Biocomposites from Chicken Feathers Waste
Dhoolappa Melinamani*, Lakshmishree KT, Sundareshan S and Prasad RV
Department of Veterinary, Veterinary College, Karnataka Veterinary Animal and Fisheries Science University, Shivamogga, India
- *Corresponding Author:
- Dhoolappa Melinamani
Department of Veterinary,
Veterinary College,
Karnataka Veterinary Animal and Fisheries Science University,
Shivamogga,
India,
Tel: 9448160837;
E-mail: drdsm2011@gmail.com
Received date: September 21, 2022, Manuscript No. IPRRWM-22-14615; Editor assigned date: September 26, 2022, PreQC No. IPRRWM-22-14615 (PQ); Reviewed date: October 04, 2022, QC No. IPRRWM-22-14615; Revised date: December 28, 2022, Manuscript No. IPRRWM-22-14615 (R); Published date: January 05, 2023, DOI: 10.36648/IPRRWM.8.1.001
Citation: Dhoolappa M, Lakshmishree KT, Sundareshan S, Prasad RV (2023) Biocomposites from Chicken Feathers Waste. J Waste Recycl Vol:8 No:1
Abstract
Nowadays, the sustainable production of consumer products is the major challenge faced by modern societies and future generations, a ter decades of reliance on fossil resources which, on one hand, did generate economic growth and prosperity but, on the other hand, has le t serious ecological, geopolitical and social legacies. In this alarming context, the concept of bioeconomy is developing and promotes as a new sustainable and knowledge-based economic model centered on the use of renewable biomass/waste derived agro-industrial and municipal wastes. Millions of tons of chicken feather gather in the waste stream every year. Traditional disposal strategies such as incineration, burial in land ills are expensive, difficult and generate greenhouse gases or pose danger to the environment. In spite of advances in the biomedical ield, there are no ideal keratin based biomaterials (biocomposite) available for clinical use till date, but their unique properties, such as remarkable biocompatibility and propensity for self-assembly, biodegradability, mechanical strength and natural profusion, make them good candidates for future applications. Although there is scope for better utilization of the keratin-based wastes, at present there are no sustainable strategies developed for making good use of these wastes. There is no systematic and translational study on morphological aspects of keratin based wastes such as hoof, feather and wool which have potential biomedical applications.
Keywords
Keratin; Biocompatibility; Biodegradability; Biopolymers; Chicken Feathers (CF)
Introduction
The morphology of bovine hoof, chicken feather, ovine wool and their potential biomedical applications were studied in the present work. The surface morphological studies of feather and wool showed rough surface due to presence of hooks and the cuticle cells respectively which played an important role in interfacial adhesion and mechanical bonding in biocomposites [1]. The biocomposites were prepared using starch based bioresin, chitosan and then reinforced with short and long keratin biofibres. Scanning electron micrographs of fractured surfaces of biocomposite showed good dispersion of matrix by short ibres when compared to long ibres. The bioinsoles of varying thickness were prepared by sandwiching the bio ibres between short ibre biocomposite (bottom layer) and bioscaffold (upper layer) to obtain desirable physical properties similar to that of insoles available in the market. Hence, the study was undertaken to understand the morphology of chicken feather, ovine wool and their potential biomedical applications were studied and discussed in the present work.
Making good use of wastes from renewable resources is an attractive concept from both commercial and environmental perspectives is a challenging task. If the animal wastes can be reused, we can turn the waste into wealth. Keratin based wastes can be recycled made eco-friendly by many ways in order to ensure sustainable practices in livestock/poultry farming and reduce the environmental threats. Keratin like other natural biopolymers viz., collagen and chitosan can be used for making biomaterials in tissue repair and regeneration. Such applications would enhance the value of utilization of keratins, as recent reports support the acceptance of keratin as a material for biotechnology and biomedical applications [2]. The traditional disposal methods (incineration, burial) are expensive, difficult and generate greenhouse gases or pose danger to the environment therefore require difficult control measures.
In spite of advances in the biomedical ield, there are no ideal keratin based biomaterials (bioscaffolds and biocomposite) available for clinical use till date, but their unique properties, such as remarkable biocompatibility and propensity for selfassembly, biodegradability, mechanical strength and natural profusion, make them good candidates for future applications [3]. During last decades, study has attentive on the extraction, puri ication, characterization and applications of keratin protein from Chicken Feathers (CF), wool ibers and hoof. Although there is scope for better utilization of the keratin-based wastes, at present there are no sustainable strategies developed for making good use of these wastes. In this regard, we have initiated to develop proof of concept to exploit the potential to convert these wastes into useful consumer products. The present need is to ind the green and eco-friendly methods for the extraction of keratin biomass with the minimal usage of harmful acids and chemicals. The use of keratin biomass for biomedical applications is one of the areas of research, still needs to be explored. There is no systematic study on morphological aspects of keratin based wastes such as hoof, feather and wool which have potential biomedical applications. Hence, the study was taken with the following objective; to study the morphology of chicken feather and wool for their potential biomedical applications.
Materials and Methods
Keratin based wastes such as chicken feather and wool were collected from slaughter house in Shivamogga and stored at room temperature for morphological studies and further processing for production of biofibres. The morphological studies undertaken were gross morphology, microscopy and morphometry [4]. After morphological characterization, the chicken feather and wool were further used for production of keratin based biofibres, development of biocomposites and bioinsoles.
Morphological studies of feather and wool
The architectural arrangement of feather parts such as shaft or rachis and their branching pattern of the fibres in chicken feather were elucidated by gross morphology. For scanning electron microscopy, both the samples of feather and wool samples were processed as previously described by Bozzola and Russell. Briefly, the specimens were fixed in 2.5% gluteraldehyde in 0.1 M phosphate buffer (pH 7.2) for 24 hours at 4oC and post fixed in 2% aqueous osmium tetroxide for 4 hours. Dehydrated in series of graded alcohols and dried to critical point drying with CPD (EMS-850) unit by using liquid corbon dioxide. The processed samples were mounted over the stubs with doublesided carbon conductivity tape and a thin layer of gold coat over the samples were done by using an automated sputter coater (Model-JEOL JFC-1600) for 3 minutes and scanned under scanning electron microscope (SEM-Model: JOEL-JSM 5600) at required magnifications as per the standard procedures at RUSKA Lab, college of veterinary science, PVNRTVU, Rajendranagar, Hyderabad, India. For morphometrical analysis, physical properties of feather and wool samples, namely, length, diameter and moisture content were determined. Length was measured in millimeter (mm) using measuring scale and the average length of randomly selected fibres (n=30) was calculated. Similarly, the diameter was measured in microns (μ) using digimatic caliper (Mitutoyo, Japan). Average diameter was calculated for the same samples (n=30). For measuring the moisture content, one gram of feather and wool samples was taken and dried in hot air oven at 500oC until no further loss in weight was noticed. During drying process, the weight of the fibres was recorded at every 24 hours interval. The weight of the fibres which did not change even after 3 times recording was considered as Final Weight (Wf) [5]. The moisture content of the samples was calculated and expressed as percentage as follows.
Moisture content=Wi-Wf/Wi X 100
Where,
Wi=The initial weight (g)
Wf=The final weight (g)
Finally, the average moisture content of the samples was determined for randomly selected samples (n=30).
Production of keratin based biofibres from feather and wool
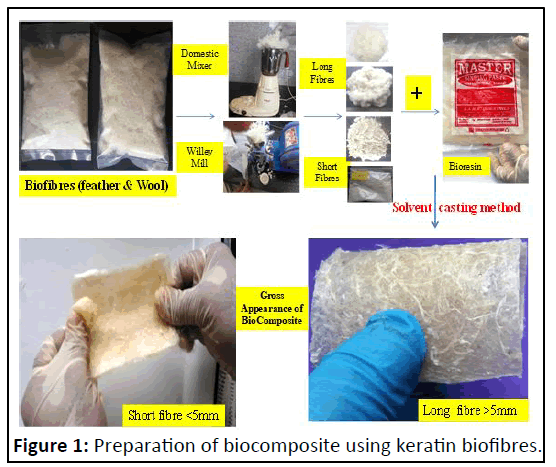
Biofibres were prepared in the laboratory, using 30 g of keratin wastes separately for feather and wool fibres as previously described with little modifications. Raw keratin based wastes collected from local slaughter houses at Shivamogga were washed using 4% soap detergent solution. The samples were decontaminated with 4% sodium hypochlorite adjusted to pH 10.0 with 1 M sodium hydroxide for one hour by agitating using domestic kitchen mixer (Prestige) and then cleaned with 70% Ethyl Alcohol (EtOH) for one hour. The cleaned and wet keratin materials were dried to constant mass in hot air oven at 50oC. The size of dried keratin materials was reduced (comminution) using kitchen mixer within closed container for 5 minutes, sheared, shredded, rubbed and fluffed the keratin wastes into a suitable product called long biofibre of more than10 mm in length [6]. These fibres were subjected to pulvarization using willey mill to get less than 5 mm sized fibres called short biofibres (Figure 1).
Development of keratin based biocomposite
Both short and long biofibres were used separately to prepare short fibre biocomposite and long biofibres biocomposites respectively. Chitosan (85% deacetylated chitin from shrimps) from sigma aldrich and starch gum from local market were procured. Casting is one of the most common techniques for processing lab scale composites. Chitosan-starch-keratin fibres were used to fabricate biocomposite by the solution casting method as previously described by Mathew, et al. Briefly, chitosan solution was prepared by dispersing 0.2 g of chitosan in 100 ml of acetic acid solution (1%, v/v) and stirred. Then, starch gum solution was prepared by mixing 5 g of starch in 100 ml of water and glycerol added as a plasticizer (1% v/v), this solution was heated beyond its gelatinization point (90°C) for 10minutes, after it was stirred and cooled to room temperature (25°C). A series of chitosan-starch composite films were prepared by mixing 100 ml of chitosan solution with 100 ml of starch solution (5:95, chitosan:starch). Short and long keratin biofibers prepared were added at 30% (w/w) as previously reported by Salhi, et al.
The mixture was stirred for 20 minutes at room temperature. The resulting mixture was poured into glass plates to allow for gel formation and later cooled to room temperature to obtain biocomposites in film forms for further morphological analysis.
Morphological analysis of biocomposites
Both short and long biofiber biocomposites were further subjected to light microscopic and ultramicroscopic morphological analysis. The light microscope (Olympus CH 20i) was used to study microscopic morphology under high power (40X) to know the homogeniciny of the biofibre and matrix in the biocomposite. The ultramicroscopic morphology of biocomposite was studied by Scanning Electron Microscope (SEM-Model: JOEL-JSM 5600). Briefly, the samples of all composites were fractured using universal testing machine, to observe by SEM the behavior of the reinforcement and matrix [7]. Fractured samples were mounted on metal stubs and were vacuum-coated with gold at 7 × 10-2 mB using argon in a sputter coater (Model-JEOL JFC-1600-EMS 550) scanned under Scanning Electron Microscope (SEM-Model: JOEL-JSM 5600) at required magnifications as per the standard procedures at RUSKA lab, college of veterinary science, PVNRTVU, Rajendranagar, Hyderabad, India.
Results and Discussion
The chicken feather was made up of the three parts; a central axis called rachis/shaft, the secondary structures (the barbs that are attached to the rachis and the third structure (barbules) which were connected to the barbs. The barbs at one end of the feather were firm, compact, closely knit, while those at the other end are downy, i.e. soft, loose and fluffy. These findings were similar to that of observation recorded by McGovern, whereas Huda and Yang and Tseng reported that substructures of the feather present an extensive and tortuous hydrophobic surface. The barbs at one end of the feather were firm, compact and closely knit, while those at the other end were downy, i.e. soft, loose and fluffy. The down feather provides insulation, while the flight feather provides an airfoil, protects the body from moisture the skin from injury and provides colors for displays.
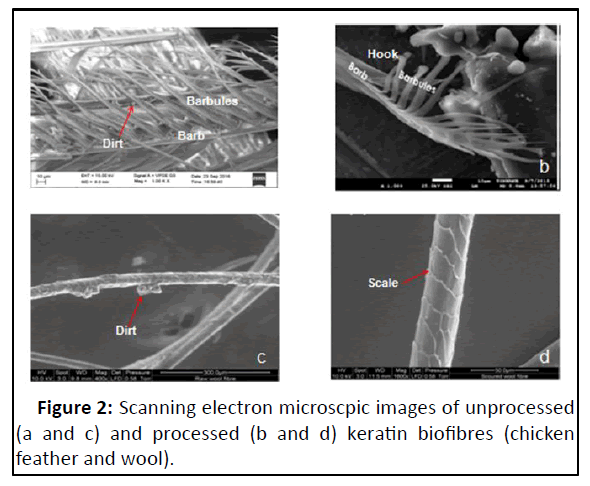
Ultramicroscopically, surface morphology of feather showed barbules with hook at its ends, while that of wool fibre showed the outer layer of cuticle cells (Figure 2). These observations corroborated with studies of Saravanan and Dhurai wherein they reported that the micro-fibrils of feather were twisted forming a helix that is responsible for the fibre’s high mechanical strength. The barbs had branches called barbules, which could contribute to the resilience property of the feathers. Further they opined that the cleave lines or striations along the fibres gave rise to a certain surface roughness, which could contribute to interfacial strength that in addition to the high length to diameter ratio reached for the fibre could be useful for reinforcing composites. The surface studies of raw feather and wool fibres by SEM showed variable quantities of wax, acids, dirt, vegetable matter, moisture and other contaminants in the present study. These findings were similar to that of observations made by Tseng who described the impurities coated the entire feather and particulates were trapped by layers of barbules and hooked barbicels holding adjacent barbs together. These substructures present an extensive and tortuous hydrophobic surface.
Morphometrically, the average length, diameter and moisture content of feather and wool fibres were 15 mm, 5 μ, 10% and 68 mm, 10 μ, 11% respectively. The average length and diameter of feather was lesser than that of the wool fibresss. In the present study, the observed moisture content value of feather (9%-11%) was lesser than the value (12%-13% in its as-received state) recorded by Kar and Misra. Schmidt and Line concluded that keratin became more strongly bound when water was removed from feather fibres giving higher stiffness. Therefore keratin biofibres of feather and wool origin were dried in hot air oven at 70°C for overnight to reduce the moisture content so as to make the biofibres stronger. Feughelman reported that the single fibre strength of feather was approximately double the strength of wool, but the elongation at break was very low. From this it could be understood that feather broke easily than wool during mechanical processing. Barbs and barbules are sold commercially as feather fibre used to reinforce polymers. Strength and modulus of the single feather is up to 300 MPa and 6 GPa which was better than most of wool fibers.
Production of keratin based biofiber from feather and wool
In spite of wider anticipated applications of keratin waste, due to lack of processing methods, we currently have a limited ability to utilize them for different applications. Therefore, the present study was proposed to optimize the process to produce clean fibre from chicken feather and wool wastes, which serve as basic biomaterial for production of various biobased products such as biocomposites, bioinsoles, for multiple biomedical applications. Tang, et al. clearly defined two kinds of biopolymers; first from living creatures and another which was essential to be polymerized but were biodegradable and come from renewable resources. Keratin fibers were strictly nonabrasive, low density, biodegradable, renewable, eco-friendly, insoluble in organic solvents, hydrophobic behavioral, warmth retention and cost effective too. These properties made it a suitable material for using in high structural reinforcement in polymer based composites.
In the present study, unprocessed feather appeared straw-like and the barbs were stuck to the rachis in a greasy tangle. Raw wet feather turned brown after 2 days when left at room temperature presented distinct putrid smell. These observations were due to the presence of variety of microorganisms on their parts of body as reported by Tompkin, et al. Bansal, et al. reported that casting of biocomposite by using chicken feather as fiber, it has to be cleaned first in order to remove the dirt and other unwanted particles present on the feather surface. Feathers are generally washed with running water and partial ethyl alcohol (C2H5OH) to obtain clean, sanitized and odor free feathers [8]. The fibers were obtained conferring to a patented process by barone and schmidt. Ethanol has been shown by Griffith to remove a fatty layer from the surface of feathers and the removal of this layer.
After washing in regular tap water, feathers had their barbs fanned out, but a significant odour remained. Water holding in the feather turned it black after a few days due to bacterial decomposition, which may be due pathogenic bacteria such as Enterobacter, Escherichia and Salmonella. A carcass surface could contain as much as 100 to 1,000 mesophilic bacteria per cm2 and member of the family Enterobacteriaceae detection was used to indicate food quality and safety. This suggested that the use of disinfectant was necessary during processing of feathers. The surface of the unprocessed feather and wool fibres contained variable quantities of wax, acids, dirt, vegetable matter, moisture and other contaminants. Hence, unprocessed feather required pre-treatment, starting with decontamination using 4% sodium hypochlorite adjusted to pH 10.0 with 1 M sodium hydroxide for one hour, then cleaned with 70% Ethyl Alcohol (EtOH) for one hour to ensure process hygiene cleaning to remove impurities that cause objectionable odour, discoloration and equipment fouling. Augurt and Asten also used in their study the chlorine bleach such as 5% (w/v) Sodium Hypochlorite (NaOCl) at pH 10.0 at 25°C and with this colony proliferation was halted or delayed. The similar efforts were made by Bansal, et al. to clean the chicken feathers using water and hair drying shampoo. After completely washing the feathers two to four times in a bucket it was then kept in an open atmosphere for 24 hours. Results obtained showed complete dry, clean and less sticky appearance. Thus the cost effective method was produced but it has lesser effectiveness as compared to other chemical processes. Shalwan and Yousif studied the significance of alkaline treatment on different natural fibers and the different polymer matrix and found that Tensile Strength (TS) and wear strength of treated fibers were better than untreated. So, the concept of using clean feathers came into existence in order to achieve better results. Jagadeeshgouda, et al. also explained the washing method of chicken feather in order to remove dirt and moisture content from the feather. Chemical treatment (using NaOH) helped to remove moisture content from the fibers thereby improving strength of the materials.
The size of dried keratin materials was reduced using domestic mixer within closed jar for 5 min, fluffed the feather into a suitable biofibres. Morphological studies revealed that the fiber surface was not damaged by this method. Fibre product consisted of barbs with intact barbules and thin rachis with barbs sheared off. The processed wool surface showed overlapping cuticle cells without impurities after the cleaning process. The processed feather and wool fibres had rough surface due to presence of hooks and the cuticle cells respectively. The observations of biofibres produced in the present study were similar to that of findings made by Tseng.
Composites differed in the amount of fiber, fiber type, fiber length, fiber orientation and possibly fiber hybridization. In general, short-fiber composites were used in lightly loaded or secondary structural applications, while continuous (long) fiberreinforced composites are utilized in primary applications and are considered high-performance structural materials. Tao and Jie reported that the natural fiber/Poly Lactic Acid (PLA) composites were prepared with ramie and jute short fiber as reinforcement and PLA as matrix. The mechanical and thermal properties of the composites were better than those of plain PLA. When the content of the fiber is 30%, the composites could get the best mechanical properties. Gautam reported that the short fibers obtained from human hair were found to possess high impact resistance, strength, stiffness and hydrophobic nature. Their low cost, low density and large aspect ratio could make them good reinforcing materials in polymer matrix to make composites. Randomly oriented short keratin fibers with 1% weight percentage were reinforced into HDPE matrix to prepare composite sheet. Hence, in the present study, short keratin fibres of less than 5 mm were produced by pulverization method using willey mill.
Development of keratin based biocomposite
Composite material can be defined as a mixture of material with two or more unlike constituents, which were differing in forms, insoluble in each other, chemically inhomogeneous and physically distinct. Gautam reported that the bio-composite was a material formed by a matrix (resin) and a reinforcement of bio fibers (from renewable resources). With wide-ranging uses from environment-friendly biodegradable composites to biomedical composites for drug/gene delivery, tissue engineering applications and cosmetic orthodontics, they often mimic the structures of the living materials involved in the process in addition to the strengthening properties of the matrix that was used but still providing bio compatibility.
Bio-composites were characterized by the fact that the bolsters (glass or carbon fiber or talc) are replaced by bio fiber (wood fibers, hemp, flax, sisal, jute). These bio-composites are emerging as a viable alternative to glass-fiber reinforced composites especially in automotive and building product applications. The combination of bio-fibers such as kenaf, hemp, flax, jute, henequen, pineapple leaf fiber and sisal with polymer matrices from both non-renewable and renewable resources to produce composite materials that are competitive with synthetic composites requires special attention. Bio fiber–reinforced polypropylene composites had attained commercial attraction in automotive industries. Bio fiber-polypropylene or bio fiberpolyester composites are not sufficiently eco-friendly because of the petroleum-based source and the non-biodegradable nature of the polymer matrix. Using bio fibers with polymers based on renewable resources will allow many environmental issues to be solved. By embedding bio-fibers with renewable resource-based biopolymers such as cellulosic plastics; polylactides; starch plastics; polyhydroxyalkanoates (bacterial polyesters) and soybased plastics, the so-called green bio-composites were continuously being developed. In the present study, we have made an attempt to prepare biocomposites from keratin based wastes.
Preparation of biocomposite
A staple length range requirements for any fibres to use them for weaving was 40 mm-50 mm. In the present study, the length range in the feather barbs was about 5 mm-25 mm, so it was difficult to process them for yarning or weaving; hence chopped mat concept was used to obtain the nonwoven mats (composites) that provide equal strength in all directions.
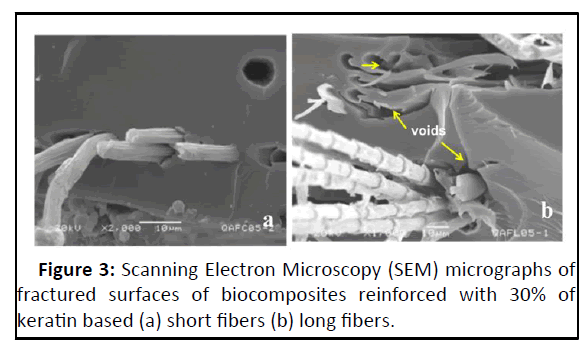
In the present study, three biopolymers have been combined synergistically to obtain a composite: Chitosan-starch as matrix and keratin based biofibers such as feather and wool as reinforcement. Incorporation of keratin biofibers in a natural matrix composed of chitosan and starch is an innovative way of preparing eco-friendly composites fully supported by natural resources. An important observation made in the present study was the difference in the physical nature between short and long fibre composites. The uniform dispersion of the short biofibres with matrix was due to the polysaccharide matrix and keratin which had high degree of compatibility. Here the matrix adhered to the fiber surface, enabling good embedding without fiber entanglements or rachis agglomerates. The matrix appeared with striations and deformations around the fiber; this is evidence of the strength transmitted between fiber matrix interfaces. Few voids were located in the surface caused by slips of fibers, but in general, good dispersion evident fiber-polymer interactions were noticed. The studies indicated the compatibility of the matrix with the biofibres with effective fibre-polymer interactions and even dispersion. The similar observations were recorded by Barone, et al. and Saucedo- Rivalcoba, et al. wherein short keratin biofibres reinforced with polyethylene and polyurethane matrix respectively for preparation of biocomposites. In contrast, the long biofiber composites had long and more evident fibres which were not wetted completely by the matrix. This resulted in reduced interfacial adhesion which in turn affected the efficiency in transmission of strength between fibre and matrix.
The scanning electron micrographs of long biofibre composites revealed the presence of zones with a high quantity of fibers, the majority appeared oriented to the load direction. In contrast to the short fibre composite, long fibers were close together they were not well dispersed, forming bundles that could cause certain weak sites (Figure 3). Mart nez-Hernández, et al. reported similar observations in their study. Brostow, et al. linked the concept of brittleness to the storage modulus (E), according to their affirmation a material with high ‘E’ will not be brittle. Keratin biofibre reinforcement decreased brittleness in composites, since for all composites ‘E’ was higher than the value for the matrix. This was due to the achieved transference of strength from the fiber to the matrix and consequence of adequate interface dispersion.
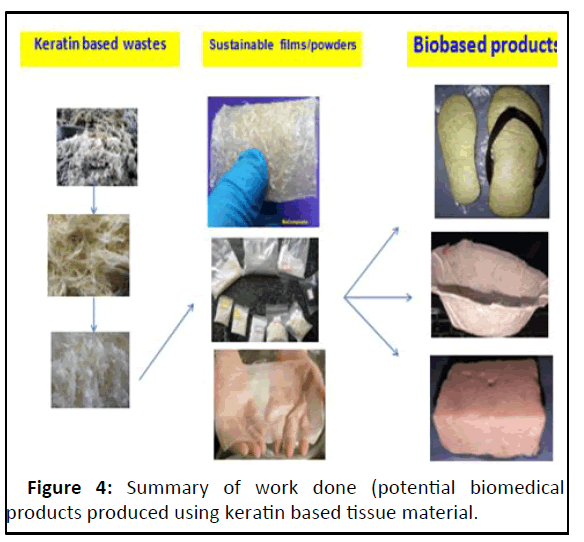
From the morphological point of view, the presence of honey comb structures makes poultry feather fibers to have low density and also provides air heat insulating capabilities unlike any other natural or synthetic fibers. The relevant composites showed a decrease in mechanical and thermo physical properties with increasing poultry feather products transition was clearly observed at 30% content of fibre as reinforcing material. Therefore, the content of the biofibres as reinforcing material in the composite was limited to 30% in our study (Figure 4).
Conclusion
The keratin based wastes of livestock origin such as hoof, chicken feather and sheep wool are potential sources for intermediary products such as biofibres, keratin-powder and biocomposits having multiple applications including bioinsole, larvicidal preparations and bioscaffolds etc.
Short fibre biocomposites are superior to long fibre biocomposites as they exhibited effective fibre-matrix interactions and even dispersion resulted in reduced the brittleness and increased the mechanical strength when compared to long fibre composites. Short fibre biocomposites are appropriate for the development of bioinsole. The bioinsoles of varying thickness were prepared by sandwiching the biofibres between short fibre biocomposite (bottom layer) and bioscaffold (upper layer) to offer additional offloading and antibacterial properties.
References
- Saravanan K, Dhurai B (2012) Exploration on amino acid content and morphological structure in chicken feather fibres. J Text Apparel Tech Manag 7:1-6
[Googlescholar] [Indexed]
- Li Z, Reimer C, Picard M, Mohanty AK, Misra M (2020) Characterization of chicken feather biocarbon for use in sustainable biocomposites. Front Mater 7:3
[Crossref] [Googlescholar] [Indexed]
- Carrillo F, Rahhali A, Canavate J, Colom X (2013) Biocomposites using waste whole chicken feathers and thermoplastic matrices. J Reinf Plast Compos 32:1419-1429
[Crossref] [Googlescholar] [Indexed]
- Aranberri I, Montes S, Azcune I, Rekondo A, Grande HJ (2017) Fully biodegradable biocomposites with high chicken feather content. Polymers 9:593
[Crossref] [Googlescholar] [Indexed]
- Casadesus M, Macanas J, Colom X, Canavate J, Alvarez MD, et al. (2018) Effect of chemical treatments and additives on properties of chicken feathers thermoplastic biocomposites. J Compos Mater 52:3637-3653
[Crossref] [Googlescholar] [Indexed]
- Molins G, Alvarez MD, Garrido N, Macanas J, Carrillo F (2018) Environmental impact assessment of Polylactide (PLA)/chicken feathers biocomposite materials. J Polym Environ 26:873-884
[Crossref] [Googlescholar] [Indexed]
- Bansal G, Jain Y, Ahmed Y, Kishore C, Agarwal V (2021) A comprehensive study on experimental analysis and development methods of chicken feather fiber reinforced bio composites. Mater Today: Proc 46:10310-10314
- Pourjavaheri F, Jones OA, Czajka M, Martinez‐Pardo I, Blanch EW, et al. (2018) Design and characterization of sustainable bio‐composites from waste chicken feather keratin and thermoplastic polyurethane. Polym Compos 39:620-632

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences