Cystic Fibrosis â??An Overview
S.Sridevi1,.M.Rooth Vasantha1, P.Aparanji2, .G.Sudhakar1
1Department of Human Genetics, Andhra University, Visakhapatnam, Andhra Pradesh, India
2Department of Biochemistry, Andhra University, Visakhapatnam, Andhra Pradesh, India
- *Corresponding Author:
- Sridevi Suvvari
Department of Human Genetics, Andhra University, Visakhapatnam, Andhra Pradesh, India
Tel: 9849460350
E-mail: suvvarisridevi@gmail.com
Received Date: June 23, 2021; Accepted Date: October 8, 2021; Published Date: October 18, 2021
Citation: Suvvari S, Vasantha RM, Aparanji P, Sudhakar G (2021) Cystic Fibrosis –An Overview Genet Mol Biol Res Vol:5No:6
Abstract
One of the disorder with single genetic autosomal recessive mode of inheritance that affects the respiratory system is Cystic Fibrosis (CF). This disease is mostly observed in Caucasians, and is caused by a variant in a gene located on the long arm of chromosome 7(7q31). The gene which is associated with this disease is cystic fibrosis transmembrane regulator (CFTR) gene. It codes for chloride transport and helps in mucin secretion through the epithelial cells of the nasopharynx and also in cytophysiological regulation of mucous transport. Mutation in this gene causes excess secretion of gelatinized mucin, and failure of mucous transport which causes accumulation of mucous plaques in lungs which leads to inflammation in the alveoli. This accumulation of mucin causes rapid development of severity of bacterial infections by micro organisms like Pseudomonas aeruginosa and Asperigillus fumigatus which become lethal due to respiratory failure.
Key words
Cystic Fibrosis; CFTR gene; Mucin, Pseudomonas aeruginosa; Asperigillus fumigatus
Introduction
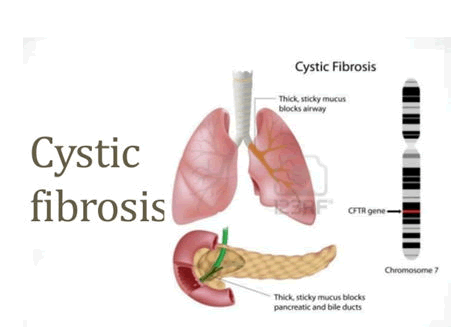
Cystic Fibrosis (CF) is a single genetic autosomal recessive disease. It is observed in 1 in 3500 births. There is nopermanent curefor it. The only future intervention method that can help in treatment is gene mapping and gene therapy or abnormal gene alteration, which can reduce the severity of symptoms and improve the quality of life. Close monitoring and early diagnosis is recommended to slow the progression of CF. If both the parents are carriers then there is 25% probability that the offsprings would be affected in this case due to transmission of single gene mutant allele. This disease is mostly observed in Caucasians, and is caused by a variant in a gene located on the long arm of chromosome 7(7q31)[Fig 1].
Figure 1: schematic diagram of affected lungs and pancrease along with representation of CFTR gene mutation in chromosome 7q32.
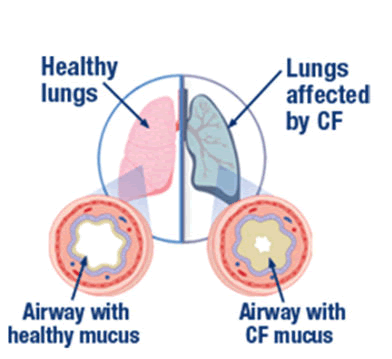
The gene which is associated with this disease is cystic fibrosis transmembrane regulator (CFTR) gene[1]. It codes for chloride transport and helps in mucin secretion through the epithelial cells of the nasopharynx. There are many gene mutations that are associated with cystic fibrosis, out of which the common defect is delta F508 where deletion of three nucleotides causes the absence of phenylalanine at the 508th codon [2, 3] and this mutation causes a huge production of CFTR protein which is the main reason for excess production of mucin in the respiratory, digestive and reproductive organs. Repeated pulmonary infection in cystic fibrosis leads to damage of epithelial cells of lungs followed by pressure on right ventricle and death of the individual [4,5] [Fig2].
Figure 2: Diagram showing the difference between healthy lungs and cystic fibrosis affected lung
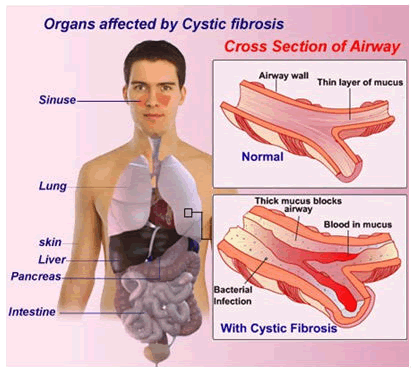
It is also one of the reasons for digestive problems as the pancreatic ducts become blocked with excess mucous secretions and reduced pancreatic enzymes. Pancreatic blockage causes malabsorption syndrome and sometimes liver gets damaged leading to liver cirrhosis. Infertility in men occurs due to blocking of vas deferens with mucous secretions. Other major organs which can be affected are small intestine, liver and rectum. These defects deprive the life span of the affected individual [Fig 3]. Grades of cystic fibrosis are given by observing the different histopathological variations. If there is accumulation of excess amount of mucin secretions in lungs and various other parts then it is differentiated as Grade I. If deterioration of exocrine part of pancrease is observed then it is regarded as Grade II. Degeneration of pancrease along with accumulation of lipids in the pancreatic parenchyma along with obesity and symptoms of premature aging is declared as Grade III [6]. Fibrosis with complete destruction of exocrine glands and with dispersed islets of Langerhans is denoted as Grade IV.
Genetic factors cause acute infection in the CF effected lungs. Elevated Immune response like excessive white blood cells in submucosa of respiratory tract occurs. Adaptive immunity in cystic fibrosis results in enhancement of T-helper cells, interleukins and cytokines in airway submucosa [7]. Decrease in immunity in cystic fibrosis causes the increase of infections by microorganisms like Pseudomonas aeruginosa and Asperigillus fumigatus causing depletion in normal functioning of lungs thereby reducing the life span of the CF patient [8]. These microorganisms are observed in large amounts in the sputum of the CF patients who are repeatedly using nebulised antibiotics. These infections can be controlled at an early stage if antimicrobial or immunomodulating interventions are used. Cultures and molecular based Real Time Polymerase Chain Reactions (RT-PCR) are repeatedly performed on mucous secretions from the spitted sputum and bronchoalveolar lavage fluid of CF patients for prior detection of P.aeruginosa but these results may vary with culture media.
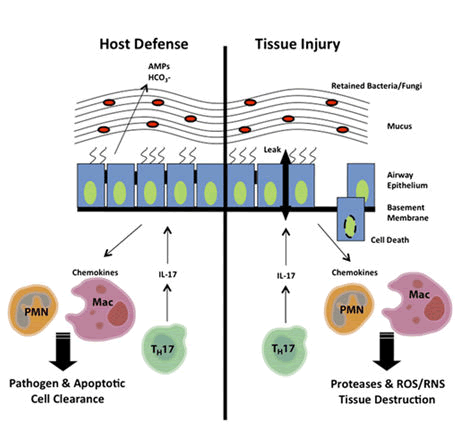
Next generation sequencing would be more helpful for accurate detection of P.aeruginosa and it would be helpful in increasing the survival rate of CF patients. The novel invitro studies showed that the allergic broncho pulmonary asperigillosis CF patients have vitamin D deficiency which causes decrease in T-helper cells(Th17) and increase in tumour growth factor-β (TGF-β) surface expression [Fig 4].
AMPs=antimicrobial peptides; Mac=macrophages; PMN= polymorphonuclear cells, RNS=reactive nitrogen species; ROS=reactive oxygen species, Th17==T helper 17 cells.
More than 20 variants of CFTR DNA over expressions identified through Next Generation Sequencing (NGS) were confirmed by Sanger sequencing. Previously only lung failure in CF patients can be predicted depending on the cystic fibrosis hospital based registry which holds the patient’s data. This approach is used to ensure the care of the people suffering from this condition. The data from the cystic fibrosis registry is an essential factor for medical understanding of personalized healthcare.
Presently, denova artificial intelligence technologies help in prediction of clinical implications, progression of the disease and targeted drug therapy for chronic cystic fibrosis. There are many complications that need to be prevented or to improve health conditions in CF individuals. These challenges can be overcomed by using machine learning approach.
References
- Nelson L Turcios; Cystic Fibrosis lung disease: An overview; Respiratory Care ; 2020,65(2)233-251
- Pallin M. Cystic fibrosis vigilance in arab countries: the role of genetic epidemiology. Respirology. 2019; 2492:93-94.
- Wilschanski M, and Durie P R. Patterns of GI disease in adulthood associated with mutations in the CFTR gene.Gut;2007;56(8),1153-1163
- Ameen N, Silvia M, Bradbury NA; Endocytic trafficking of CFTR in health and disease; J Cyst Fibros, 2007, 6 (1):1-14.
- Sander D B,Bittner R.C.L, Rosenfeld M et al.,Pulmonary exacerbations are associated with subsequent FEV1 decline in both adults and children with cystic fibrosis; Pediatr Pulmonol.2011;46:393-400
- AmbeshP, Lal H., Pancreatic Lipomatosis: Complete Replacement of Pancrease by Fat; J Clin Diagn Res.2015 Oct,9(10).
- Karen K, Reid A,Moore JE et al., Coinfection with Pseudomonas aeruginosa and Aspergillus fumigatus in Cystic fibrosis; European Respiratory Review 2020:;29(158):200011.
- Amanda Cambraia, Mario Campos Junior, Veronica Marques Zembrzuski et al., “Next-Generation Sequencing for Molecular Diagnosis of Cystic Fibrosis in a Brazillian Cohort” Disease Markers, Vol. 2021,8 pages.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences