ISSN : 2393-8854
Global Journal of Research and Review
Capsule Based Aerosol Generation-Test Article Conservation during the Conduct of in vivo Lead Optimization Respiratory Safety Assessment Studies
S Moore*, R Goodway , S Cracknell and G Paul
Global Lead of Inhalation Sciences and Engineering, Covance Laboratories, UK
- *Corresponding Author:
- S Moore
Global Lead of Inhalation Sciences and Engineering,
Covance Laboratories,
UK,
E-mail: simon.moore@covance.com
Received Date: March 18, 2020; Accepted Date: March 26, 2020; Published Date: April 04, 2020
Citation: Moore S (2020) Capsule Based Aerosol Generation–test article conservation during the conduct of in vivo lead optimization respiratory safety assessment studies. Glob J Res Rev Vol.7 No.1:49.DOI: 10.36648/2393-8854.7.1.49
Copyright: © 2020 Moore S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
In both clinical and non-clinical environments the inhaled delivery of drug substances to conscious subjects will typically require larger quantities of the test article than any other route of administration. This difference is greatest for dry powder formulations in which charge and proximity effects are most pronounced, but it is also due to the losses and inefficiencies in the delivery device and aerosol delivery system. Minimizing such losses, particularly when conducting in vivo lead optimization studies in rodents, provides a means of reducing the cost of drug development. Decreasing the amount of test article requirement enables the conduct of investigations utilising inhaled route earlier in a development programme, when the test article is most likely to have limited availability and greatest cost. Over the last three years GSK and Envigo have successfully collaborated to identify alternative aerosol generation methods to minimize powder usage for inhaled delivery to conscious non-clinical species. The principle alternative delivery methodology, intratrachael insufflation, achieves particulate deposition dissimilar to conscious inhaled delivery and can produce artefactual toxicological and pharmacological results. Results of work conducted by Envigo and GSK to characterize and redesign a capsulebased aerosol generator are presented. If further work is successfully concluded, the doser should facilitate the use of the inhaled route earlier in in vivo lead optimization studies, so increasing the quality of candidate drugs and reducing compound attrition precipitated by findings in later, resource intensive in vivo studies.
Keywords
Aerosol; Capsule; Envigo capsule doser; Test articles
Powder Aerosol Generator Assessment
Following a review of available powder aerosol generators, a capsule-based doser designed and manufactured by Envigo (Envigo capsule doser) was selected for further evaluation and refinement. The primary benefit identified for this doser was the potential to utilize the entirety of any test article dispensed. This contrasts with commercially available particulate aerosol generators that incur losses to dead volume as a consequence of their relatively large test article reservoirs. These losses contribute significantly to overall test article usage, particularly for aerosol production in shorter studies of 1-7 exposures. The doser sequentially loads, single-pierces and then discharges the contents from individual hydroxypropyl methyl cellulose (HPMC) size 2 capsules through a pneumatically operated cycle. A small compressed air pulse and venturi effect are used to aerosolize the contents of each capsule[1-4].
Envigo Capsule Doser
The results presented1 (Tables 1 and 2) for this doser showed good potential to significantly reduce overall test article usage even though it had been originally designed to operate at a significantly higher airflow (50 L/minute) than used for the evaluation.
| Analytical concentration (μg/L) | ||
|---|---|---|
| Sample number | Undiluted micronised test article | 40% test article with lactose |
| 1 | 25.8 | 8.60 |
| 2 | 23.4 | 7.56 |
| 3 | 27.9 | 0 |
| 4 | 26.7 | 0 |
| Overall mean | 26.0 | 8.08 |
| Coefficient variation | 7.3 | 9.1 |
Table 1: Analytical aerosol concentration of Envigo capsule doser.
| Test article | MMAD (μm) | Geometric standard deviation |
|---|---|---|
| 1 mg undiluted | 2.3 | 2.03 |
| 1 mg 40% blend | 2.5 | 2.24 |
Table 2: Analytical particle size distribution data of the Envigo capsule doser
The doser generated atmospheres with a mass median aerodynamic diameter (MMAD) that was within the respirable range for rodents (1-3 μm)2 for both blended and undiluted test article (Table 2). The achieved values were similar to those found for the same test article when used in studies using commercially available powder generators. The UPLC-UV data were consistent, however, the duration of aerosol sampling exceeded 3 minutes and was not of a suitable duration to characterize the pulstile delivery (three capsule/minute) of aerosol into the chamber.
The other issue with this device was the aerosol generation efficiency (calculated as the ratio of analyzed to nominal test article concentrations) was lower than anticipated when compared with some standard commercial powder generators. Consequently, the doser was redesigned and a new prototype produced to improve utilization of the test article.
Redesigning the Envigo Capsule Doser
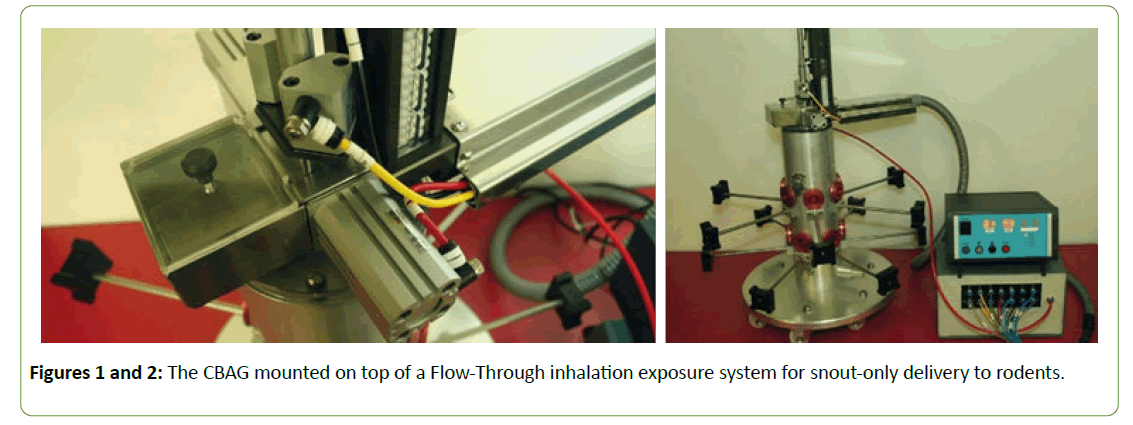
The Capsule Based Aerosol Generator (CBAG) The doser (Figures 1 and 2) was completely redesigned and rebuilt to include the following features:+In-line double piercing of the capsules+ Capsule contents discharged downwards rather than upwards+Adual capsule feed Greater flexibility in the control systems to permit variation of the air discharge volume and pulse timeframe to maximize capsule content ejection+Greater actuation rate flexibility (time between capsules discharges) +And, critically, a lower overall operating airflow to reduce test article usage.
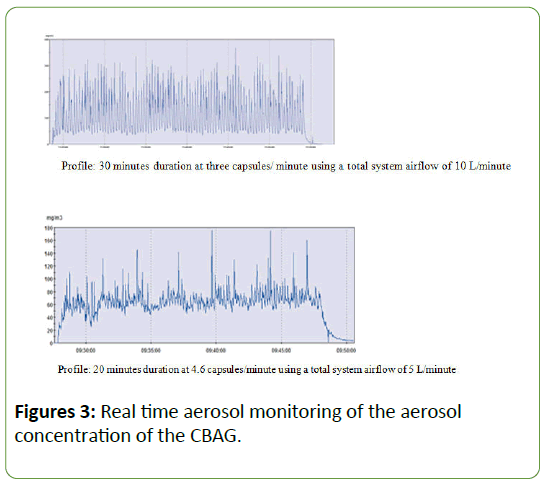
The prototype CBAG was evaluated3 with three drug materials that were selected based on their dissimilar physical characteristics. These were:+Fluticasone propionate (FP) +Tiotropium bromide +GSK-CMPD: 3-{8-(2,6-difluorophenyl)-2- [(1H-imidazol-2-yl methyl) amino-7-oxo-7,8-dihydropyrido[2,3- D]pyrimidin-4-yl}-4-methyl-N-2-thiazolylbenzamide monomethanesulfonate For these trials HPMC size 2 capsules were again used and the capsule fill weight employed was varied between 0.1 and 1 mg (for FP only) to investigate the relationship between the two dosers. However, for these trials, the capsules were filled using a semi-automated system for capsule loadings greater than 0.3 mg, as well as manually. Aerosol concentration was determined using an HPLC-UV method of analysis. Additionally, a continuous real-time dust monitor was connected to the exposure system and used to specifically monitor the consistency of aerosol generation at one second intervals by providing qualitative assessment. As expected for a capsule based system, a pulsatile powder aerosol concentration was observed, which was particularly marked when delivering three capsules/minute with an airflow of 10 L/ minute (Figure 3). The operating parameters were then modified by increasing the capsule delivery rate and reducing the airflow to 5 L/minute. These conditions achieved a more consistent and less pulsatile aerosol concentration.
Further trials were conducted over a broader range of target aerosol concentrations (0.07 to 119 μg/L) using an airflow of 5 L/minute and, like the Envigo capsule doser, the redesigned doser gave a linear relationship (Figure 4) with respect to content of drug substance. However, this observation is of greater significance due to the dissimilar physical properties of the individual test articles.
Varying the capsule loading of undiluted FP from 0.1 to 1 mg/ capsule (Figure 5) also gave a linear relationship with respect to aerosol concentration (20 to 119 μg/L). This was consistent with the previously presented results1 using the original Envigo capsule doser. The mean MMAD for these three test articles varied between 2.7 and 5.0 μm under various operating conditions3 , which was larger than expected and greater than was observed with the original Envigo capsule doser. These data are presented in Table 3. No explanation is currently available for the larger than expected particle size, however, the authors suspect it is related to the low exposure system airflow rather than the CBAG doser. Another factor that may have impacted results is the static charge associated with generation of aerosol from capsules, this may have resulted in increased particle aggregation as evidence of charge accumilation was readily observed during trials with the spray dried FP test article.
| Test article | GSK-CMPD | Tiotropium | FP | Spray dried FP | |
|---|---|---|---|---|---|
| Doser | Envigo capsule | CBAG | CBAG | CBAG | CBAG |
| Blend strengths Used(w\w) | 36% and 91% | 5% and 85% | 5% and 80% | 0.01%, 0.1%,1%, 5%, 10% and 99% | 25% and 99% |
| Airflow (L/minute) | 10 and 20 | 5 and 13 | 5, 7 and 10 | 5 | 5 |
| MMAD (μm) | 2.3-2.5 | 2.7-4.4 | 3.3-5.0 | 3.3-4.9 | 5.6-5.8 |
| Average generation efficiency | 12% | 24% | 19% | 21% | 11% |
1In terms of active and correcting for purity
Table 3: Blend strengths, airflow, analytical particle size distribution and generation efficiency data for the three different test articles.
Comparison between EnvigoCapsule Doser and CBAG Dosers
Although over 80 trials have been conducted with these two dosers, a direct comparison between the two is currently difficult to make as modifications were made to the dosers during the course of the trials and the number and nature of operating parameter.
These include:
• Type of chamber (modified large commercially available, medium sized and narrow Flow-Through chamber)
• A wide range of airflows
• Piercing and airburst time could not be varied for the Envigo capsule doser
• Capsule actuation rate per minute (one capsule delivered every 20, 30 or 40 seconds for the Envigo capsule doser and one capsule delivered every 10, 13 or 20 seconds for the CBAG)
• Different batches of blended test material
However, when comparing the Envigo capsule doser and CBAG, it is clear that the new doser provided greater consistency between and within trials, and improved generation efficiency with the same test article (Table 3).
The reason for the low generation efficiency of the spray dried FP was considered to be due to static charge with the capsules.
Reduction of the Particle Size
Based on the particle size results, further modifications to the doser were made to reduce the particle size and additional preliminary trials were conducted. The generator outlet nozzle was modified to include the option of a baffle and to permit the introduction of a jet to aid particle deaggregation (Table 4). This reduced the particle size for most samples to an acceptable MMAD range for rodents2.
| Formulation | % blend strength Mass | Median Aerodynamic Diameter (μm) | ||
|---|---|---|---|---|
| - | - | Nozzle only | Nozzle with jet (no baffle) | Nozzle with jet and baffle |
| Tiopium | 5% | 4.7 | 3.7 | 3.5 |
Table 4: Analytical particle size distribution data with and without the modified nozzle, jet and baffle.
Compound Requirement Assessment
The data in Figure 6 represents a comparison between two commonly used powder generators, Wright Dust Feed (WDF) and Rotating Brush Generator (RBG) with an Envigo-modified WDF (reduced dead space) and the CBAG. This represents the minimum undiluted test article usage for a single exposure of 30 minutes duration with 250 g animals and the respiratory minute volume equation derived by Alexander et al4.
The mass of test article that would be required for standard WDF and RBG generators is significantly greater than that required for the modified WDF and for the Envigo capsule doser. The lower requirement for the modified WDF generator when compared with the standard WDF is due to a decrease in the canister depth and thus a lower overall unusable test article ‘dead space’ (reduced from 8 mm to 2 mm in depth). The higher usage for the RBG generator is principally due to the required higher operating airflow for generation relative to the other dosers (even when minimal operating settings are used) and the relatively high test article dead volume in the powder reservoir. The significant advantage of the CBAG is its ability to utilize the entire dispensed test article, other generators require that a proportion of the test article is used to fill dead volume in a powder reservoir.
Conclusion
The capsule based aerosol generator (CBAG) successfully generated aerosols from three test articles with dissimilar physical characteristics. There was a linear and proportional relationship between aerosol concentration and formulation loading of the test article when capsules are filled with 1 mg of total formulation. There was a linear and proportional relationship between aerosol concentration and capsule loading. Following system modifications to include a jet and baffle option in the nozzle, the particle size, for most samples was within the respirable range acceptable for rodents2. The test article requirement to achieve a delivered dose of 300 μg/kg has decreased from 950 mg using standard commercial generators, to 15 mg using the CBAG for a single exposure. This means the limiting factor for supply is the amount required for chemical validation and analysis rather than technical dosing considerations. The CBAG offers a significant cost saving in test article production over commercially available dosers as it minimizes dead volume waste.
Acknowledgement
The authors would like to thank all Envigo and GlaxoSmithKline staff, who have been involved in this project.
References
- Moore S, Goodway R, Hardy C, Coombs D, Allen A, et al., (2011) Inhalation Capsule Doser - Conserving Test Article in Small Scale Inhalation Exposures, Society of Toxicology Annual Meeting logy, USA.
- OECD (2009) Subchronic Inhalation Toxicity: 90-day study OECD Guidelines for the Testing of Chemicals.
- Paul GR, Somers GI, Moore SA, Goodway RE (2012) The Capsule Based Aerosol Generator – Conserving Test Article in Small Scale Inhalation Exposures. Respiratory Drug Delivery 2: 525-530.
- Alexander D, Collins C, Coombs D, Gilkison I, Hardy C, et al., (2008) Association of Inhalation Toxicologists (AIT) working party recommendation for standard delivered dose calculation and expression in non-clinical aerosol inhalation toxicology studies with pharmaceuticals. Inhalation Toxicology 20: 1179-1189.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences