ISSN : 2634-7806
Journal of Clinical and Molecular Pathology : Open Access
Cytoxicity of Gold NP over Mouse Gastric Stem Cells: Short Commentary
Ali Hilal-alnaqbi1*, Sawsan Dagher1, Razan Alkhatib1 and Mohamed Al-Rubeai2
1Department of Mechanical Engineering, BioEngineering, UAE University, P.O. Box 15551, Al-Ain, UAE
2School of Chemical and Bioprocess Engineering, University College Dublin, Dublin 4, Ireland
- *Corresponding Author:
- Ali Hilal-alnaqbi
Department of Mechanical Engineering, BioEngineering
UAE University, P.O. Box 15551, Al-Ain, UAE
Tel: +971 3 7135130
E-mail: alihilal@uaeu.ac.ae
Received date: January 17, 2017; Accepted date: February 09, 2017; Published date: February 13, 2017
Citation: Hilal-alnaqbi A, Dagher S, Alkhatib R, Al-Rubeai M (2017) Cytoxicity of gold NP over mouse gastric stem cells: Short Commentary . J Clin Mol Pathol 1: 06.
Copyright: © 2017 Hilal-alnaqbi A et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Short Commentary
Raman spectroscopy (RS) is an emerging lased based optical technique exploited extensively in the field of bio-medical research [1]. The principle involved in RS is when a monochromatic light is incident on an analyte, originates scattered light after interacting with sample and a small fraction of the incident light will be in elastically scattered and is used to construct Raman spectrum [2]. It is a more powerful tool to characterize any biological system, diagnose disease in tissue, cells and to understand the basics in biology [3]. RS is nondestructive, label free technique that identifies the fingerprint of any molecule and their applications include cell state identification, differentiation of cultivated stem cells, and observation of intra cellular composition and distribution of bio molecules which corresponds too many kinds of cell activities [4]. Currently cells staining and sorting and Biomarkers are used to sort and characterize stem cells. These techniques can be tedious, time consuming and error-prone and talking samples from cells cultures may cause mechanical stress and therefore cause chemistry changes to the cells surface. Furthermore, Biomarkers are limited to specific cells and not easily translated to adult stem cells. RS provides details regarding the structure, chemical bond, nature of its environment (hydrogen bonding, etc.,) plays key role in the field of biology [5].
Practical applications are limited because Raman signals are incredibly weaker compared to fluorescence signals. Numerous methods have been developed to enhance the signal and among them using noble metal nano structures, SERS technique is quite interesting [6]. Surface enhanced Raman spectroscopy (SERS) is a powerful vibrational spectroscopy technique which is nondestructive, good sensitivity and the method of preparation is simple [7]. They have been extensively used for biomedical, cancer markers in live cells, sensing, imaging and therapeutic applications [8,9]. SERS may amplify the intensity of the signal, to target biological molecule in trace amounts [10], and simultaneous examination of chemical structure and capable of detecting the single molecule [11,12] and widely used in different fields of research [13-18]. Gold nanoparticles are safe, biocompatible, chemically inert and for their physiochemical properties used as a promising material for biomedical applications [19]. Gold nanoparticles can be used as surface enhanced Raman scattering (SERS) substrates to amplify the Raman scattering intensities of molecules adsorbed to their surfaces by means of localized surface plasmon resonance (LSPR) [20,21].
Au Nps and Ag Nps shows interest in the emerging field of nano biotechnology and medicine, their efficiency in optical and spectroscopic property, advances in their synthesis and fabrication, have brought these particles in the prominent field of nano technology research and their applications in therapy and imaging [22] and various fields [23]. Application of nanoparticles in the field of biotechnology is increasing interest and their effects exerted on body, there is possible of toxicity and is an issue to be discussed [24] and more research article and reviews have been published on Cytoxicity of nanoparticles [25-29]. Cytoxicity of silver nano particles were studied over human mesenchymal stem cells [30] and studies on PC-12 cells shows changes in gene expression and interference with signal transduction after exposure of silver nano particles [31,32]. Cytoxicity of silver and gold nanoparticles in various cell systems have been studied both in vivo and in vitro conditions [33-35], human skin (HeLa), human leukaemia (K562), human breast carcinoma (SK-BR-3) and HepG2 cells lines [36-38]. Gold nanoparticles shows cytoxicity effect on epithelial cells and human carcinoma cell line [39-41] and their effects of gold nanoparticles were studied in vitro on Balb/3T3 mouse fibroblasts [42], human dermal fibroblasts [43] adipose derived stromal cells [44]. Gold nanoparticles were shown to induce cytoxicity in cos-1 cells [45], HeLa cells [46], human alveolar type II like cell line A 549 and NC1H441 in vitro [47] and toxicity of gold nano particles were studied on human glioma cell line LN-229 and human glioma stem cell line HNGC-2 [48]. Several groups have shown minimal toxicity with gold nanoparticles and more studies need to perform.
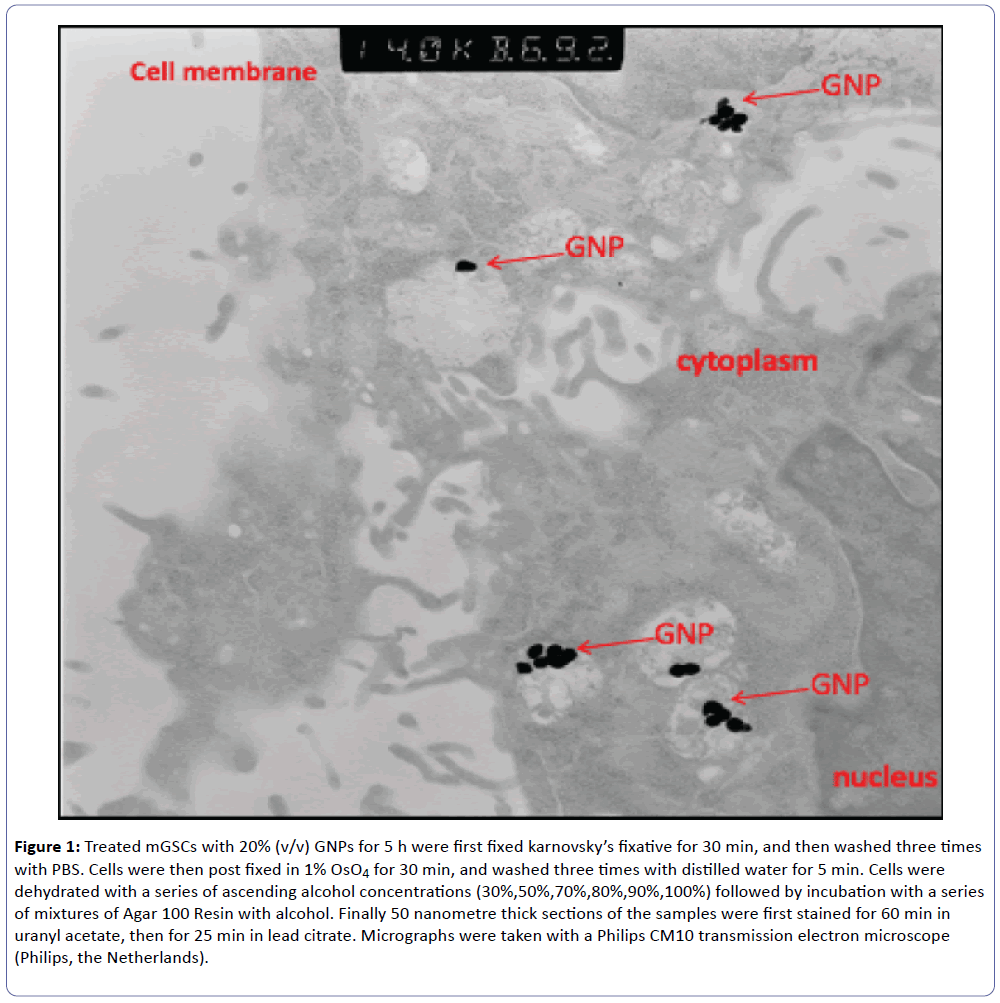
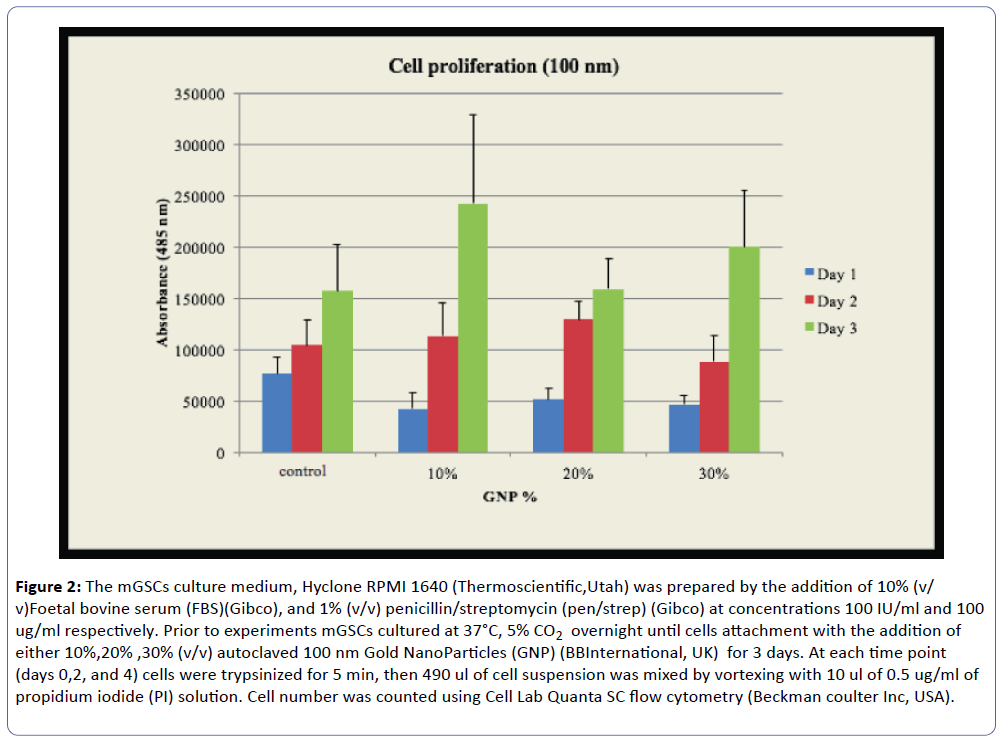
Our laboratory in UAEU studied the cytoxic effect of gold nanoparticles on mouse gastric cells for SERS. The stomach is lined by different types of epithelial cells which are generated continuously by gastric stem (GS) cells. These cells are rare and difficult to identify. Previous reports demonstrated the use of gold nanoparticle-based surface-enhanced Raman scattering (SERS) for probing the differentiation of embryonic stem cells [49]. As a first step to use SERS as a tool to identify and characterize GS cells we tested the effect of gold nanoparticles (GNP) on growth and viability of GS cells. Trypsinized mouse GS cells were incubated with GNP either directly or after forming embryoid bodies using the hanging drop method. Transmission electron microscopy (TEM) was used to localize GNP inside cells (Figure 1), whereas Pico Green assay were used to measure cell proliferation. TEM confirmed the intracellular localization of GNP (Figure 2). Pico Green assay showed that the number of GS cells treated with GNP increased by 32.8% within 3 days in comparison to untreated cells. We are currently employing SERS to identify and characterize GS cells. In conclusion, GNP does not affect the viability and cell proliferation. Embryoid bodies cultured in differentiation media show a slight morphological difference (not shown here) which is yet to be determined by Raman.
Figure 1: Treated mGSCs with 20% (v/v) GNPs for 5 h were first fixed karnovsky’s fixative for 30 min, and then washed three times with PBS. Cells were then post fixed in 1% OsO4 for 30 min, and washed three times with distilled water for 5 min. Cells were dehydrated with a series of ascending alcohol concentrations (30%,50%,70%,80%,90%,100%) followed by incubation with a series of mixtures of Agar 100 Resin with alcohol. Finally 50 nanometre thick sections of the samples were first stained for 60 min in uranyl acetate, then for 25 min in lead citrate. Micrographs were taken with a Philips CM10 transmission electron microscope (Philips, the Netherlands).
Figure 2: The mGSCs culture medium, Hyclone RPMI 1640 (Thermoscientific,Utah) was prepared by the addition of 10% (v/ v)Foetal bovine serum (FBS)(Gibco), and 1% (v/v) penicillin/streptomycin (pen/strep) (Gibco) at concentrations 100 IU/ml and 100 ug/ml respectively. Prior to experiments mGSCs cultured at 37°C, 5% CO2 overnight until cells attachment with the addition of either 10%,20% ,30% (v/v) autoclaved 100 nm Gold NanoParticles (GNP) (BBInternational, UK) for 3 days. At each time point (days 0,2, and 4) cells were trypsinized for 5 min, then 490 ul of cell suspension was mixed by vortexing with 10 ul of 0.5 ug/ml of propidium iodide (PI) solution. Cell number was counted using Cell Lab Quanta SC flow cytometry (Beckman coulter Inc, USA).
References
- Das RS, Agarawal YK (2011) Raman spectcroscopy: Recent advancements, techniques and applications. Vibrational spectroscopy 57: 164-176.
- Bumbrah GS, Sharma RM (2016) Raman spectroscopy – Basic principle, instrumentation and selected applications for the characterization of drugs of abuse. Egyptian J Forensic Sci.6: 209-215.
- Downes A, Elfick A (2010) Raman Spectroscopy and Related Techniques in Biomedicine. Sensors 10: 1871-1889.
- Nakamura MJ, Hotta K, Oka K (2013) Raman spectroscopic imaging of the whole ciona intestinalis Embryo during development. Plos one 8: e 71739.
- Kundu PP, Narayana C (2012) Raman based imaging in biological application- a perspective. J. Med., Allied sci. 2: 41-48.
- Sathuluri RR, Yoshikawa H, Schimizu E, Saito M, Tamiya E (2011) Gold nanoparaticle-based surface enhanced raman scattering for non invasive molecular probing of embryonic stem cell differentiation. Plos ONE 6: e22802.
- Hong S, Li X (2013) Optimal Size of Gold Nanoparticles for Surface-Enhanced Raman Spectroscopy under Different Conditions. J Nanomater 2013: 1-9.
- Kneipp K, Haka AS, Kneipp H, Badizadegan K, Yoshizawa N, et al. (2002) Surface-Enhanced Raman Spectroscopy in Single Living Cells Using Gold Nanoparticles. App Spectrosc 56: 150-154.
- Sun QJ, Walker GC (2013) Functionalized surface enhanced Raman scattering gold Nanoparticles: size correlation of optical and spectroscopic properties and stabilities in solutions. J Undergraduate Life Sci 7: 46-53.
- MacLaughlin CM, Parker EPK, Walker GC, Wang C (2013) Evaluation of SERS labeling of CD20 on CLL cells using optical microscopy and fluorescence flow cytometry”. Nanomedicine: NBM 9: 55-64.
- Zavaleta CL, Smith BR, Walton I, Doering W, Davis G, et al. (2009) Multiplexed imaging of surface enhanced Raman scattering nanotags in living mice using noninvasive Raman spectroscopy. PNAS 106: 13511-13516.
- Sabur A, Havel M, Gogotsi Y (2008) SERS intensity optimization by controlling the size and shape of faceted gold nanoparticles. J. Raman Spectrosc. 39: 61-67.
- Dijkstra RJ, Scheenen WJ, Dam N, Roubos EW, terMeulen JJ (2007) Monitoring neurotransmitter release using surface-enhanced Raman spectroscopy. J Neurosci Methods 159: 43–50.
- Moskovits M (2010) Spectroscopy: expanding versatility. Nature 464: 357.
- R. A. Alvarez-Puebla and L. M. Liz-Marz´an, “SERS-based diagnosis and biodetection,” Small, vol. 6, no. 5, pp. 604–610, 2010.
- Mannelli I, Marco MP (2010) Recent advances in analytical and bioanalysis applications of noble metal nanorods. Anal Bioanal Chem. 398: 2451–2469.
- Vo-Dinh T, N.Wang H, Scaffidi J (2010) Plasmonicnanoprobes for SERS biosensing and bioimaging. J Biophotonics 3: 89–102.
- Kneipp J, Kneipp H, Wittig B, Kneipp K (2010) Novel optical nanosensors for probing and imaging live cells. Nanomedicine 6: 214–226.
- Gerber A, Bundschuh M, Klingelhofer D, Groneberg AD (2013) Gold nanoparticles: recent aspects for human toxicology. J Occup Med Toxicol 8: 32.
- Njoki PN, Lim IS, Mott D, Park H, Khan B (2007) Size Correlation of Optical and Spectroscopic Properties for Gold Nanoparticles. J. Phys. Chem. 111: 14664-14669.
- Kneipp K (2007) Surface-enhanced Raman scattering. American Institute of Physics 2007. 60: 40-46.
- Liu A, Ye B (2013) Application of gold nanoparticles in biomedical researches and diagnosis. Clin Lab 59: 23–36.
- Ravindran A, Chandran P, Khan SS (2013) Biofunctionalized silver nanoparticles: Advances and prospects,” Colloids and Surfaces B. Biointerfaces 105: 342– 352.
- Fratoddi I, Venditti I, Cametti C, Russo MV (2014) How Toxic are Gold Nanoparticles? The State-of-the-Art. Nano Res. 2014.
- Yah CS (2013) The toxicity of gold nanoparticles in relation to their physicochemical properties. Biomedical Research 24: 400–413.
- Chen H, Shao L, Li Q, Wang J (2013) Gold nanorods and their plasmon properties. Chem. Soc. Rev. 42: 2679–2724.
- Khlebtsov N, Dykman L (2011) Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem. Soc. Rev. 40: 1647–1671.
- Hussain SM, Braydich-Stolle LK, Schrand AM, Murdock KO, Yu RC, et al. (2009) Toxicity evaluation for safe use of nanomaterials: Recent achievements and technical challenges. Adv. Mater 21: 1549–1559.
- Fadeel B, Garcia-Bennett AE (2010) Better safe than sorry: understanding properties of inorganic nanoparticles manufactured for biomedical applications. Adv. Drug Delivery Rev 62: 362–374
- Pan Y, Neuss S, Leifert A, Wen F, Simon U, et al. (2007) Size dependent cytotoxicity of gold nanoparticles. Small 3: 1941–1949.
- Fratoddi I, Venditti I, Cametti C, Palocci C, Chronopoulou L, et al. (2012) Functional polymeric nanoparticles for dexamethasone loading and release. Coll. Surf. B: Biointerfaces 93: 59–66.
- Johnston HJ, Hutchison G, Christensen FM, Peters S, Hankin S, e al. (2010) A review of the in vivo and in vitro toxicity of silver and gold nanoparticles: particle attributes and biological mechanisms responsible for the observed toxicity. Crit. Rev. Toxicol 40: 328–346.
- Hackenberg S, Scherzed A, Kessler M, Hummel S, Technau A, et al. (2011) Silver nanoparticles: evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicology Letters 201: 27-33.
- Hussain SM, Javorina AK, Schrand AM, Duhart HM, Ali SF, et al. (2006) The interaction of manganese nanoparticles with PC-12 cells induces dopamine depletion. Toxicol Sci 92: 456–463.
- Wang J, Rahman MF, Duhart HM, Newport GD, Patterson TA, et al. (2009) Expression changes of dopaminergic system-related genes in PC12 cells induced by manganese, silver, or copper nanoparticles. Neurotoxicology 30: 926–933.
- Nowrouzi A, Meghrazi K, Golmohammadi T, Golestani A, Ahmadian S, et al. (2010) Cytotoxicity of subtoxic AgNP in human hepatoma cell line (HepG2) after long-term exposure. Iran. Biomed. J. 14: 23–32.
- Li PW, Kuo TH, Chang JH, Yeh, JM, Chan WH, (2010) Induction of cytotoxicity and apoptosis in mouse blastocysts by silver nanoparticles. Toxicol. Lett. 197: 82–87.
- Kim YS, Song MY, Park JD, Song KS, Ryu HR, et al. (2010) Subchronic oral toxicity of silver nanoparticles. Part. Fibre Toxicol 7: 20.
- Tarantola M, Pietuch A, Schneider D, Rother J, Sunnick E, et al. (2011) Toxicity of gold-nanoparticles: synergistic effects of shape and surface functionalization on micromotility of epithelial cells. Nanotoxicology 5: 254–268.
- Zhang XD, Wu HY, Wu D, Wang YY, Chang JH, et al. (2010) Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int. J. Nano medicine 5: 771–781.
- Soenen SJ, Manshian B, Montenegro JM, Amin F, Meermann B, et al. (2012) Cytotoxic Effects of Gold Nanoparticles: A Multiparametric Study. ACS nano 6: 5767–5783.
- Coradeghini R, Gioria S, García CP, Nativo P, Franchini F, et al. (2013) Size-dependent toxicity and cell interaction mechanisms of gold nanoparticles on mouse fibroblasts. Toxicol Lett. 217: 205-16.
- Mironava T, Hadjiargyrou M, Simon M, Jurukovski V, Rafailovich MH (2010) Gold nanoparticles cellular toxicity and recovery: effect of size, concentration and exposure time. Nanotoxicology 4: 120-37.
- Mironava T, Hadjiargyrou M, Simon M, Rafailovich MH (2014) Gold nanoparticles cellular toxicity and recovery: adipose Derived Stromal cells. Nanotoxicology 8: 189-201.
- Goodman CM, McCusker CD, Yilmaz T, Rotello VM (2004) Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem 15: 897-900.
- Pernodet N, Fang X, Sun Y, Bakhtina A, Ramakrishnan A, et al. (2006) Adverse effects of citrate/gold nanoparticles on human dermal fibroblasts. Small 2: 766-773.
- Pan Y, Neuss S, Leifert A, Fischler M, Wen F, et al. (2007) Size-dependent cytotoxicity of gold nanoparticles. Small 3: 1941-1949.
- Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, et al. (2006) PEG-modified gold nanorods with a stealth character for in vivo applications. J Control Release 114: 343-347.
- Sathuluri RR, Yoshikawa H, Shimizu E, Saito M, Tamiya E (2011) Gold nanoparticle-based surface-enhanced Raman scattering for noninvasive molecular probing of embryonic stem cell differentiation. PloS one 6: e22802.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences