Development of an Efficient Callus Derived Regeneration System for Garlic (Allium sativum L.) from Root Explant
Nadeem Khan1,2*, Muhammad Fayyaz Chaudhary1,3, Arshad Mehmood Abbasi4, Sabaz Ali Khan4, Abdul Nazir4 and Ghulam Mujtaba Shah5
1 Department of Microbiology, Quaid-i- Azam University, Islamabad, Pakistan
2 Department of Breeding and Genomics, MKS (Pty) Ltd, Lancefield, VIC, Australia
3 Department of Nano Science and Technology, Preston University, Islamabad, Pakistan
4 Department of Environmental Sciences, COMSATS Institute of Information Technology, Abbottabad, Pakistan
5 Department of Botany, Hazara University Mansehra, Khyber Pakhtunkhwa, Pakistan
- *Corresponding Author:
- Nadeem Khan
Department of Breeding and Genomics, MKS (Pty) Ltd, 3422 Melbourne Road, Lancefield, VIC 3435, Australia.
Tel: +61 3 9354 5780
E-mail: khan_m_nadeem@yahoo.com, nadeem@magnuskahl.com
Received Date: November 14, 2017; Accepted Date: November 17, 2017; Published Date: November 21, 2017
Citation: Khan N, Chaudhary MF, Abbasi AM, Khan SA, Nazir A, et al. (2017) Development of an Efficient Callus Derived Regeneration System for Garlic (Allium sativum L.) from Root Explant. J Plant Breed Agric. Vol.1 No.1:3
Abstract
In the current research, an efficient protocol has been devised for production of calli and then regeneration for the important medicinal crop garlic (Allium sativum L.) via roots explants which were derived from the cloves on MS medium. Results showed that optimum calli production could be obtained on MS medium with 2, 4-D (0.5 mg/l) and Kin (0.5 mg/l). Calli were then regenerated into shoots after 4-6 weeks on regeneration medium i.e., MS medium with BA (0.5 mg/l) alone or BA (1.0 mg/l) along with 0.5 mg/l Kin. Also, optimum rooting could be observed on MS medium with growth hormones IAA (2.0 mg/l) and NAA (0.5 mg/l). The shoots obtained from calli developed into plantlets on plain ½ strength MS medium. This optimized protocol may be a step forward towards making in vitro garlic plantlets for various tissue culture studies and could be a useful tool in future for genetic transformation of this medicinally important everyday vegetable crop.
Keywords
Allium sativum L; Callus induction; Garlic; in vitro plantlets; Plant regeneration; Root segments
Introduction
Garlic (Allium satium L.) is a monocot species and belongs to family Alliaceae and is widely cultivated for a long time as it contains medicinal traits. It is well known for its antibiotic, antitumour, and antiantherosclerosis effects as well as for its positive effects against certain cardiovascular diseases [1-4]. Due to these reasons, there is an increasing demand of the garlic production worldwide. However, this increasing demand of garlic production needs attention for genetic improvement of this important crop. So far, clonal selection of wild varieties or spontaneous mutants are the only sources of garlic breeding due to garlic sexually sterile available germplasm and thus obligate apomictic [5,6]. There are two strategies to improve the current garlic cultivars) to develop new garlic cultivars via sexual hybridization [7] and ii) to develop more efficient transformation system [8]. Such kinds of system have ready been reported onion and shallot, which are close relatives of garlic [9]. Abo El-Nil [10] reported for the first-time regeneration from bulb leaf discs and stem tips explants. Thereafter, it was also documented for basal plate, leaf, receptacle and flower buds [11-15]. But, in this case the plantlets regeneration was found to be much low. Hence, mainly shoot or stem disc are mostly used for the tissue culture purposes of this medicinally important vegetable crop [16-21]. However, interestingly, Haque and his colleagues [22,23] have documented successful regeneration of garlic via root tip but without the intervening callus phase.
Garlic regeneration system has been reported for root-tip explant [22-27]. However, the problem is that there is only one root tip on a root which can be used for the regeneration. Probably because of this difficulty, Myers and Simon [28] used root segments from shoot tip-derived plantlets for callus induction and regeneration. There are reports on the formation of calli and the regeneration in different alliaceous plants [29-32]. Unfortunately, the systematic and comparative studies of the effects of various plant growth hormones on callus formation from root segments and subsequent shoot and root proliferation is scared and inconclusive. A successful garlic transformation system is highly dependent on an efficient and reliable regeneration system that is applicable to a wide range of cultivars.
Intensive work, therefore, needs to be undertaken in the view to develop an effective and efficient protocol including proper callus induction and optimization of medium for rapid production of multiple numbers of micro propagating units in the form of shoots and roots. The main objective of present study is to establish a rapid and efficient regeneration system using root segments as explants, which could be further utilized in genetic transformation and genetic improvement program of garlic.
Materials and Methods
Current research was carried out at the Department of Microbiology, Quaid-i-Azam University, Islamabad Pakistan.
Plant material
Local garlic (Allium sativum L.) variety commonly cultivated in different regions of the country was used in this research. The garlic bulbs were purchased from the local market of Islamabad. All the plant material procured for the present study was disease free and maintained under clean and healthy environment.
in vitro root development
First of all, the outer protective leaf sheaths were removed from garlic cloves mechanically. Then these were surface sterilized with 96% (v/v) ethanol, for 5 min then with 0.1% HgCl2 by dipping the cloves in the solution for 5-7 minutes the cloves were then rinsed five times with sterilized distilled water for 5 minutes each under complete aseptic conditions. Disinfected cloves were laid on sprouting medium i.e. half strength liquid MS medium, and then placed under cool white florescent light with a photoperiod of 16/8 hrs (light/darkness). The temperature was maintained at 25 ± 1°C. After 3-4 days, root tips segments (3-4 cm) were cut, which were used as explants for callogenesis and subsequent morphogenesis.
Callus induction
For callus induction, root explants were cut into 1 cm long segments and then were placed on MS medium (Murashige and Skoog in 1962) [33] with 3% (w/v) sucrose, 0.8% (w/v) plant agar and various combinations of auxin and cytokinin as mentioned in Table 1. Auxins were including 2,4-dichlorophenoxyacetic acid (2,4-D), naphthalene acetic acid (NAA), indole-3- acetic acid (IAA), and indole-3-butyric acid (IBA), while Kinetin (Kn) include Zeatin (Z) and benzyladenine (BA). Both these hormones were used in different combinations and concentrations for callus induction (Table 1). These hormones were either autoclaved or filter sterilized using 0.22 μm sterile filters prior to use. For callus induction, ten explants were placed in each per petri dish and were sealed with parafilm and placed in the 16/8 (L/D) conditions. Data for callus induction were recorded regularly.
| S No | Combination and concentration of plant growth regulators (mg/l) | Callogenic response after 8 weeks | Callus morphology |
|---|---|---|---|
| 2,4-D | |||
| I | 01 | - | No callus formation |
| Ii | 02 | - | No callus formation |
| Iii | 03 | ++ | Compact, nodular, yellowish white |
| Iv | 04 | +++ | Compact, nodular, yellowish white |
| V | 05 | ++++ | Compact, nodular, yellowish white |
| Vi | 06 | ++ | Compact, nodular, yellowish white |
| Vii | 07 | - | No callus formation |
| Viii | 08 | - | No callus formation |
| Ix | 09 | - | No callus formation |
| X | 10 | ++ | Compact, nodular, yellowish white |
| Xi | 15 | - | No callus formation |
| Xii | 20 | - | No callus formation |
| Xiii | 25 | - | No callus formation |
| Xiv | 30 | - | No callus formation |
| Xv | 35 | - | No callus formation |
| 2,4-D+NAA | |||
| I | 05+05 | ++++ | Compact, nodular, yellowish green with white spot |
| Ii | 05+10 | +++ | Compact, nodular, yellowish green |
| Iii | 05+15 | ++ | Compact, nodular, yellowish green |
| Iv | 10+05 | - | No callus formation |
| V | 15+10 | - | No callus formation |
| Vi | 20+10 | - | No callus formation |
| Vii | 25+20 | - | No callus formation |
| 2,4-D+IAA | |||
| I | 05+05 | +++ | Compact, nodular, yellowish white |
| Ii | 05+10 | +++ | Compact, nodular, yellowish white |
| Iii | 10+05 | - | - |
| Iv | 10+10 | - | - |
| V | 15+05 | - | - |
| Vi | 20+10 | - | - |
| Vii | 20+15 | - | - |
| Viii | 25+15 | - | - |
| 2,4-D+IBA | |||
| I | 05+05 | +++ | Compact, nodular, yellowish green |
| Ii | 05+15 | +++ | Compact, nodular, yellowish green |
| Iii | 10+05 | - | - |
| Iv | 15+05 | - | - |
| V | 15+10 | - | - |
| Vi | 15+15 | - | - |
| Vii | 20+05 | - | - |
| Viii | 20+10 | - | - |
| Ix | 20+15 | - | - |
| X | 20+20 | - | - |
| Xi | 25+25 | - | - |
| 2,4-D+Kin | |||
| I | 05+03 | ++ | Compact, nodular, yellowish white |
| Ii | 05+04 | ++++ | Compact, nodular, yellowish white |
| Iii | 05+05 | ++++ | Compact, nodular, yellowish white |
| Iv | 05+06 | +++ | Compact, nodular, yellowish white |
| V | 05+07 | ++ | Compact, nodular, yellowish white |
| Vi | 05+10 | ++ | Compact, nodular, yellowish white |
| Vii | 10+05 | + | - |
| 2,4-D+BA | |||
| I | 05+02 | ++ | Compact, nodular, yellowish white |
| Ii | 05+03 | +++ | Compact, nodular, yellowish white |
| Iii | 05+04 | ++++ | Compact, nodular, yellowish white |
| Iv | 05+05 | ++++ | Compact, nodular, yellowish white |
| V | 05+07 | ++ | Compact, nodular, yellowish white |
| Vi | 05+10 | ++ | Compact, nodular, yellowish white |
| Vii | 10+05 | + | Compact, nodular, yellowish white |
| Viii | 10+10 | + | Compact, nodular, yellowish white |
| Ix | 15+10 | _ | _ |
| X | 20+05 | _ | _ |
| IAA+Kin | |||
| I | 05+05 | ++ | Compact, nodular, yellowish white |
| Ii | 05+10 | +++ | Compact, nodular, yellowish white |
| Iii | 10+05 | + | Compact, nodular, yellowish white |
| Iv | 10+10 | - | - |
| V | 15+05 | - | - |
| Vi | 20+05 | - | - |
| Vii | 20+10 | - | - |
| Viii | 20+10 | - | - |
| Ix | 20+15 | - | - |
| X | 25+20 | - | - |
| IAA+BA | |||
| I | 05+05 | + | Compact, nodular, yellowish white |
| Ii | 10+05 | - | - |
| Iii | 15+10 | - | - |
| Iv | 20+10 | - | - |
| V | 20+20 | - | - |
| Vi | 25+20 | - | - |
| Vii | 25+25 | - | - |
| 2,4-D+Kin+BA | |||
| I | 05+03+03 | ++ | Compact, nodular, yellowish white |
| Ii | 05+02+03 | ++ | Compact, nodular, yellowish white |
| Iii | 05+05+05 | ++++ | Compact, nodular, yellowish white |
| Iv | 10+05+05 | + | Compact, nodular, yellowish white |
| V | 05+10+05 | +++ | Soft, yellowish white |
| Vi | 05+10+10 | + | Compact, nodular, yellowish white |
| Vii | 10+10+10 | - | |
| 2,4-D+IAA+NAA | |||
| I | 05+05+05 | +++ | Compact, nodular, yellowish white with green spots |
| Ii | 05+10+05 | +++ | Compact, nodular, yellowish white |
| Iii | 05+10+10 | + | Compact, nodular, yellowish white |
| Iv | 10+10+10 | - | |
| Key: -: No Callogeneic Response; +: 40-50g; ++: 50-60g; +++: 60-70g; ++++: 70-80g | |||
Table 1: Effect of various combinations and concentrations of different plant growth regulators on callogenesis from root segment explants.
Plant regeneration
For shoot and root regeneration, calli were transferred to plant regeneration medium (MS medium having pH 5.8 with various combinations and concentrations of the two growth hormones i.e., auxins and cytokinins; Tables 2 and 3). The growth conditions for calli regeneration were an ambient temperature of 25 ± 1°C with a 16/8 h photoperiod. In order to provide fresh nutrients and avoid any damage, the explants were subcultured after every four weeks to fresh MS medium. Data were regularly recorded for the root and shoot regeneration.
| S No | Combination and concentration of plant growth regulators (mg/l) | Shooting response after 4-6 weeks (150-200mg of callus) | General characteristics of leaves |
|---|---|---|---|
| BA | |||
| I | 025 | + | Green, narrow |
| Ii | 05 | ++++ | Green, narrow |
| Iii | 10 | +++ | Green, narrow |
| Iv | 20 | ++ | Green, narrow |
| V | 30 | ++ | Green, narrow |
| Kin | |||
| I | 05 | ++ | Green, narrow |
| Ii | 10 | +++ | Green, narrow |
| Iii | 15 | ++ | Green, narrow |
| iv | 20 | ++ | Green, narrow |
| V | 25 | ++ | Green, narrow |
| vi | 30 | +++ | Green, narrow |
| BA+Kin | |||
| I | 05+05 | ++ | Green, narrow |
| Ii | 10+05 | +++ | Green, narrow |
| iii | 15+05 | ++ | Green, narrow |
| iv | 20+10 | ++ | Green, narrow |
| V | 30+10 | - | |
| Kin+NAA | |||
| I | 10+05 | +++ | Green, narrow |
| Ii | 15+05 | ++ | Green, narrow |
| iii | 20+05 | +++ | Green, narrow |
| iv | 30+05 | ++ | Green, narrow |
| BA+NAA | |||
| I | 10+05 | +++ | Green, narrow |
| Ii | 15+05 | +++ | Green, narrow |
| iii | 20+05 | ++ | Green, narrow |
| iv | 30+05 | ++ | Green, narrow |
| Kin+Z | |||
| I | 05+05 | +++ | Green, narrow |
| Ii | 10+05 | ++ | Green, narrow |
| iii | 15+05 | ++ | Green, narrow |
| BA+Z | |||
| I | 05+05 | ++ | Green, narrow |
| Ii | 10+05 | +++ | Green, narrow |
| iii | 15+05 | ++ | Green, narrow |
| Key: -: No Shooting Response; +: 5-10 Shoots; ++: 15-25 Shoots; +++: 25-35 Shoots; ++++:>35 Shoots | |||
Table 2: Response of in vitro shooting from callus to different plant growth regulators.
| S No | Combination and concentration of plant growth regulators (mg/l) | Shooting response after 6 weeks (100mg of callus) | General characteristics of leaves |
|---|---|---|---|
| IBA+NAA | |||
| I | 10+05 | ++ | Single, thin whitish |
| Ii | 20+05 | +++ | Single, thin whitish |
| iii | 30+10 | ++ | Single, thin whitish |
| IAA+NAA | |||
| I | 10+05 | +++ | Single, thin whitish |
| ii | 20+05 | +++ | Single, thin whitish |
| iii | 30+10 | ++ | Single, thin whitish |
| IBA+IAA | |||
| I | 10+05 | ++ | Single, thin whitish |
| ii | 20+05 | ++ | Single, thin whitish |
| iii | 30+10 | ++ | Single, thin whitish |
| BA+NAA | |||
| I | 05+10 | - | - |
| ii | 05+20 | - | - |
| iii | 05+30 | ++ | Single, thin whitish |
| Key: -:No Roots; +: 3-7 Roots; ++: 7-10 Roots; +++:>10 Roots | |||
Table 3: In vitro rooting from callus culture under various plant growth regulators.
Results
Callus induction as affected by growth hormone auxins
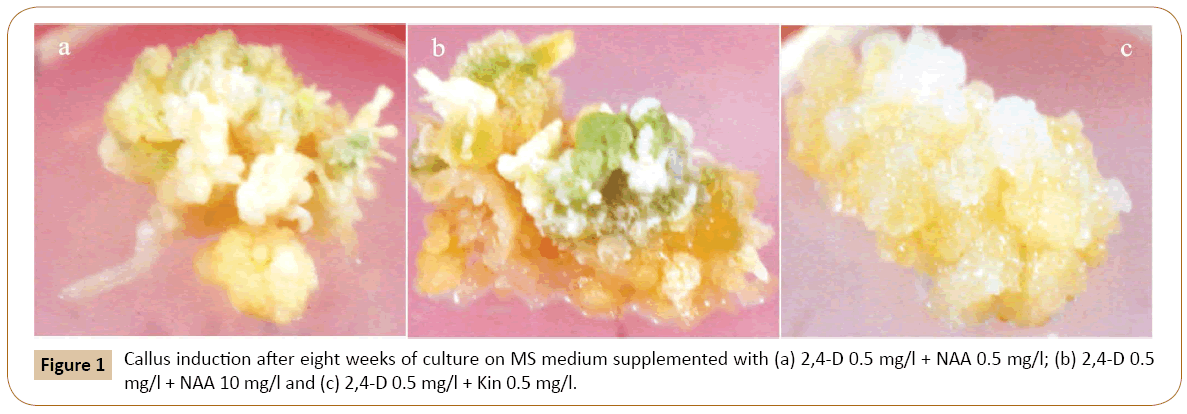
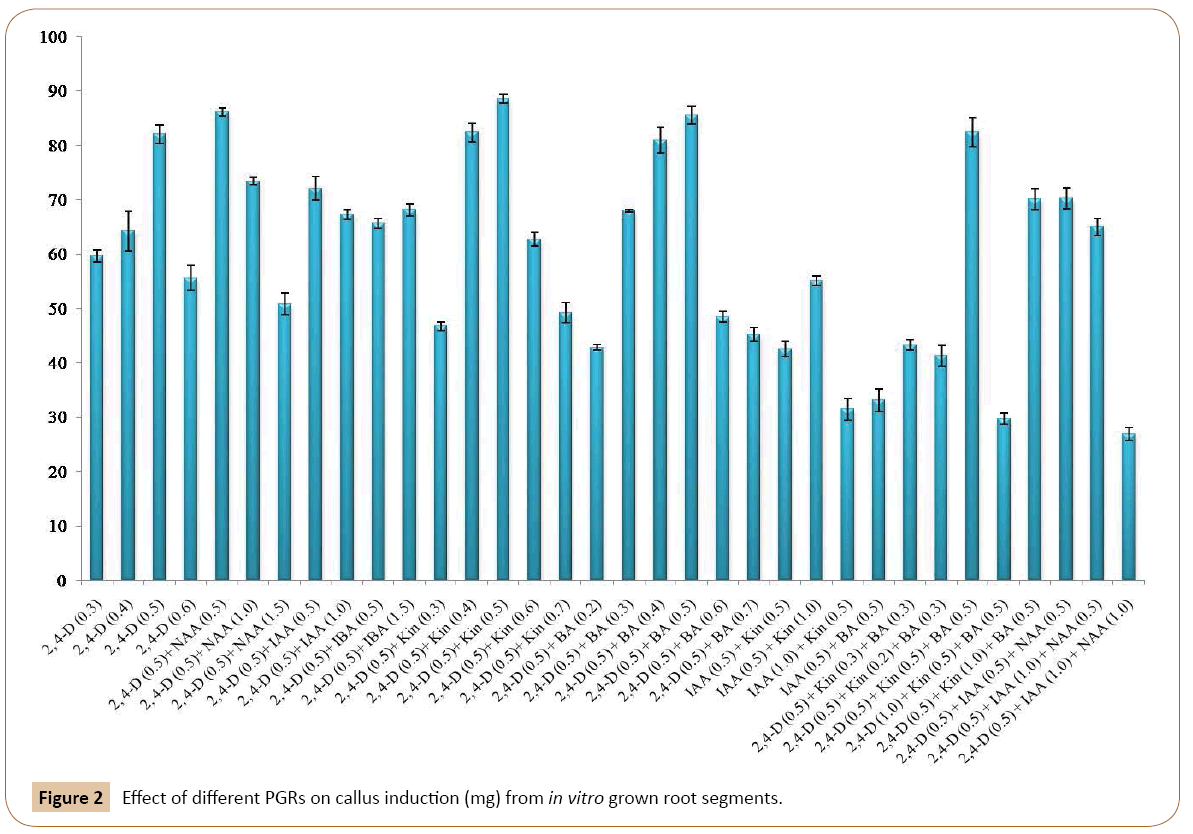
The callus induction started after 20-25 days on only when the root segments became swollen towards their edges. Optimum calli were induced on MS medium containing 2, 4-D (0.5 mg/l) which was followed by 0.4 mg/l and 0.3 mg/l of 2, 4-D respectively. The calli obtained were of compact and nodular in morphology and yellowish white in color. When, MS medium was with 0.5 mg/l 2, 4-D and 0.5 mg/l NAA then optimum calli induction were observed. By increasing the concentrations of NAA (1.0 mg/l) and keeping the 2, 4-D concentration same (0.5 mg/l) we found similar results (Figures 1a-1c). The callus was compact, nodular and yellowish white in color. All other concentrations of this combination failed to give positive response towards callus induction. Out of different concentrations of 2, 4-D and IAA (Table 1), three concentrations responded positively toward the callus induction. MS medium with 0.5 mg/l of 2, 4-D and 0.5 mg/l of IAA showed the optimum results. Second optimum results were obtained when 0.5 mg/l of 2, 4-D was used in combination with 1.5 mg/l IAA. Calli were yellowish white in color, compact and nodular. Other concentrations of this combination of auxins (2, 4-D and IAA) used during the experiment failed to respond in callus induction (Table 1). The root segment derived explants were also cultured on MS medium with different concentrations of 2, 4-D and IBA. It was found that the MS medium with 0.5 mg/l 2, 4-D and 0.5 mg/l IBA showed the optimum results. Similar results were recorded on MS medium with 0.5 mg/l of 2, 4-D and 1.5 mg/l of IBA. Calli were compact, nodular and yellowish white in color. All the other used concentrations of this combination failed to produce callus (Table 1). Similarly, callus initiation on MS medium with 0.5 mg/l of 2, 4-D, 0.5 mg/l of IAA and 0.5 mg/l NAA was observed after eight weeks of inoculation. It was found that at this combination and concentration the root explants gave good result. The callus produced is soft in texture and yellowish white in color. This was followed by 0.5 mg/l of 2, 4-D, 1.0 mg/l of IAA and 0.5 mg/l of NAA. The remaining concentrations failed to respond positively (Table 1). The effects of different PGRs on callus development are given in Figure 2.
Effects of 2, 4-D and cytokinins (Kin and BA) on callus induction
MS medium with 0.5 mg/l 2, 4-D and 0.5 mg/l Kin exhibited optimum callogenensis which was followed by 0.5 mg/l 2, 4-D and 0.4 mg/l Kin (Figures 2). Other concentrations of this combination also responded positively towards callus induction from root segments used as explants (Table 1). The resultant callus was yellowish white in color with compact and nodular morphology. Out of four concentrations mentioned in Table 1, the excellent callus development was obtained on MS medium with 0.5 mg/l 2, 4-D and 0.5 mg/l BA. Similarly, 0.5 mg/l 2, 4-D together with 0.4 mg/l BA also responded very well towards callus formation (Figure 2). The other concentrations of this combination also responded positively towards callus formation (Table 1). In this research, 2,4-D was also used simultaneously in combination with Kin and BA in different concentrations. Of these three concentrations of 2, 4-D, Kin and BA tested for callus formation (Table 1), good callus formation was recorded on MS medium with 0.5 mg/l 2, 4-D, 0.5 mg/l BA and 0.5 mg/l Kin. This hormonal concentration and combination produced nodulated, compact and yellowish white callus. It was followed by MS medium with 0.5 mg/l 2, 4-D, 0.3 mg/l Kin and 0.3 mg/l BA were used (Figure 2). The other concentrations of this combination also responded to produce good callus (Table 1). Efforts were also made to induce callus only with IAA and Kin without 2, 4-D. For this purpose, three concentrations of IAA and Kin were tested. It was found that IAA in combination with Kin exhibited positively towards callus induction. The best results were obtained when MS medium was supplemented with 0.5 mg/l IAA and 1.0 mg/l Kin. Similar results have been observed with 0.5 mg/l of IAA and 0.5 mg/l of Kin. However, the rest of the treatments from same combination did not response to callus induction (Table 1). Beside this various concentration of IAA and BA were tested for callogenic response. Of these combinations and concentrations 0.5 mg/l IAA and 0.5 mg/l BA responded positively towards the callus formation. All the other concentrations failed to induce callogenesis.
Effects of PGRs on in vitro shooting from root segments derived callus
The effects of different plant growth hormones on shoot differentiation from callus has been investigated in the present study. For this purpose, callus cultured from root segments was inoculated on MS medium with auxins and cytokinins in different combinations and concentrations.
Shoot induction with BA
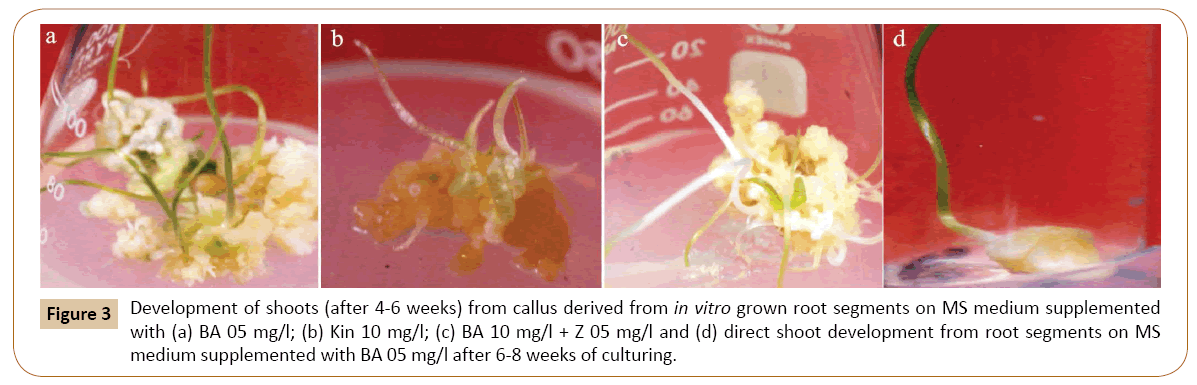
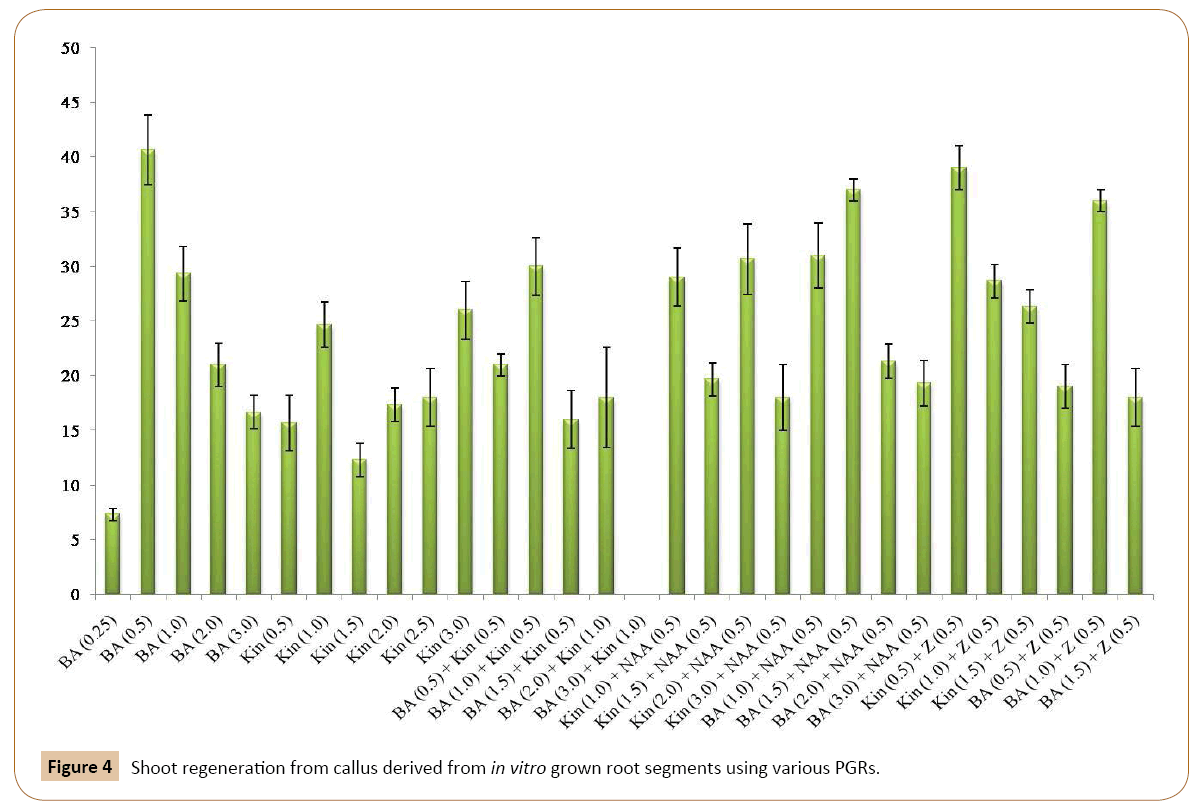
For this, the callus cultures were transferred to MS medium containing BA in different concentrations. All the five concentrations of BA tested produced shoots from callus (Table 2). Of these concentrations of BA, excellent results were obtained on 0.5 mg/l BA as shown in Figures 3a-3d, by producing lush green shoots. Initially small protuberances were appeared on the surface of calli which were then converted into shoots. Further growth of shoots continued until the cultures were maintained on different medium of the same concentration after 4-6 weeks. To analyze the effect of Kin on shoot induction, the calli were cut put on MS medium with Kin (Table 2). Shooting was induced on all the concentrations tested, however, the shooting response was rather slow. The medium containing 1.0 mg/l Kin responded very well towards shooting. Similar results were obtained with 3.0 mg/l of Kin (Figure 4) (Tables 2). A large number of small outgrowths appeared on surface of the callus and later on these outgrowths resulted into shoot formation (Figure 3b). The shoots were lush green in color and were kept on growth for few weeks and then subculture on medium with same concentrations.
Figure 3: Development of shoots (after 4-6 weeks) from callus derived from in vitro grown root segments on MS medium supplemented with (a) BA 05 mg/l; (b) Kin 10 mg/l; (c) BA 10 mg/l + Z 05 mg/l and (d) direct shoot development from root segments on MS medium supplemented with BA 05 mg/l after 6-8 weeks of culturing.
Effects of BA, Kin and NAA on shoot induction
It was found that all the concentrations of the Kin and BA were effective in shoot formation (Table 2). However, MS medium with 1.0 mg/l BA and 0.5 mg/l Kin produced optimum shoots. Also, all the four concentrations and combinations of BA and NAA were effective in producing shoots from the callus (Table 2) and optimum shoots with regenerated medium containing 1.5 mg/l BA and 0.5 mg/l NAA followed by 1.0 mg/l BA and 0.5 mg/l NAA. To evaluate the effect of Kin and NAA on shoot induction four different combinations of Kin and NAA were tested (Table 2) and all the combinations resulted in shoot formation from callus. The shooting response was rather slow in root tip explants. Shoots with green, narrow leaves were obtained on the MS medium with 1.0 mg/l of Kin and 0.5 mg/l of NAA, followed by 2.0 Kin and 0.5 mg/l NAA.
Effects Z with Kin and BA on shoot induction
The effect of Kin and Z on shoot induction from callus was also monitored in the present investigation. It was found that the best shooting is achieved when MS medium was supplemented with 0.5 mg/l of Kin and 0.5 mg/l of Z (Figure 4). Other concentrations of this combination responded moderately to shoot regeneration. Similarly, the best shooting was achieved when calli were inoculated on MS medium with 1.0 mg/l of BA and 0.5 mg/l of Z (Figure 3c). Other concentrations of this combination responded moderately to shoot regeneration.
Direct shoot induction from root segments
When root tips were placed on MS medium with BA 1.0 mg/l and 0.5 mg/l, the small shoots were generated from one end of the root tip. The root tips become greenish and swollen at one end after about 4 weeks of inoculation on the medium. The swollen portion then protruded out to give small shoots (Figure 3d).
Effects of different PGRs on in vitro root formation from callus
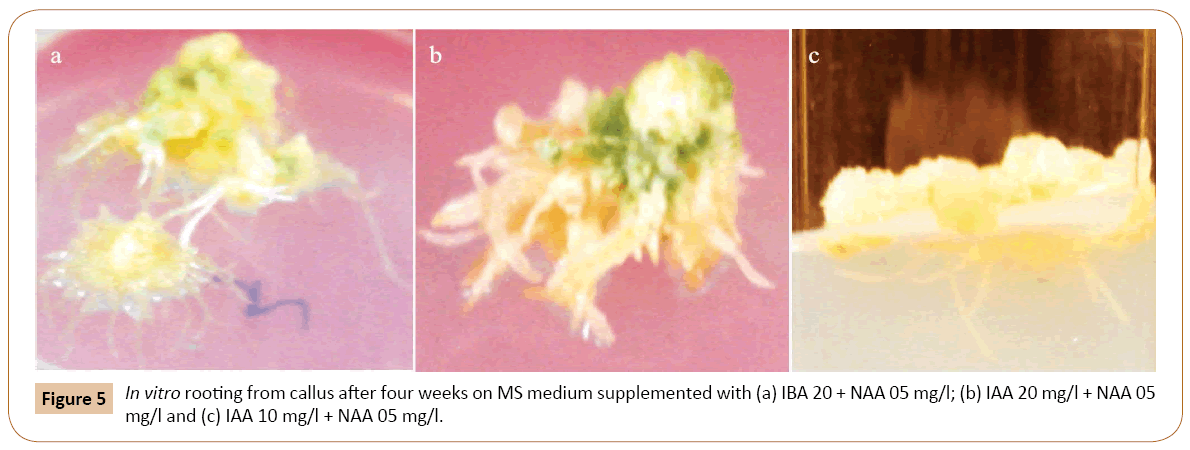
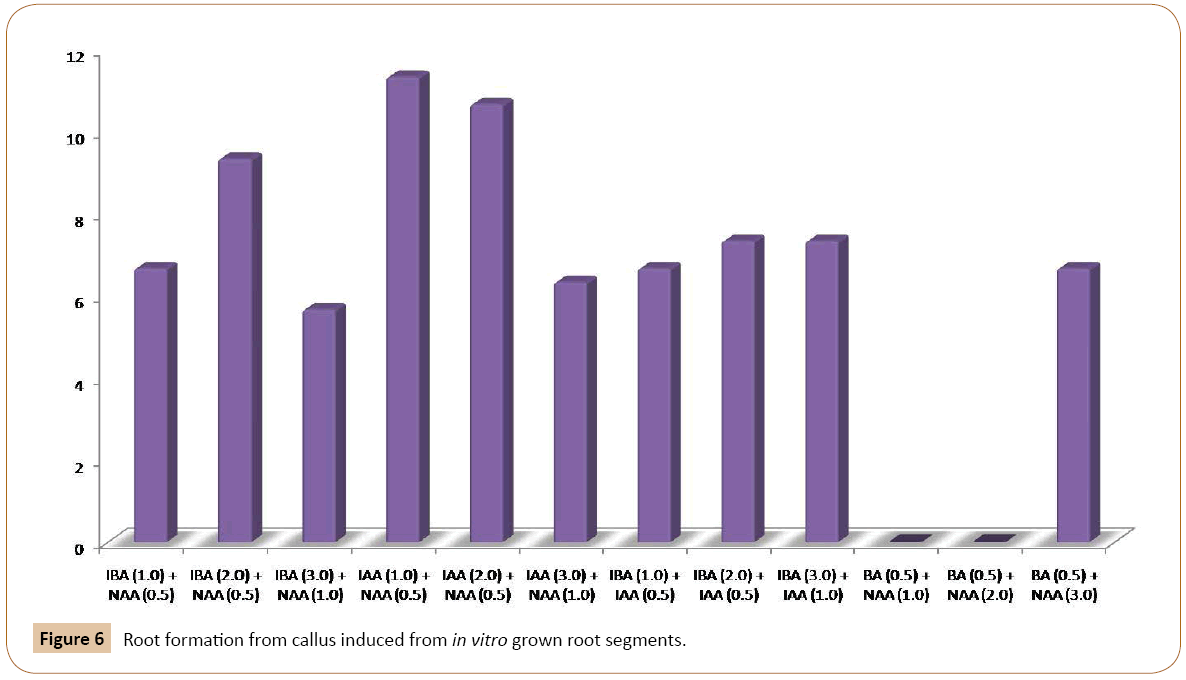
The effect of different PGRs on root differentiation has been investigated in the current research. Callus cultured obtained from in vitro grown root segments, were put on MS medium with various combinations and concentrations of auxins and cytokinins. When these cultures were placed on MS medium containing different concentrations and combinations of auxins themselves or high auxins to cytokinins ratio, produced rooting on the surface of the medium initially. The roots then grew deep into the medium. Good rooting occurred on the medium with 2.0 mg/l IBA and 0.5 mg/l NAA (Figure 5a). The roots were whitish without branching. Other combination tested also produced the same roots from the calli on the surface of the medium (Table 3). Of the three concentrations of IAA and NAA tested, responded positively to root induction from callus. However, best rooting occurred when MS medium was supplemented with 2.0 mg/l IAA and 0.5 mg/l NAA. This was followed by 1.0 mg/l IAA and 0.5 mg/l NAA concentration (Figures 5b and 5c). The roots were whitish, single and without root hair. Root induction was also obtained from callus culture of garlic with various concentrations of IBA and IAA (Table 3).
Discussion
Plant tissue culture serves as a platform for the plant biotechnology and hence become of much importance in the current advancement of this important research area. This is true especially for multiplication and conservation of those plants which are vegetatively propagated or having unviable seeds [34-37]. This thus helps in the conservation of important plant species through in vitro culture techniques. The prime objective of tissue is also the production of pathogen free plants. This can be achieved through the in vitro culture of these important plant species such those of medicinal importance, by growing them on artificial growth medium with plant growth hormones in an optimized condition. For example, large number of plantlets could be regenerated from a single seed or even single cell [38].
Garlic is one of the several important vegetable crops within the Allium family and is widely grown for its culinary and medicinal properties. Its seeds are not viable because of non-fertile flowers and is therefore only propagated through vegetative parts [39,40]. Moreover, it is very susceptible to viral, nematodes and fungi diseases [41,42]. On the other hand, bulb production is costly because garlic clove has low efficiency of propagation [43]. In this study, we put efforts to develop an efficient regeneration system for garlic, using its roots as explant. Here we reported, the optimum callus induction with 2, 4-D. Low concentrations rather than higher concentrations of this hormone favor the optimum callus induction (Table 1). These results are in line with those of Fereol et al. [44] who found that low concentrations (0.3-0.5 mg/l) induced the highest callus frequency from root tip and leaf explants in garlic on modified B5 medium. Organogenic calli were also successfully induced on MS medium with even low concentration of 2, 4-D (2.2-4.5 μM) from in vitro grown cloves under dark conditions [27]. During present study different concentrations of 2, 4-D and NAA have been used to induce callus from root segments in garlic (Table 1). The best callus induction was obtained when low concentrations of this combination were added to MS basal medium (Table 1). Similarly, the effect of different concentrations of 2, 4-D and IBA were monitored to induce callus from in vitro grown root segment explants. These explants exhibited the best callus induction when MS medium was supplemented with 0.5 mg/l 2, 4-D and 0.5 mg/l IBA (Table 1). This was studied for the first time to induce callus from garlic root explants on MS medium. This indicated that IBA can be used potentially with 2, 4-D to induce callus for subsequent regeneration of garlic plantlets.
It could be concluded from the present investigation that auxins and cytokonins at about equal concentrations promote callus induction from root segments in garlic. The present findings also supported the results of Robledo et al [27] who induced organogenic callus from root tip explants inoculated on MS medium with 2.2- 4.5 μM 2, 4-D and 2.3- 4.6 μM Kin. When 2, 4-D and BA were used in combinations, the optimum results were recorded with 0.5 mg/l 2, 4-D and 0.5 mg/l BA. Shimonaka et al. [45] also succeeded in inducing callus from basal leaf explants of another close relative of garlic i.e., A. fistolum L. in Gamborg medium with 4.5 μM 2, 4-D and 0.44 μM BA after two-month incubation. The present results revealed that in case of root explants 0.5-1.0 mg/l 2,4-D is more effective in callus induction regardless of the concentrations and combinations of other PGRs (Table 1). It was also observed that BA and Kin together were not efficient in producing callus from root segment in basal MS medium. However, when MS medium was supplemented with 0.5 mg/l of 2, 4-D, Kin and BA each, then it resulted in the best callus production (Table 1). Similarly, when root segments were placed on basal MS medium with supplement of 0.5 mg/l 2, 4-D, IAA and NAA, optimum callus induction was observed after eight weeks of inoculation. This finding reveals the potential of various auxins and cytokinins that may enhance the induction of callus production from different explants.
Effects of different PGRs were tested on shoot regeneration from callus of garlic. For this purpose, calli were grown with various auxins and cytokinins (Table 2). The calli cultures were transferred to MS medium with different concentrations of BA which responded positively towards shoot formation. These results are in accordance with the findings of Fereol et al. [44], who showed that when medium is supplemented with 0.5 mg/l BA, the embryogenic callus of garlic subsequently develop into shoot apex within four weeks. Robledo-Paz et al [27] obtained shoots from root tip explants with 4.4 μM BA. Barandiaran et al. [25] successfully regenerated the shoots from the callus derived from axenic root tips of garlic in B5 medium with BA (3.0 mg/l). However, it is also found that the higher concentrations of BA promoted abnormalities in shooting of garlic [44]. The best shoot regeneration occurred at 1.0 mg/l of Kin for root explants derived calli of garlic (Table 2). Successful regeneration of roots was obtained from callus derived from axenic root tips on B5 medium with much higher concentrations of Kin i.e., 10.0 mg/L [26]. When MS basal medium was supplemented with Kin and BA, the best shooting was occurred at a concentration of BA (1.0 mg/l) and Kin (0.5 mg/l) from root derived callus. These results show the efficiency of different cytokinins for shoot formation from callus.
Conclusion
The purpose of the present study was to induce callogenesis and subsequent organogenesis in garlic. The effects of nature, combinations and concentrations of plant hormones and explant types (in vitro grown young root segments) on induction of callus and subsequent regeneration has been investigated. Efforts were made to regenerate shoots from callus cultures of garlic by using different concentrations of Kin and NAA. The callus responded well to shoot formation on MS medium with 1.0 mg/l Kin and 0.5 mg/l NAA (Figure 2) (Table 2). The present results are in close line with the findings of Kim et al. [46] who showed that media containing 3.0 mg/l of both Kin and NAA exhibited the best shoot formation from garlic explants. However, in the current research, this was achieved by using relatively low concentrations of the same combination. Barandiaran et al. [25] also succeeded to develop shoots from callus cultures from axenic root tips of garlic on B5 medium having Kin (10.0 mg/l) and IAA (2.0 mg/l) instead of NAA. For shoot induction, the calli obtained from root segments were cultured on MS medium with different concentrations of BA and NAA (Table 2). The best shoot regeneration was achieved with BA (1.5 mg/l) and NAA (0.5 mg/l) from root segment explants derived callus (Figure 2). The present results are in accordance with the findings of Hasegawa et al. [42] who successfully produced shoots from callus cultures of garlic with 2.0 mg/l BA and 0.02 mg/l NAA. Shimonaka et al. [45] also succeeded to produce shoots from callus cultures of A. fistolusium L. on ½ strength MS medium with BA (1.0 μM) and NAA (0.5 μM). The present results are also in line with that of Kim et al. [45] who were able to regenerate the shoots from callus culture of garlic on 3.0 mg/l of both BA and NAA. The present findings are also consistent with the results of Ali and Metwally [47] who applied the similar combinations and concentrations for shoot induction.
For shoot induction, different concentrations of Z together with BA and Kin were used. When calli, obtained from root tip explants were transferred to MS medium, the best shooting was achieved with 0.5 mg/l Kin and 0.5 mg/l Z. Other concentrations of this combination responded moderately to shoot regeneration (Figure 2). The callus derived from root segments also exhibited the best shooting response with 1.0 mg/l BA and 0.5 mg/l Z indicating that BA and Z can be used together for subsequent in vitro shoot induction from callus. When root tips were placed on MS medium with 0.5 and 1.0 mg/l BA, the small shoots were generated from one end of the root tip. The root tips become greenish and swollen at one end after about four weeks of inoculation on the basal MS medium. The swollen portion then protruded out and small shoots were produced from it. The calli were subjected to root formation on MS medium with different concentrations of auxins. The best rooting response was achieved with IBA 2.0 mg/l and NAA 0.5 mg/l (Figure 6) (Table 3). Of the various concentrations of IAA and NAA tested, IAA (2.0 mg/l and 1.0 mg/l) together with NAA (0.5 mg/l) exhibited the optimum rooting. Similarly, different concentrations of IBA and IAA were also effective in root formation from callus cultures of garlic on basal MS medium (Figure 6) (Table 3). For complete plantlet formation, the best results were achieved when shoots were inoculated on ½ strength MS medium with 3.0% sucrose.
These results indicated that A. sativum L. could be micropropagated at large scale from root segments explants. These findings may be useful not only for micropropagation of Allium spp., but also for the genetic engineering through plant transformation for the production of medicinally active compounds through cell suspension cultures.
References
- Campbell JH, Efendy JL, Smith NJ, Campbell GR (2001) Molecular basis by which garlic suppresses atherosclerosis, J Nutr 13: 1006-1009.
- Milner JA (2001) A historical perspective on garlic and cancer. J Nutr 131: 1027-1031.
- Rahman K (2001) Historical perspective on garlic and cardiovascular disease. J Nutr 13: 977-979.
- Hile AG, Shan Z, Zhang ZS, Block E (2004) A version of European starlings (Sturnus vulgaris) to garlic oil treated granules: Garlic oil as an avian repellent. Garlic oil analysis by nuclear magnetic resonance spectroscopy. J AgriFood Chem 52: 2192-2196.
- Koul AK, Gohil RN (1970) Causes averting sexual reproduction in Allium sativum Linn. Cytologia 35: 197-202.
- Koul AK, Gohil RN (1971) Further studies on natural triploidy in viviparous onion. Cytologia 36: 253-261.
- Kamenetsky R, Rabinowitch HD (2001) Floral development in bolting garlic. Sex Plant Repro 13: 235-241.
- Kondo T, Hasegawa H, Suzuki M (2000) Transformation and regeneration of garlic (Allium sativum L.) by Agrobacterium-mediated gene transfer. Plant Cell Reports 19: 989-993.
- Zheng SJ, Khrustaleva LI, Henken B, Sofiari E, Jacobsen E, et al. Agrobacterium tumefaciens-mediated transformation of Allium cepa L: The production of transgenic onions and shallots. Mol Breed 7: 101-115.
- Abo El-Nil MM (1997) Organogenesis and embryogenesis in callus cultures of garlic (Allium sativum L.). Plant Sci Lett 9: 259-264.
- Al-Zahim MA, FordLloy BV, Newbury HJ (1999) Detection of somaclonal variation in Garlic (Allium sativum L.) using RAPD and cytological analysis. Plant Cell Reports 18: 473-477.
- Wang HL, Kang YQ, Zhang CJ (1994) Embryogenesis via culture of garlic sprout leaf. Acta Agric Bor Sin 9: 92-94.
- Zheng SJ, Henken B, Sofiari E, Jacobsen E, Krens FA, et al. (1998) Factors influencing induction, propagation and regeneration of mature zygotic embryo-derived callus from Allium cepa L. Plant Cell Tissue Organ Cul 53: 99-105.
- Xue HM, Araki H, Shi L, Yakuwa T (1991) Somatic embryogenesis and plant regeneration in basal plate and receptacle derived-callus cultures of garlic (Allium sativum L). J Japan Society Horticul Sci 60: 627-634.
- Suh SK, Park HG (1998) Somatic embryogenesis and plant regeneration from flower organ culture of garlic (Alliumsativum L.). Korean J Plant Tissue Culture 15: 121-132.
- Kehr AE, Schaeffer GW (1976) Tissue culture and differentiation of garlic. HortScience 11: 422-423.
- Nagasawa A, Finer JJ (1988) Development of morphogenic suspension cultures of garlic (Allium sativum L.). Plant Cell Tissue Organ Cult 15: 183-187.
- Choi SY, Paek KY, Jo JT (1993) Plantlet production through callus culture in Allium sativum L. J Korean Society Hort Science 34: 16-28.
- Ayabe M, Taniguchi K, Sumi SI (1995) Regeneration of whole plants from protoplasts isolated from tissue-cultured shoot primordial of garlic (Allium sativum L.). Plant Cell Reports 15: 17-21.
- Ayabe M, Sumi SI (1998) Establishment of a novel tissue culture method, stem-disc culture, and its practical application to micropropagation of garlic (Allium sativum L.). Plant Cell Reports 17: 773-779.
- Myers JM, Simon PW (1999) Regeneration of garlic callus as affected by clonal variation plant growth regulators and culture conditions over time. Plant Cell Reports 19: 32-36.
- Haque MS, Wada T, Hattori K (1997) High frequency shoot regeneration and plantlet formation from root tip of garlic. Plant Cell Tissue Organ Cult 50: 83-89.
- Haque MS, Wada T, Hattori K (1999) Anatomical changes during in vitro direct formation of shoot bud from root tips in garlic (Allium sativum L.). Plant Production Sci 2: 146-153.
- Haque MS, Wada T, Hattori K (1998) Efficient plant regeneration in garlic through somatic embryogenesis from root tip explants. Plant Production Sci 1: 216-222.
- Barandiaran X, Martin N, Rodriguez-Conde MF, Di Pietro AD, Martin J (1999a) An efficient method for callus culture and shoot regeneration of garlic (Allium sativum L.). HortScience 34: 348-349.
- Barandiaran X, Martin N, Rodriguez-Conde MF, Di Pietro AD, Martin J (1999b) Genetic variability in callus formation and regeneration of garlic (Allium sativum L.). Plant Cell Reports 18: 434-437.
- Robledo PA, Villalobos AVM, Jofre GAE (2000) Efficient plant regeneration of garlic (Allium sativum L.) by root-tip culture. In Vitro Cellular Develop Biol-Plant 36: 416-419.
- Myers JM, Simon PW (1998) Continuous callus production and regeneration of garlic (Allium sativum L.) using root segments from shoot tip-derived plantlets. Plant Cell Reports 17: 726-730.
- Suzuki S, Nakano M (2001) Organogenesis and somatic embryogenesis from callus cultures in MuscariarmeniacumLeichtl. Ex Bak. In Vitro Cellular Develop Biol-Plant 37: 382-387.
- Okazaki K, Koizumi M (1995) Callus formation and regeneration of some species of Lilium. Acta Horticulturae 392: 97-106.
- Wickremesinhe ERM, Holcomb EJ, Arteca RN (1994) A practical method for the production of flowering Easter lilies from callus cultures. Scientia Horticulturae 60: 143-152.
- Arzate-Fernández AM, Nakazaki T, Okumoto Y, Tanisaka T (1997) Efficient callus induction and plant regeneration from filaments with anther in lily (LiliumlongiflorumThunb.). Plant Cell Reports 16: 836-840.
- Murashige T, Skoog S (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15: 473-497.
- George EF, Sherrington PD (1984) Plant propagation by tissue culture. Handbook and directory of commercial laboratories. Exegetics Ltd., Basingstoke, UK.
- Dodds JH (1991) In vitro methods for conservation of plant genetic resources. Chapman & Hall, London, UK.
- George EF (1993) Plant propagation by tissue culture. Part 1. The technology. Exegetics Ltd., Basingstoke, UK.
- Das DK, Nirala NK, Reddy MK, Sopory SK, Upadhyaya KC (2006) Encapsulated somatic embryos of grape (Vitis vinifera L.): An efficient way for storage and propagation of pathogen free plant material. Vitis 45: 179-84.
- Fay MF (1992) Conservation of rare and endangered plants using in vitromethods. In Vitro Cellular Develop Biology-Plant 28: 1-4.
- Etoh T (1986) Fertility of the garlic clones collected in Soviet Central Asia. J Japan SocHort Sci 55: 312-319.
- Novak FJ (1990) Allium tissue culture, In:Rabinowitch HD, Brewster JL (eds) Onions and allied crops CRC Press, Boca Raton, Fla. pp. 234-250.
- Laborde CJA (1986) Coexistence of garlic white rot with commercial production in Central Mexico. In: EntwistleAR(ed). Proceedings of the Third International Workshop on Allium white rot, Wellesbourne, UK. Leamington Spa: Printing Associates. pp. 24-40.
- Davies JLM (1994) Chemical control of Allium white rot: A review. In: Entwistle AR,Melero-Vara JM (eds). Proceedings of the Fifth International Workshop of Allium white rot. Cordoba, Spain: Instituto de Agricultura Sostenible. pp. 7-8.
- Hasegawa H, Sato M, Suzuki M(2002) Efficient plant regeneration from protoplasts isolated from long-term, shoot primordia-derived calluses of garlic (Allium sativum). J Plant Physiol 159: 449-452.
- Fereol L, Chovelon V, Causse S, Michaux-Ferriere N, Kahane R (2002) Evidence of a somatic embryogenesis process and plant regeneration and acclimatization in garlic (Allium sativum L.). Plant Cell Reports 21: 197-203.
- Shimonaka M, Hosoki T, Tomita M, Yasumuro Y (2002) Production of somatic hybrid plants between Japanese bunching onion (Allium fistulosum L.) and bulb onion (Allium cepa L.) via electrofusion. J Japanese Society for Horticultural Sci 71: 623-631.
- Kim EK, Hahn EJ, Murthy HN, Paek KY (2003) High frequency of shoot multiplication and bulblet formation of garlic in liquid cultures. Plant Cell Tissue Organ Cult 73: 231-236.
- Ali A,Metwall EE (1992) Somatic embryogenesis and plant regeneration as a tool for garlic improvement. Egypt J Appl Sci 7: 727-735.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences