ISSN : 2573-0320
Journal of Transmitted Diseases and Immunity
Cryptococcal neorformans Antigenemia among HIV-Infected Patients in North Eastern Nigeria
Baba Waru Goni1*, Ibrahim Musa Kida1, Ismaila Adamu Saidu2, Haruna Yusuph1, Michael Brown3, Bukar Bakki1, Babajide Ajayi4, Ballah Denue1, Abubakar Yerima1, Sabiu Abdu Gwalabe5 and Abubakar Sahabi Mohammed1
1Department of Medicine, College of Medical Sciences, University of Maiduguri, Maiduguri, Nigeria
2Faculty of Infectious Tropical Diseases, London School of Hygiene and Tropical Medicine, London, UK
3School of Health and Human Sciences, University of Essex, Colchester, UK
4Department of Immunology, University of Maiduguri Teaching Hospital, Maiduguri, Nigeria
5Department of Medicine, Abubakar Tafawa Balewa University Teaching Hospital, Bauchi, Nigeria
- *Corresponding Author:
- Goni BW
Department of Medicine, University of Maiduguri, PMB 1069, Maiduguri, Borno State, Nigeria
Tel: +234 800 000 0000
E-mail: babawaru@gmail.com
Received date: December 27, 2016; Accepted date: February 23, 2017; Published date: February 28, 2017
Citation: Goni BW, Kida IM, Saidu IA, et al. Cryptococcal neorformans Antigenemia among HIV-Infected Patients in North Eastern Nigeria. J Transm Dis Immun. 2017, 1:1.
Abstract
Background: Cryptococcus neoformans infection is a common fungal infection that is cosmopolitan in distribution and causes a life-threatening disease among HIV-infected patients especially in advanced disease. Screening for cryptococcal antigen (CrAg) in patients enrolling in ART programs may identify those at risk of cryptococcal meningitis and permit targeted use of pre-emptive therapy.
Methods: This was a cross survey study of 215 consecutive HIV-infected patients at an ART treatment centre were screened for cryptococcal antigenaemia using the cryptococcalantigen lateral flow assay (CrAg LFA). Study subjects were assessed for clinical features of Cryptococcus neoformans infection. CD4+ cell count, haemoglobin concentration as well as HIV-1 RNA viral load was also compared between cryptococcalantigen positive and negatives.
Results: 37 (16.7%) patients were positive for cryptococcal antigenaemia. Cryptococcal antigen positive subjects had a lower median CD4+ cell count (58 cells/uL vs. 273 cells/uL, p<0.001) and higher median viral load (log 3.6 copies/mL vs. log2.3 copies/mL, p=0.05) compared to cryptococcal antigen negative subjects. The commonest predictors of positive CrAg status in the study were; low BMI, low Hb, fever and cough.
Conclusion: The study has shown the prevalence of cryptococcal antigenaemia to be high among the study subjects attending this service. Therefore, screening of patients receiving care at this centre may help in identifying individuals who are at risk of cryptococcalmeningitis for prophylactic treatment.
Keywords
Cryptococcal antigenaemia; Heterobasidiomycetous; Neurological sequel
Introduction
It has been over a century since Cryptococcus neoformans was discovered from an environmental source [1,2]. Cryptococcus neoformans is an encapsulated, heterobasidiomycetous fungus that is worldwide in distribution as an opportunistic pathogen particularly among HIV patients. The spectrum of clinical infection ranges from a complete asymptomatic state to more severe lifethreatening disseminated disease. Cryptococcus neoformans primarily enters the body through the respiratory tract, but it has a particular predilection for the central nervous system (CNS).
Cryptococcal neoformans is not generally considered as part of normal human flora [3-8]. It is usually commonly isolated in immunocompromised patients e.g., AIDS patients, patients on immunosuppressive therapy, head and neck cancers, lymphoproliferative diseases, etc. Studies done in the USA gave a predicted overall incidence of cyptococcosis in the pre-AIDS era in the United States to be around 0.8 cases per million persons per year [9,10]. However, during the peak of the HIV/AIDS epidemic in the USA in the early 1990s the incidence reached almost 5 cases per 100,000 persons per year in several major cities. With the roll out of Highly Active Antiretroviral Therapy (HAART) in the developed world in the mid-1990s there was a significant fall in the overall incidence to almost its pre-AIDS state.
Available data have shown that the prevalence of Cryptococcus neoformans infection among HIV-infected patients is very high in most parts of the world particularly in sub-Saharan Africa, which is considered the epicentre of the HIV/AIDS pandemic [11,12]. The total population of sub-Saharan Africa is estimated to be about 800 million (i.e., 11.4% of the global population), but the region is home to about 60% of the total number of people infected with HIV in the world (22.6 million). In addition, sub- Saharan Africa is the poorest region of the world where the evil triads of poverty, ignorance and infectious diseases/malnutrition thrive in a synergistic fashion [12,13]. Studies have shown the prevalence to be in the range of 15-45% among HIV-infected patients especially in advanced disease [13,15]. In many studies in sub-Saharan African, Cyptococcus neoformans is one of the commonest isolates among culture positive meningitis in the setting of HIV/AIDS [13-15]. Further studies have elucidated that the risk of Cryptococcal meningitis was higher among Africanborn HIV-infected individuals even after migrating to the West. Other similar studies done in Asia e.g., in Thailand has also shown higher incidence of Cyptococcal meningitis among cohorts of HIV-infected patients. Research has shown that C. neoformans is a common cause of life-threatening opportunistic infection in up to 40% of HIV-infected patients in sub-Saharan Africa and about 17% of AIDS patients’ world-wide [16].
The Center for Disease Control and Prevention (CDC) reported in one of its surveys that up to 6% of patients with AIDS in the United States had developed Cryptococcal infection. HIV associated Crytococcal infection now accounts for about 80% to 90% of the total cases of Cyptococcal infection worldwide. The outcome of Cryptoccal neoformans infection in immunocompromised patients (e.g., HIV/AIDS) is often grave. Furthermore, patients that experienced clinical recovery from the infection often require a lifelong secondary prophylaxis therapy. Studies have shown that the mortality rate due to Cryptococcal meningitis in the setting of HIV/AIDS is in the range of 25-30%. In addition, about of 40% of survivors would end up with a neurological sequela in the form of cranial palsies, hydrocephalus, loss of vision, mental retardation, etc. Relapse rate is also common i.e., up to 25% in some series. Cryptococcal meningitis is a common and often fatal opportunistic infection in HIV-infected individuals, especially in Africa and Asia. In North East Thailand, cryptococcal disease is second only to tuberculosis as an AIDS-defining illness. In a Bangkok Hospital; mortality of patients with HIV-associated cryptococcal meningitis was 43% with a mean time to death of 14 days, despite treatment with full conventional doses of amphotericin B.
Methods
Study site
This was a cross-sectional study conducted at the University of Maiduguri Teaching Hospital, North Eastern Nigeria. Ethical Approval for the study was obtained from the Medical Ethics Review Committee of the University of Maiduguri Teaching Hospital, London School of Hygiene and Tropical Medicine Ethical Committee, UK. Informed written consents were obtained from each individual patient before commencing the study. Patients had the unlimited liberty to deny consent or opt out of the study at any stage without any consequences.
Population
Sample size was calculated using Epi Info statistical software based on study power of 80% (i.e., β=0.8) and α set at 0.05 for cross-sectional descriptive study. Two hundred and fifteen people living with HIV aged 18 years and older who were referred to the Antiretroviral Treatment (ART) Clinic at the University of Maiduguri Teaching Hospital, Nigeria. The participants who consented to participate were consecutively recruited. Participants were referred to the clinic after testing positive on screening for HIV-infection either from TB treatment centres (DOTS), antenatal clinics, or from other health facilities in the region.
Procedure
Cases of newly diagnosed HIV-infected subjects were tested for Cryptococcus neoformans antigen by Cryptococcal Antigen Lateral Flow Assay (CrAg LFA) method. Structured questionnaires were administered to each study subject after obtaining an informed consent. Data about socio-demographic characteristics, clinical signs and symptoms as well as CD4+ cell counts; HIV-1 RNA viral load and haemoglobin concentration were obtained from each study subjects. Data were entered into a computer database and analyse using STATA statistical package (StataCorp 4905 Lakeway Drive, Texas USA).
Laboratory
Blood samples were taken in the clinic by a trained phlebotomist and samples were subsequently sent to the immunology laboratory at the UMTH; where samples were analysed for Cryptococcal antigen using the CrAg LFA method, as well as CD4 cell count, HIV-1 RNA viral RNA and Haemoglobin concentration by a trained laboratory scientist working with the ART programme.
The qualitative crag lateral flow assay (CrAg LFA)
This is an immune-chromatographic test system for the qualitative or semi-quantitative detection of the capsular polysaccharide antigens of Cryptococcus species complex (Cryptococcus neoformans and Cryptococcus gatti) in serum, plasma and cerebrospinal fluid (CSF). The CrAg LFA test is a prescription-use laboratory assay, which can aid in the diagnosis of cryptococcosis. The CrAg LFA is dipstick sandwich immunechromatographic assay. Specimens and specimen diluent were added into appropriate test tubes, and the lateral flow device was placed into the tubes. The test used specimen wicking to capture gold-conjugated, anti-CrAg monoclonal antibodies and goldconjugated control antibodies deposited on the test membrane.
Results interpretation: The reactions were interpreted as follows:
1. The presence of two lines (test and control), regardless of the intensity of test lines, indicates positive results.
2. A single control line indicated a negative result.
3. If the control line does not appear, the results were interpreted as invalid and the tests were repeated.
HIV-screening test
Immunoblot method was used to screened patients for the presences of HIV antibody (Immunoblot Assay) for the detection of IgG antibodies against HIV-1 and HIV-2 antigens; Immunitics Incorporation, CA, USA).
Results interpretation: (1). The bands developed were identified and compared to the positive control strip by aligning them with matching bands on the reference card provided with the test kits. (2). Presence of a band compared with positive strip was interpreted as a positive result while the absence of a band was interpreted as negative result.
CD4 cell count by flow cytometric analysis
CD4-PE fluorescence was analysed on a Partec Flow Cytometer (Partec Healthcare and Immunology, Partec GmbH, A Flugplatz 13. D-02828 Gorlitz, Germany) with an excitation light source of 488 nm or 532 nm (blue or green solid state laser). To count CD4+ T-cells, the test tubes with 840 μL of the ready prepared venous blood samples were transferred to the Partec Flow Cytometer and count analysis were carried out automatically. The dilution factor was set at 42. Counting results were displayed automatically as CD4+ T-cells per μL of whole blood.
HIV-1 RNA viral load test: This was analysed using the Amplicor method (AMPLICOR HIV-1 MONITOR TEST VERSION 1.5)
Haemaglobin concentration test: Auto analyzer was used to test for haemoglobin concentration (Haematology Auto Analyser- MIDARY BC-3200):
Data analysis
Data were entered into a computer data based and analysed using Stata statistical package version 12.1 (Statacorp, 4905 Lakeway Drive, College Station, Texas 79845 USA). Mean, median, standard deviation and interquartile range (IQR) were generated. Percentages were compared using Chi-square test, while Fishers exact was used where frequency cells were less than 5. Student t-test was used to compare mean of continuous variables. Multivariate logistic regression analysis was used to analyze for predictors for cryptococcal antigen prevalence as well as to adjust for potential confounders, mean (± 2SD) and median (IQR) were compared by Chi square/student t-test and Mann Whitney test methods for normally distributed and skewed data respectively where necessary. A p-value of 0.05 was considered as strong evidence against the null hypothesis.
Results
Table 1 shows the socio-demographics and clinical characteristics of the participants. A total of 215 HIV-infected patients were studied. Females constituted 60% of the study subjects. Their age range was 22-66 years with a mean of 38 years (SD ± 9), 42% of the study subjects were within the age category of 30-39 years. One-fifth had tertiary education, while 14.0% and 34.9% had Islamic and no education respectively (Table 1).
| N (%) | |
|---|---|
| Gender | |
| Male | 89 (41.4) |
| Female | 126 (58.6) |
| Age group (year) | |
| <30 | 39 (18.3 |
| 30 – 39 | 89 (41.8) |
| 40 – 49 | 62 (29.1) |
| ³50 | 23 (10.8) |
| Educational level | |
| None | 75 (34.9) |
| Primary | 54 (25.2) |
| Secondary | 44 (20.5) |
| Tertiary | 42 (19.5) |
| Marital status | |
| Single | 19 (8.8) |
| Married | 147 (68.4) |
| Divorced | 15 (7.0) |
| Widowed | 34 (15.8) |
| CD4+ count (cells/ul) | |
| <50 | 21 |
| 50-99 | 26 |
| 100-199 | 26 |
| >200 | 107 |
Table 1: Socio-demographics and clinical characteristics of the participants N=215.
Laboratory characteristics
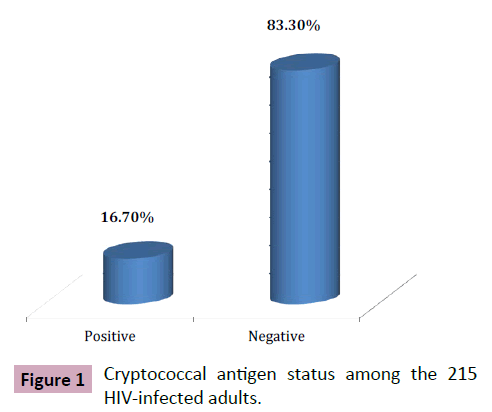
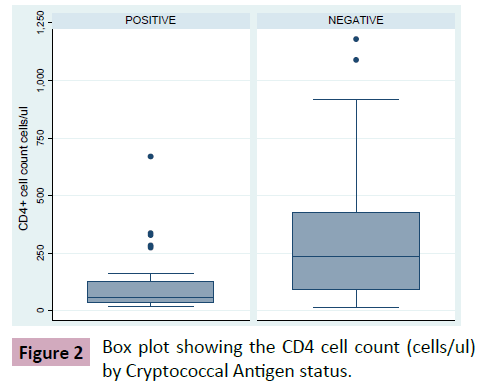
The prevalence of Cryptococcal antigenamia (by CrAg LFA qualitative method) was 16.7% (Figure 1). Median CD4 count was 58 cells/μl (IQR: 36-129) and 237 (IQR: 90-425) amongst Cryptococcal antigen positive and negative patients respectively (Figure 2). This finding was statistically significant (p-value<0.001). Mean Hb levels were 9.6 g/dL (SD: ± 2.3) and 11 g/dL (SD: ± 2.0) amongst Cryptococcal antigen positive and negative patients respectively. This finding was statistically significant (p-value 0.09). In addition, there were more CrAg positive subjects with CD4 cell count less than 100 cells/μL compared to negatives i.e., 45.5% vs. 24.0% (Table 2). Viral load was available for 75 patients (median viral load for Cryptococcal antigen positive: log3.6 copies/mL (IQR: 2.3-4.6), cryptococcal antigen negative: log2.3 copies/ml (IQR: 2.3-3.6); p-value (Figures 1 and 2) (Table 2).
| CD4 cell count (cells/ul) | CrAg Positive N (%) | CrAg Negative N (%) |
| <50 | 3 (13.7) | 19 (12.0) |
| 50-99 | 7 (31.8) | 19 (12.0) |
| 100-199 | 5 (22.7) | 21 (13.1) |
| >200 | 7 (31.8) | 100 (62.9) |
| Total N (%) | 22 (100) | 159 (100) |
Table 2: Proportion of CD4 cells count by CrAg antigen status.
Data were available for 89 HIV-infected adults to compute the BMI. The mean BMI was 22 kg/m2 (cryptococcal antigen positive: 22 kg/m2, cryptococcal antigen negative: 21 kg/m2; p-value=0.6). Table 3 below shows the percentage distribution of neurological symptoms by cryptococcal antigen status. Fever (38%) was the mostly reported amongst the clinical symptoms (positive: 18.5%, negative: 81.5%). This finding was not statistically significant (p-value=0.59). Approximately 18% reported cough, this was higher amongst those that were cryptococcal antigen negative (68.4%) compared to positives (31.6%). This finding was statistically significant (p-value=0.01). One-fifth reported skin rash, which was higher amongst those that were cryptococcal antigen negative (67.4%) compared to positives (32.6%). This finding was also statistically significant (p-value=0.01). Logistic regression was fitted to determine the risk of cryptococcal antigen prevalence (Table 4) following a multivariate model. Risk of CrAg LFA antigen positivity that was found to be statistically significant were BMI, Haemoglobin, fever and cough (Tables 3 and 4).
| Cryptococcal Antigen status | ||||
|---|---|---|---|---|
| Neurological symptoms | Total | Positive n(%) | Negative n(%) | P-value |
| Fever | 81 | 15 (18.5) | 66 (81.5) | 0.59 |
| Headache | 66 | 16 (24.2) | 50 (75.8) | 0.05* |
| Loss of consciousness | 6 | 2 (33.3) | 4 (66.7) | 0.26 |
| Seizures | 7 | 2 (28.6) | 5 (71.4) | 0.33 |
| Cough | 38 | 12 (31.6) | 26 (68.4) | 0.01* |
| Weight loss | 54 | 10 (18.5) | 44 (81.5) | 0.68 |
| Skin rash | 43 | 14 (32.6) | 29 (67.4) | 0.01* |
| Note=*statistically significant at p < 0.05 | ||||
Table 3: Neurological/clinical Symptoms by Cryptococcal Antigen status.
| Variable | Crude OR (95% C.I) | p-value | Adjusted OR (95% C.I) | p-value |
|---|---|---|---|---|
| Age | 0.99 (0.95-1.03) | 0.621 | 0.91 (0.81-1.03) | 0.135 |
| Gender | ||||
| Female | 1 | 1 | 0.785 | |
| Male | 1.33 (0.65-2.73) | 0.44 | 0.77 (0.12-4.88) | |
| BMI | 1.03 (0.92-1.15) | 0.62 | 1.36 (1.02-1.80) | 0.037* |
| Cd4 count | 1.00 (0.99– 1.00) | 0.001* | 1.00 (0.99-1.00) | 0.952 |
| Hb | 0.70 (0.57-0.85) | <0.001 | 0.56 (0.36-0.95) | 0.032* |
| Fever | ||||
| No | 1 | 1 | ||
| Yes | 1.22 (0.59– 2.53) | 0.59 | 0.04 (0.01-0.71) | 0.028* |
| Cough | ||||
| No | 1 | 1 | ||
| Yes | 2.94 (1.31-6.60) | 0.009 | 18.05 (1.58-206.09) | 0.020* |
| Skin rash | ||||
| No | 1 | 0.003* | 1 | |
| Yes | 3.29 (1.51-7.17) | 2.36 (0.38-14.50) | 0.354 | |
| Headache | ||||
| No | 1 | 1 | ||
| Yes | 2.06 (0.99-4.30) | 0.053 | 1.16 (1.34-10.12) | 0.891 |
| Weight loss | ||||
| No | 1 | 1 | ||
| Yes | 1.18 (0.53-2.64) | 0.687 | 1.13 (0.13-9.96) | 0.912 |
| Note=*statistically significant at p<0.05 | ||||
Table 4: Predictors of cryptococcal antigen prevalence.
Discussion
The prevalence of cryptococcal antigenaemia among the study population was found to be 16.7%, among these 51.4% were males and 48.6% females. This nearly equal male to female ratio in the prevalence rate may suggest same level of exposure to environmental risk factors for the acquisition of Cryptococcus neoformans rather than differences in susceptibility to infection between the two sexes. However, some studies have reported sexual differences in prevalence rate between male and females. The authors in such studies did not give any plausible explanation for gender variation in prevalence rate, however increased male sex exposure to environmental risk factors for the acquisition of cryptococcal infection may be possible explanation. Majority of the study subjects i.e., both cryptococcal antigen positives and negative subjects were in the age range 22-66 years, this favorably compares with findings from similar studies in Africa (Dzoyem et al.) [17-19]. It should be noted that this is the sexually active age group and the most infected with the HIV. Additionally, this age group may be actively engaged in activities that could predispose them to environmental risk factor such as farming (contact with soil contaminated with birds’ droppings) and exposure to birds’ guano. The overall prevalence 16.7% favourably compares with those reported by Jacinta et al. in Uganda (19%) and that of Osazuwa et al. in southern Nigeria which reported a cryptococcal antigen prevalence rate of 12.7% among antiretroviral naïve HIV-infected patients [20]. Similarly, Micol et al. and Jarvis et al. reported cryptococcal antigenaemia prevalence of 18% and 13% in Cambodia and South Africa, respectively. Dzoyem and colleagues reported relatively lower cryptococcal antigen prevalence rate of 8.5-9.68% in Yaounde, Cameroon. The results from this study demonstrate a relatively high prevalence (16.7%) of cryptococcal antigenemia in a semi-urban setting among HIV-infected patients. In a community clinic in a rural setting in Tororo, Uganda, a prevalence of only 5.3% was reported. The low prevalence rate in the Ugandan study may be due to the fact that only asymptomatic patients participated in the study [21-31]. The high prevalence in our study warrants the need to screen for and diagnose cryptococcal infection among patients with severe immunosuppression prior to initiation of ART. This may prevent unmasking of sub-clinical infection, especially in patients who are asymptomatic due to immune reconstitution [32].
On the other hand, Kumar et al. reported a prevalence rate of up to 60% among HIV-infected patients presenting with a diagnosis of meningitis in Northern India. This relatively high prevalence rate could be explained by the used of highly select group of patients presenting with features of meningitis in the study. The differences in prevalence rate in the various studies could be due to variations in study populations, exposure to environmental risk factors, sensitivity of test kits used, differences in study design and selection criteria and different biological specimens (e.g., urine, CSF or Blood). The serum cryptococcal antigen test has been shown to be a cost effective way of early diagnosis as well as prevention of fatal cryptococcal infection in the setting of AIDS. This is because screening for serum cryptococcal antigen is highly sensitive, specific and cost effective in preventing death in HIV-infected patients with CD4 counts of 100 cells/mm3 or less. In a study on cost effectiveness analysis in Uganda the findings suggest that the number needed to test and treat with CrAg screening and fluconazole treatment to prevent one cryptococcal meningitis case is 11.3 (95% CI 7.9-17.1) at a cost of US$190 (95% CI, $132-$287) while the number needed to test and treat to save one life is 15.9 (95% CI 11.1-24.0) at a cost of $266 (95% CI $185- $402) [33,34].
The frequently reported clinical symptoms that may suggest cryptococcal infection were: fever (38%), headache (31%), weight loss (25%), skin rash (20%) and cough (18%). However, cough and skin rash were found to be statistically more common among cryptococcal antigen negative subjects than positives. These clinical symptoms are not specific to cryptococcal infection alone; as such other opportunistic infections especially in the setting of HIV/AIDS may present with a similar clinical picture. Cough and fever for example are common clinical symptoms of pulmonary tuberculosis and various studies have shown that pulmonary TB is the commonest opportunistic infection as well as the cause of mortality among HIV-infected patients in sub-Saharan Africa [35,36]. Furthermore, more than one opportunistic infection could exist in the same HIV-infected patient especially in advanced disease. The following factors were found to be independently associated with cryptococcal antigenaemia: low BMI, low haemoglobin concentration, fever and cough. These findings are similar to those reported by Jacinta and colleagues in Uganda as well as Micol et al. in Cambodia. Low BMI and Anaemia (i.e., low haemoglobin concentration) may be due to malnutrition, which in turn predisposes to a dysfunctional immune system, which could have also predisposed patients to the risk of cryptococcal infection [37,38]. The following findings have been reported in malnourished HIV-infected patients: suppression of antigen specific arms of immune system and several generalized host defence mechanisms, including decreased T-cell primary antibody response, memory response and atrophy of lymph tissues. Peripheral lymphocytes and natural killer cells activities have been found to be reduced in malnourished patients [39]. Low BMI (under 15.4 kg/m2) was reported to be an independent factor associated with cryptococcal antigenemia in Cambodia [31]. Furthermore, low BMI, anaemia, fever and cough may point to an already established cryptococccal infection or presence of other opportunistic infections such as tuberculosis, disseminated bacterial infection, etc. These conditions may in themselves further predispose these patients to cryptococcal infection as a result of further immune suppression. In resource-limited settings, screening of patients with AIDS indicator conditions (i.e., opportunistic diseases) for cryptococcal infection may be more clinically relevant.
The study also revealed that cryptococcal antigen positive subjects had statistically significant low median CD4 cell count and a high median viral load. This may suggest that this group of patients were more immunosuppressed and therefore had higher risk of AIDS related mortality and morbidity. These findings are in concordance with those of Micol et al. in Cambodia. A plausible postulate for this could be; cryptococcal infection setting up an inflammatory process in which cytokines and other immune mediators are released (e.g., IL-1, interferon gamma, etc.) subsequently resulting in increased HIV-1 RNA replication and decreased in CD4 cell count [40-43]. Reverse causality could also be another possible explanation. Some of the potential explanation of this study could be from the limitations of the test method used. The cryptococcal antigen lateral flow assay is not suitable for performing a screening test for the general population especially in areas with low prevalence of cryptococcal infection. This is simply because the predictive value of the positive or negative serologic test depends on the pre-test likelihood of cryptococcal disease being present. Prozoning phenomenon (High doze hook effect) can results in false negative CrAg LFA test because of the fact that extremely high concentration of cryptococcal antigen can result in weak test line and, in extreme cases yield negative test results. Semi-quantitative procedure was used, in two suspected weakly positive results in this study to rule out prozoning. Another limitation of the CrAg LFA assay is that performance characteristics have not been established for matrices other than serum, plasma and cerebrospinal fluid. A common draw back of the CrAg LFA assay include the fact that testing haemolysed serum samples could lead to false negatives due the high background colour on the strip. Precautions were taken in this study to make sure that all test samples were stored in EDTA bottles. The assay has not been evaluated for potential interference related to specimens pre-treatment with mercaptoethanol or with specimens including the following substances: Vaginal cream, caffeine, ascorbic acid, itraconazole, amphotericin B, acetaminophen, or acetylsalicylic acid. Lumbar puncture was not done on patients who were cryptococcal antigen positive because of non-consent and logistic constraint. The prevalence of cryptococcal antigenaemia was high among HIV-infected patients at this service. CrAg positive patients had a lower median CD 4 cell count and higher median viral load than negatives. Routine screening of HIV-infected patients at this facility for cryptococcal antigen may give an opportunity to commence pre-emptive therapy in order to prevent the fatal consequences of cyrptococcal meninigitis in the setting of HIV/ AIDS in at risk individuals.
Acknowledgements
We wish to express our sincere appreciation to the Faculty of Infectious Tropical Diseases LSHTM UK, where the work was conceived by the first author (with support from his course director and supervisor) as his summer project for the MSc TMIH Masters programme in 2012. We also want to extend our special thanks to the patients and staff at the Department of Infectious Diseases and Immunology UMTH Maiduguri Nigeria, where the work was conducted.
Conflict of interest
All the authors declared that there was no conflict of interest during the preparation and submission of this manuscript.
References
- Mendel GL, Bennet JE, Dolin R (2014) Principles and Practice of Infectious Diseases (6th edn). Philadelphia: Churchill Livingstone 2997-3009.
- Perfect JR, Casdevall A (1894) Cryptococcosis. Infect Dis Clin North Am 16: 837-874.
- Sanfelice F (1894) Contribution to the Morphology and Biology of Blastomyces that develop in some fruit juices. Ann d’ Igiene 4: 463-495.
- Buschke A (1895) A coccidian of human disease. Dtsch Med Wochenschr 21: 14.
- Verse M (1914) A case of generalised blastomycosis in humans. Ver Dtsch Path Ges 17: 275-278.
- Stoddard JL, Cutler EC (1916) Torula infection in man. Rockefeller Institute for Medical Research, Monograph 6: 1-98.
- Benham RW (1976) Cryptococcosis and blastomycosis. Ann N Y Acad Sci 50: 1299-1314.
- Kwon-Chung KJ (1976) Morphogenesis of Filobasidiella neoformans, sexual state of Cryptococcus neoformans. Mycologia 68: 821-833.
- Hull C, Heitman J (2002) Genetics of Cryptococcus neoformans. Ann Rev Genet 36: 557-615.
- Dromer F, Gueho E, Ronin O (1993) Serotyping of Cryptococcus neoformans by using a monoclonal antibody specific for capsular polysaccharide. J Clin Micobiol 31: 359-363.
- Dzoyem JP, Kechia FA, Ngaba GP, Lunga PK, Lohoue PJ (2012) Prevalence of cryptococcosis among HIV-infected patients in Younde, Cameroon. Afri Health Sci 12: 129-133.
- Jean-Michel T, Larry P, Fogg C, Biraro S, Mayanja, et al. (2003) Systematic Screening of Cryptococcal Antigenemia in HIV-Positive Adults in Uganda. JAIDS 33: 411-412.
- Jean-Michel T, Larry P, Fogg C, Biraro S, Mayanja, et al.(2003) Systematic Screening of Cryptococcal Antigenemia in HIV-Positive Adults in Uganda. JAIDS 33: 411-412.
- Bogaerts J, Rouvroy D, Taelman H, Kagame A (1999) AIDS-Associated cryptococcalmeningitis in Rwanda (1983-1992): Epidemiologic and diagnostic features. J Infect 39: 32-37.
- Hajjman A, Conn LA, Stephens DS (1999) Cryptococcosis: population based multistate active surveillance and risk factors in human immunodeficiency virus-infected persons. Cryptococcal Active Surveillance Group. J Infect Dis 179: 449-454.
- Harris JR, Lindsley MD, Henchaison S, Poonwan N (2012) High prevalence of cryptococcal infection among HIV-infected patients hospitalized with pneumonia in Thailand. Clin Infect Dis 54: 43-50.
- Musubire AK, Meya BD, Mayanja-Kizza H, Lukande R, Wiesner LD, et al. (2012) Challenges in diagnosis and management of Cryptococcal immune reconstitution inflammatory syndrome (IRIS) in resource limited settings. Afri Health Sci 12: 226-230.
- Hospenthal D, Bennett JE (2000) Persistence of cryptococcomas on neuroimaging. Clin Infect Dis 31: 1303-1306.
- Kambanga A, Meya DB, Rhein J, O’Brien M (2008) Outcomes of Cryptococcal Meningitis in Uganda Before and After the Availability of Highly Active Antiretroviral Therapy. Clin Infect Dis 46: 1694-1701.
- Van Elden LJ, Walenkamp AM, Lipovsky MM (2000) Declining number of patients with Cryptococcosis in the Netherlands in the era of Highly Active Antiretroviral Therapy. AIDS 14: 2787-2788.
- Van der Horst C, Saag MS, Cloud GA (1997) Treatment of Cryptococcal meninigitis associated with AIDS. N Engl J Med 337: 15-21.
- Lawn S, Harries A, Anglaret X, Myer L, Wood R (2008) Early Mortality among adults accessing antiretroviral treatment programs in sub-Saharan Africa. AIDS 22: 1897-908.
- Lawn SD, Bekker LG, Myer L, Orrell C, Wood R (2005) Cryptococcocal immune reconstitution disease: a major cause of early mortality in a South African antiretroviral program. AIDS 19: 2050-2052.
- Musubire AK, Meya BD, Mayanja-Kizza H, Lukande R, Weiesner LD, et al. (2012) Challenges in diagnosis and management of Cryptococcal Immune Reconstitution Syndrome (IRIS) in resource limited settings. Afri Health Sci 2: 226-230.
- Devil SJ, Scheerson R, Egan W (1991) Cryptococcus neoformans serotype A glucuronoxylamannan protein conjugate vaccines: Synthesis, characterisation and immunogenicity. Infect Immun 59: 3700-3707.
- Mukherjee J, Pirofski LA, Scharf MD (1993) Antibody-mediated protection in mice with lethal intracerebral Cryptococcus neoformans infection. Proc Natl Acad Sci USA 90: 3636-3640.
- National Population Commission of Nigeria (2007) Preliminary results of the 2006 Census; Borno State Ministry of Information and Culture. Maiduguri 3: 18-22.
- Federal Ministry of Health, Nigeria (2009) National HIV/Syphilis Seroprevalence Sentinel Survey, 2009. BOSACA NEWS 1: 10-14.
- Jacinta O, David M, Bajunwire F, Kamya MR (2012) Prevalence and factors associated with cryptococcal antigenemia among severly immunosuppressed HIV-infected adults in Uganda: a cross-sectional study. J Intl AIDS Soc 1515-7.
- Osazuwa OF, Dirisu O, Okuonghae FE (2012) Cryptococcal antigenemia in anti-retroviral naïve AIDS patients: prevalence and its association with CD4 cell count. Acta Med Iran. 50: 344-7.
- Micol R, Dromer F, Fontanet A, Sar B (2007) Prevalence, determinants of positivity and clinical utility of cryptococcal antigenemia in Cambodian HIV-infected patients. J Acquir Immune Defic Syndr 45: 555-559.
- Jarvis JN, Lawn SD, Vogt M, Bangami N, Wood R, et al. (2009) Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis 48: 856-862.
- Kumar S, Wanchu A, Chakrabarti A, Sharma A, Bambery P et al. (2008) Cryptococcal meningitis in HIV-infected patients from North Indian tertiary centre. Neurol India 56: 444-449.
- Meya DB, Manabe YC, Casenuouvo B, Cook BA (2010) Cost effectiviness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with CD4+ cell count<or=100 cells/ul who start ART therapy in resource limited settings. Clin Infect Dis 51: 448-455.
- Bamba S, Lorthlary O, Sawadogo A, Millogo A (2012) Decreasing incidence of cryptococcal meninigitis in West Africa in the era of HAART. AIDS 26: 1039-1041.
- Bicanic T, Harrison TS, (2005) Cryptococcal meninigitis. Brit Med Bull 72: 99-118.
- Hakim JG, Gangaidzo IT, Heyderman RS (2000) Impact of HIV infection on meningitis in Harare, Zimbabwe: a prospective study of 406 predominantly adult patients. AIDS 14: 1401-1407.
- Martinez E, Garcia-Viejo MA, Marcos MA (2000) Discontinuation of secondary prophylaxis for Cryptococcal Cryptococcal Meningitis in HIV-infected patients responding to HAART. AIDS 14: 2615.
- Makadzange AT, Ndhlovu CE, Takarinda K, Michael R (2010) Early versus Delayed Initiation of Antiretroviral Therapy for Concurrent HIV Infection and Cryptococcal Meningitis in Sub-Saharan Africa. Clin Infect Dis 50: 1532-1538.
- Vibhagool A, Sungkanuparph S, Mootsikapun P (2003) Discontinuation of secondary prophylaxis for cryptococcal meningitis in HIV-infected patients treated with HAART: A prospective, multicentre randomised study. Clin Infect Dis 36: 1329-1331.
- Scarborough M, Gordon SB, Whitty CJ (2007) Corticosteroids for bacterial meningitis in adults in sub-Saharan Africa. The New Engl J Medi 357: 2441-50.
- Kisenge PR, Hawkins At, Maro VP, Mchele JPD, Swai NS, et al. (2007) Low CD4 count plus coma predicts cryptococcal meningitis in Tanzania. BMC Infect Dis 7: 39.
- Lee B, Anekthanon T, Poungvarin N, Nilanont Y (2012) Etiology and risk factors of stroke in HIV-infected patients in Siriraj Hospital: A case-control stud. J Med Assoc Thai 95: S227-34.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences