cells in pathogenesis of autoimmune diseases. Cent Eur J Immunol 40: 470-476.
- Hervier B, Beziat V, Haroche J, Mathian A, Lebon P, et al. (2011) Phenotype and function of natural killer cells in systemic lupus erythematosus: excess interferon-gamma production in patients with active disease. Arthritis Rheum 63: 1698-1706.
- de Matos CT, Berg L, Michaelsson J, Fellander-Tsai L, Karre K, et al. (2007) Activating and inhibitory receptors on synovial fluid natural killer cells of arthritis patients: role of CD94/NKG2A in control of cytokine secretion. Immunology 122: 291-301.
- Dungan LS, McGuinness NC, Boon L, Lynch MA, Mills KH (2014) Innate IFN-gamma promotes development of experimental autoimmune encephalomyelitis: a role for NK cells and M1 macrophages. Eur J Immunol 44: 2903-2917.
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, et al. (2009) A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 457: 722-725.
- Bernink JH, Krabbendam L, Germar K, de Jong E, Gronke K, et al. (2015) Interleukin-12 and -23 Control Plasticity of CD127(+) Group 1 and Group 3 Innate Lymphoid Cells in the Intestinal Lamina Propria. Immunity 43: 146-160.
- Bernink JH, Mjosberg J, Spits H (2017) Human ILC1: To Be or Not to Be. Immunity 46: 756-757.
- Roan F, Stoklasek TA, Whalen E, Molitor JA, Bluestone JA, et al. (2016) CD4+ Group 1 Innate Lymphoid Cells (ILC) Form a Functionally Distinct ILC Subset That Is Increased in Systemic Sclerosis. J Immunol 196: 2051-2062.
- Klose CSN, Flach M, Mohle L, Rogell L, Hoyler T, et al. (2014) Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 157: 340-356.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences