ISSN : 2634-7164
Journal of Medical Microbiology and Immunology Research
Appraisal of EBERs, LMP-1 and EBNA-2 on the Prostate Carcinoma as Diagnostic and Originator Markers
Layla Abd AL-Kareem Waheed1*, Basim Shab Ahmed2 and Dawood Salman3
1Department of Teaching Laboratories, AL-Mustansiriya University, Iraq
2Department of Pathology, College of Medicine, AL-Mustansiriya University, Iraq
3College of Health and Medical Technology, AL-Mustansiriya University, Iraq
- *Corresponding Author:
- Waheed LAA
Department of Teaching Laboratories
AL-Mustansiriya University, Iraq
Tel: +96414168500
E-mail: maysaaposy@gmail.com
Received Date: Apr 22, 2019; Accepted Date:May 06, 2019; Published Date: May 13, 2019
Citation: Waheed LAA, Ahmed BS, Salman D (2019) Appraisal of EBERs, LMP-1 and EBNA-2 on the Prostate Carcinoma as Diagnostic and Originator Markers. J Med Microbiol Immunol Res 3:1.
Copyright: © 2019 Waheed LAA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Epstein-Barr virus (EBV) considers the first human virus to be directly implicated in carcinogenesis in different sites of body. Prostate adenocarcinoma is very common in all world. In Iraq, prostate cancer is one of the ten top cancers that affect men. 30 formalin-fixed Paraffin embedded tissue blocks were obtained from prostate carcinoma biopsies and transurethral Resection of Prostate (TURP) and, 30 formalin-fixed, paraffin embedded blocks were obtained from benign prostatic hyperplasia (BPH) collected from archives of laboratory in Ghazi Al-Hariri hospital for surgical specialties and from private histopathology laboratories in Baghdad from May 2016 to December 2016, and 20 control tissue from forensic medicine institute in Baghdad. All samples examination by In situ hybridization (ISH) to detect Epstein-Barr encoded small RNAs (EBERs), then use Immunohistochemistry (IHC) to exam EB nuclear antigen-2(EBNA-2) and Latent membrane protein-1(LMP-1). EBV can be detected by successful method In situ hybridization technique' to exam EBERs in Prostate cancer, which detected only in Prostate carcinoma groups in this study. LMP-1 and EBNA-2, can be detected by Immunohistochemistry method in Prostate carcinoma with highly percentage than benign prostate hyperplasia and control group. Finally, there is a correlation between EBERs, LMP-1 and EBNA-2 in malignant group.
Keywords
EBV; EBERs; LMP-1; EBNA-2; Prostate tissue
Introduction
Epstein-Barr virus (EBV) (type 4 human herpes), it is well known cause of many diseases, like infectious mononucleosis, multiple sclerosis and cancerous diseases [1].
There are two types of EBV infections including either lytic or latent. The former include replication of virus genome and protein transcription while in latent infection there is only limited genes expression which include two genes EBVencoded RNAs (EBER-1 and -2), 6 EBV nuclear antigens(EBNA-1,-2,-3A,-3B,-3C and LP), and 3 latent membrane proteins(LMP-1,-2A,-2B) [2].
EBV-encoded RNA 1 (EBER1) and EBER2 are translated RNAs are the most numerous viral transcripts in latent cells infected with EBV, which play a great role in the effective growth transformation of primary EBV-induced B cells.
The LMPs i.e. (LMP1, LMP2A and LMP2B) play a role in the immortalization operation and oncoprotein.
EBNA1 can be detected in all EBV-associated malignancies while EBNA1 is very stable and contains a glycine-alanine repeat sequence near its N-terminus that inhibits translation and subsequent self-replication.
The prostate is a small organ, presents in the male only and situated below the bladder and surrounds the urethra, its main function is to provide the semen with fluid.
There are many risks factors for prostate cancer (PCa) (similar to those of benign prostatic hyperplasia (BPH) include old age, family history, racial factor, diet, hormones and occupational hazard [3]. The cancer aroused at peripheral zone of gland. Unlike BPH the obstruction of urine flow is less common in cancer and only can be seen in late stage of disease.
Materials and Methods
A retrospective study was conducted on the following main groups during the period from May 2016 to December 2016.
The age of the patients ranged between 40-80 years, and the samples were collected from archives of laboratory in Ghazi Al-Hariri hospital for surgical specialties and from private histopathology laboratories in Baghdad.
Thirty 30 formalin-fixed Paraffin embedded tissue blocks were obtained from prostate carcinoma biopsies Transurethral Resection of Prostate(TURP) and 30 formalin-fixed, paraffin embedded blocks were obtained from benign prostatic hyperplasia(BPH). Twenty (20) prostate autopsies from forensic medicine institute in Baghdad after take consent from relative of patients (control group).
Primarily, the diagnosis of these tissue blocks were based on the obtained histopathological laboratory records of prostate that had accompanied each tissue block in each hospital laboratory and review the diagnosis of slides were done by consultant pathologist.
In situ hybridization detection kit from abcam lot-N63-922091071
In situ hybridization EBV probe: The Probe (Biotin-labeled) was produced by Zytofast/Germany/Cat Numbers (T-1014-40).The probe contains biotin-labeled oligonucleotides which target EBV EBER RNA.
Immunohistochemistry kits
• Anti-EBV Latent Membrane Protein-1 antibody-[D24-G] ab136633 Abcam/United kingdom (UK).
• LMP-1 primary antibody: Monoclonal mouse antibody for EBV latent membrane 1 and the clone number: (CS1, CS2, CS3, CS4).
• Anti-EBV Nuclear Antigen antibody [E1-2.5] AB8329 Abcam/United kingdom (UK).
• Monoclonal antibody for EBNA2: (ab8329) clone number (PE2).
In situ hybridization for detection of EBV by EBERs
Principle of the test: The presence of certain nucleic acid sequences in cells or tissue can be detected with in situ hybridization using labeled RNA Probes. The hybridization results in duplex formation of sequences present in the test object and the specific gene probe. It is indirectly detected using an enzyme-conjugated antibody targeting the tags: the enzymatic reaction of chromogenic substrates leads to the formation of a color precipitate that is visualized by light microscopy at 10-20X (strong blue–violet signals). Slides preparation: Serial thin sectioning of (4 μm) thickness was done for each paraffin-embedded tissue block and sticking the sections on charged slides.
CISH signals were determined for at least 10 high power fields. Nuclear fast red staining was considered a positive result for EBERs. Positive CISH signal patterns were classified as follows: (1) diffuse (D), when nuclei were fully stained; (2) punctate, when distinct dot-like intranuclear signals were noted; (3) mixed, diffuses, and punctate (D/P) if both patterns are noted [4].
Immunohistochemistry
Detection of latent membrane protein-1 and EBV nuclear antigen 2(EBNA2): This detection system detects a specific antibody bound to an antigen in tissue sections. The specific antibody is located by a biotin-conjugated secondary antibody. After that, add streptavidin-enzyme conjugate that binds to the biotin present on the secondary antibody. The complex (specific antibody, secondary antibody, and streptavidinenzyme) can visualize with an appropriate substrate/ chromogen.
The results of the study under application of the statistical package (SPSS) ver. (14.0).
Results and Discussion
Results of In-Situ Hybridization(ISH) for EBVencoded RNAs (EBERs) in studied groups
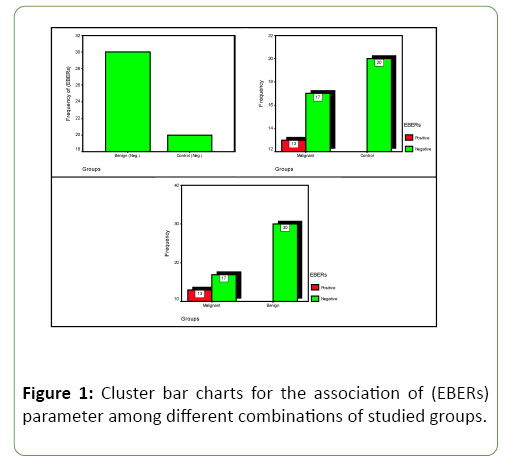
The results of ISH for detection EBV-EBERs in study group tissues were shown in Table 1 and Figure 1. The percentage of EBV results in the total group of Benign and control groups have no responding, while malignant group have high responding 13 out of 30 were positive (43.3%). Comparison significant (C.S.) between control groups and malignant group have highly significant value (HS) (p=0.001), and (HS) (p=0.000) association between malignant and benign groups, therefore studied EBERs parameter seems to be good indicator for characterized differences between study groups.
Table 1: Distribution of the studied (EBERs) parameter responding according to different combination's groups with comparisons significant.
| Groups | No. and Percent | EBERs | Total | C.S. (*) P-value | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Benign | No. | 0 | 30 | 30 | No Association (Same Responding) |
| % EBERs | 0.0% | 100% | 100.0% | ||
| Control | No. | 0 | 20 | 20 | |
| % EBERs | 0.0% | 100% | 100% | ||
| Total | No. | 0 | 50 | 50 | |
| % EBERs | 0.0% | 100% | 100% | ||
| Malignant | No. | 13 | 17 | 30 | C.C.=0.436 P=0.001 (HS) Cohort (Neg.)=0.567 (0.414-0.775) |
| % EBERs | 43.3% | 56.7% | 100% | ||
| Control | No. | 0 | 20 | 20 | |
| % EBERs | 0.0% | 100% | 100% | ||
| Total | No. | 13 | 37 | 50 | |
| % EBERs | 26% | 74% | 100% | ||
| Malignant | No. | 13 | 17 | 30 | C.C.=0.465 P=0.000 (HS) Cohort (Neg.)=0.567 (0.414-0.775) |
| % EBERs | 43.3% | 56.7% | 100% | ||
| Benign | No. | 0 | 30 | 30 | |
| % EBERs | 0.0% | 100% | 100% | ||
| Total | No. | 13 | 47 | 60 | |
| % EBERs | 21.7% | 78.3% | 100% | ||
(*) HS: Highly Sig. at P<0.01; [C.C.: Testing based on a Contingency Coefficient test].
Results obtained are nearly compatible to Saul Grinstein et al. study which showed strong EBV reactions in 36.8% of neoplastic nuclei from Seven out of 19PCa when exam by (ISH) method.
On other hand Mohammed Ali and Mohammed study [5] found that 19 of 40 (47.5%) PCa cases positive to EBERs when detection by ISH, while only 2 of 20 (10%) from BPH and not found in control group, that's mean this result agree with current study and have the same highly significant P value between malignant group and both BPH and control groups.
The percentage of EBV-RNA in PCa was found to be higher when comparison to the benign and control group reflects a possible role of the EBV-infection in the carcinogenesis of PCa.
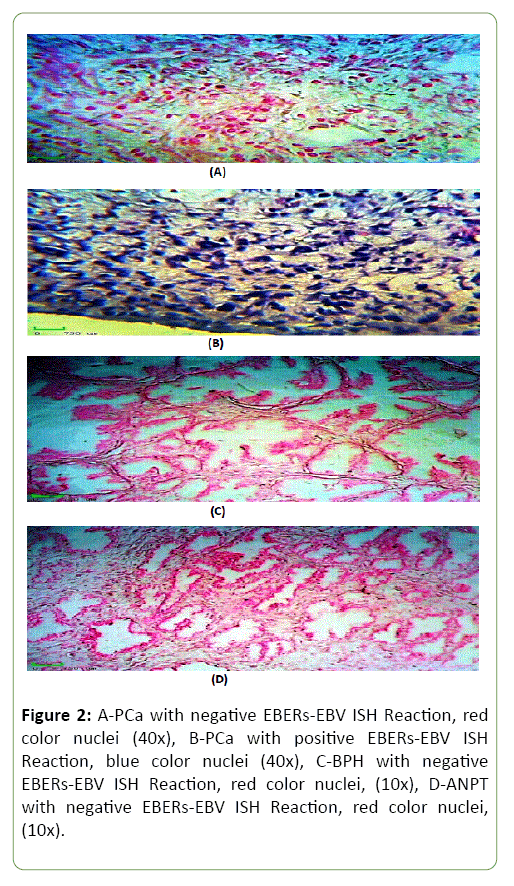
Figure 2 shows the results of ISH for detection EBV-EBERs in PCa, BPH and ANPT tissue using biotinylated-labeled EBERs- EBV probe, stained with NPT/BCIP (blue) and counter stained by Nuclear Fast Red (Red).
Latent membrane protein-1(LMP-1) parameter with different combination's groups
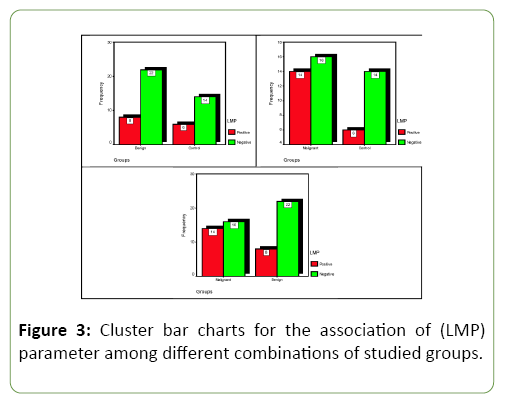
As shown in Table 2 and Figure 3, the results of Immune Histochemistry (IHC) for detection EBV-LMP-1 in study group tissues. The LMP-1was detected in 8 out of 30 results were positive (26.7%) in the Benign group and 6 out of 20 specimens were positive (30%) from control group, while malignant group have high responding 14 out of 30 were positive (46.7%). Comparison significant (C.S.) between study groups no significant results were shown.
Table 2: Distribution of the studied (LMP) parameter responding according to different combination's groups with comparisons significant.
| Groups | No. and Percent | LMP | Total | C.S. (*) P-value | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Benign | No. | 8 | 22 | 30 | C.C.=0.036 P=0.797 (NS) OR=0.848 (0.242-2.970) |
| % LMP | 26.7% | 73.3% | 100% | ||
| Control | No. | 6 | 14 | 20 | |
| % LMP | 30% | 70% | 100% | ||
| Total | No. | 14 | 36 | 50 | |
| % LMP | 28% | 72% | 100% | ||
| Malignant | No. | 14 | 16 | 30 | C.C.=0.164 P=0.239 (NS) OR=2.042 (0.618-6.748) |
| % LMP | 46.7% | 53.3% | 100% | ||
| Control | No. | 6 | 14 | 20 | |
| % LMP | 28% | 72% | 100% | ||
| Total | No. | 20 | 30 | 50 | |
| % LMP | 40% | 60% | 100% | ||
| Malignant | No. | 14 | 16 | 30 | C.C.=0.203 P=0.108 (NS) OR=2.406 (0.816-7.095) |
| % LMP | 46.7% | 53.3% | 100% | ||
| Benign | No. | 8 | 22 | 30 | |
| % LMP | 26.7% | 73.3% | 100% | ||
| Total | No. | 22 | 38 | 60 | |
| % LMP | 36.7% | 63.3% | 100% | ||
(*) NS: Non Sig. at P>0.05; OR: Odds Ratio; [C.C.: Testing based on a Contingency Coefficient test].
But odds ratio shows that twice and half time a positive outcomes concerning Malignant group are recorded in more cases toward Benign group, and therefore studied LMP seems to be somewhat good indicator for characterized differences among (Control, and Benign) in contrast of Malignant group.
No other study was found which mentioned the association between LMP-1 and prostate pathology.
However, both of prostate and breast tissue are glandular and productive tissue [6] (thus we can compare between these tissues, so Tseng et al., study which used PCR and (IHC) staining for LMP-1 protein detection was found 9 out of 20 Breast cancer (BC) (45%) were positive, this is the first study suggesting a role for EBV in the pathogenesis of fibro adenomas in the immunosuppressed patients specific localized to epithelial cells [7].
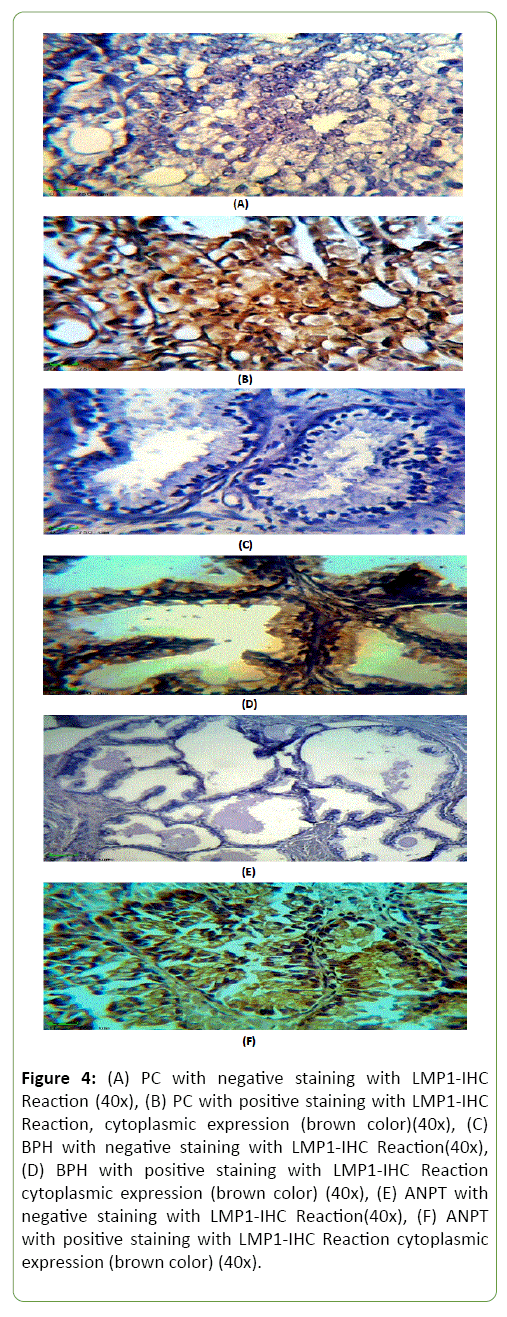
PCa, BPH and ANPT showing the results of IHC staining protein using Biotinylated Anti-LMP1 protein antibody; stained by DAB-Chromogen(Brown) and counter stained by Mayer’s Hematoxylin(Blue) (Figure 4).
Figure 4: (A) PC with negative staining with LMP1-IHC Reaction (40x), (B) PC with positive staining with LMP1-IHC Reaction, cytoplasmic expression (brown color)(40x), (C) BPH with negative staining with LMP1-IHC Reaction(40x), (D) BPH with positive staining with LMP1-IHC Reaction cytoplasmic expression (brown color) (40x), (E) ANPT with negative staining with LMP1-IHC Reaction(40x), (F) ANPT with positive staining with LMP1-IHC Reaction cytoplasmic expression (brown color) (40x).
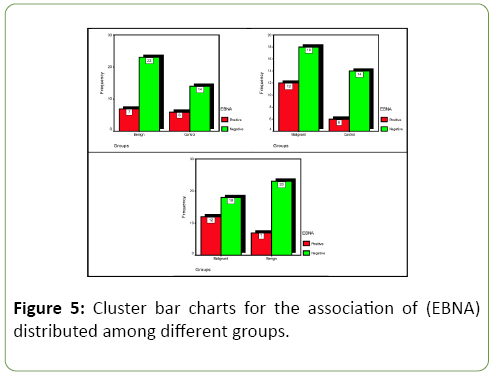
EB nuclear antigen-2(EBNA-2) parameter with different combination's groups
Table 3 and Figure 5 show association of "EBNA-2" parameter responding with different of combination's groups regarding the seven out of 30(23.3%)from benign group, 6 out of 20(30%) from control group, and 12 out of 30(40%)from malignant group that showed positive IHC reactions for EBNA-2 marker. Statistically, no significant differences (p>0.05) were found between three studied groups.
Table 3: Distribution of the studied (EBNA) parameter responding according to different combination's groups with comparisons significant.
| Groups | No. and Percent | EBNA | Total | C.S. (*) P-value | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Benign | No. | 7 | 23 | 30 | C.C.=0.074 P=0.599 (NS) OR=0.710 (0.198-2.546) |
| % EBNA | 23.3% | 76.7% | 100% | ||
| Control | No. | 6 | 14 | 20 | |
| % EBNA | 30% | 70% | 100% | ||
| Total | No. | 13 | 37 | 50 | |
| % EBNA | 26% | 74% | 100% | ||
| Malignant | No. | 12 | 18 | 30 | C.C.=0.102 P=0.470 (NS) OR=1.556 (0.467-5.182) |
| % EBNA | 40% | 60% | 100% | ||
| Control | No. | 6 | 14 | 20 | |
| % EBNA | 30% | 70% | 100% | ||
| Total | No. | 18 | 32 | 50 | |
| % EBNA | 36% | 64% | 100% | ||
| Malignant | No. | 12 | 18 | 30 | C.C.=0.176 P=0.165 (NS) OR=2.190 (0.716-6.698) |
| % EBNA | 40% | 60% | 100% | ||
| Benign | No. | 7 | 23 | 30 | |
| % EBNA | 23.3% | 76.7% | 50.0% | ||
| Total | No. | 19 | 41 | 60 | |
| % EBNA | 31.7% | 68.3% | 100% | ||
(*) NS: Non Sig. at P>0.05; OR: Odds Ratio; [C.C.: Testing based on a Contingency Coefficient test].
On reference to subject (Benign, and Malignant) combination, EBNA-2, and rather than no significant relationship at P>0.05 are recorded, but according to calculated P-value=0.165), it's more informative for that result to be reported, rather than simply stating that statistical significant was not achieved [8].
In addition to that, an odds ratio shows that more than twice time a positive outcomes concerning Malignant group are recorded in more cases toward Benign group, and therefore studied EBNA-2 seems to be somewhat good indicator for characterized differences among (Control, and Benign) in contrast of Malignant group. Unfortunately, no any other study was found in light of studied subject, but this study bind between both HPV type 18 and EBV gene (EBNA1) were obtained high and equal proportion of normal, benign, and PCa specimens [9] that’s mean not compatible with this study. But the frequency of co-infection of EBV and HPV was significantly higher in PCa (55%) than in benign (15%) and normal prostate (30%) specimens which are compatible with these results.
In addition, evidence of experiments shows that HPV and EBV work together to enhance the proliferation of cultured cervical cells [10], suggesting that may be have the same role in prostate epithelial cells [11]. Indian study was found IHC for EBNA-1 was positive in 28out of 51 malignant breast cases (54.9%) and benign breast patients also have a higher immunological response against EBNA-1, while all control group (30) was negative [12]. Indian study was compatible with this study in malignant group only which have high percentage of EBNA-1 positive reaction.
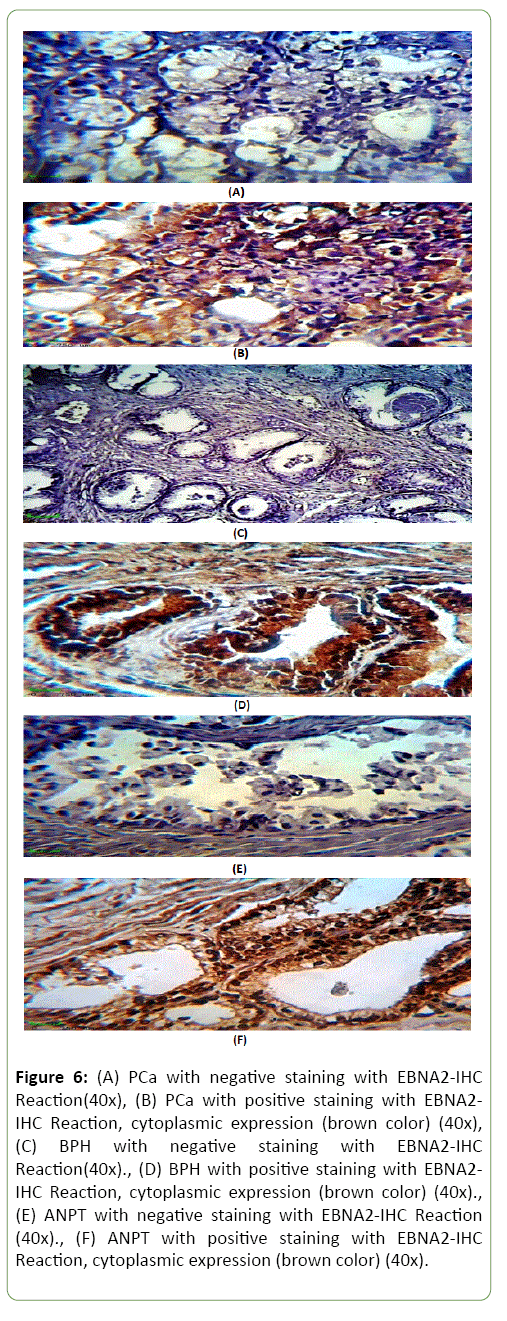
PCa, BPH and ANPT showing the results of IHC staining protein over expression using Biotinylated Anti-EBNA2 protein antibody; stained by DAB-Chromogen(Brown)and counter stained by Mayer's Hematoxylin(Blue) (Figure 6).
Figure 6: (A) PCa with negative staining with EBNA2-IHC Reaction(40x), (B) PCa with positive staining with EBNA2-IHC Reaction, cytoplasmic expression (brown color) (40x),(C) BPH with negative staining with EBNA2-IHC Reaction(40x)., (D) BPH with positive staining with EBNA2-IHC Reaction, cytoplasmic expression (brown color) (40x)., (E) ANPT with negative staining with EBNA2-IHC Reaction (40x)., (F) ANPT with positive staining with EBNA2-IHC Reaction, cytoplasmic expression (brown color) (40x).
Correlation between EBERs and other markers in malignant group
Table 4 shows significant relationship are accounted at P=0.030 between EBERs and LMP in malignant group, no other study to PCa compatible with this study but Plaza G study was detected EBERs by ISH in 96.67% of the Nasopharyngeal cancer (NPC) cases, and detected expression of LMP-I in 43.33% of the NPC cases [13]. These results compatible with current study.
Table 4: Distribution of the studied (EBERs) parameter responding with different leftover parameters in malignant group.
| Parameter | Response | No. and Percent | EBERs | Total | C.S. (*) P-value | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| LMP | Positive | No. | 9 | 5 | 14 | C.C.=0.368 P=0.030 (S) OR=5.400 (1.120-26.044) |
| % EBERs | 30% | 16.6% | 46.7% | |||
| Negative | No. | 4 | 12 | 16 | ||
| % EBERs | 13.3% | 40.0% | 53.3% | |||
| EBNA | Positive | No. | 8 | 4 | 12 | C.C.=0.359 P=0.035 (S) OR=5.200 (1.068-25.309) |
| % EBERs | 26.6% | 13.4% | 40.0% | |||
| Negative | No. | 5 | 13 | 18 | ||
| % EBERs | 16.6% | 43.3% | 60.0% | |||
(*) S: Sig. at P<0.05; NS: Non Sig. at P>0.05; [C.C.: Testing based on a Contingency Coefficient test], and OR: Odds Ratio.
On other hand, Jiang Li study was determined EBERs in 56(100%) by ISH in NPC, and LMP-1 in 24(43%) out of 56 [14]. While Jian-Yong Shao et al. study was reported: In the nuclei of the tumor cells from 87 NPC biopsies (100%) when used ISH method was detected EBERs while LMP1 in 55 from the same biopsies (63%) which appear as a dot-like cell membrane and or cytoplasmic staining signals [15]. While accounted P=0.035 between EBERs and EBNA.No any previous study mentioned this relation with current study but Mohamad Nidal Khabaz study which compatible with this study, from 92 breast carcinoma(BC) biopsies was detected 24(26%) which infected with EBV while only 3 from 49 control group biopsies with represented a statistically significant difference (p-value using χ2=0.008). These results found an association between EBV infection and BC development [16].
While other study reported highly percentage (45%) when detected both EBNA1 by PCR and EBER by ISH in (BC) patients, EBV was detected in 18/40 (45%) and 14/50 (28%) of Egyptian and Iraqi women; respectively where p=0.073, compared to 0/20 (0%) of their control groups (p<0.05) [17].The high percentage of this result may be because use PCR technique to detect EBNA marker, which is very sensitive.
These studies suggest EBV might act as a promoter for the development of BC and it might contribute to increased tumor aggressiveness in epithelial cell carcinoma.
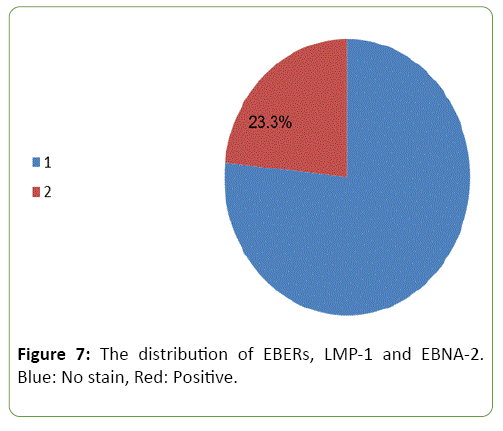
Correlation between EBERs, LMP-1 and EBNA-2 in malignant group
Figure 7 shows the distribution of EBERs, LMP-1 and EBNA-2, seven (23.3%) out of 30 samples were found positive of these markers (red area). Unfortunately, no study was found related to this subject.
Conclusions
This study was found that:
• EBERs detected only in Prostate carcinoma groups by successful method 'In situ hybridization technique'.
• LMP-1 and EBNA-2, can detect by Immunohistochemistry method in Prostate carcinoma with highly percentage than benign prostate hyperplasia and control group.
• There is a correlation between EBERs, LMP-1 and EBNA-2 in malignant group.
Acknowledgement
The authors are grateful to laboratory staff in Ghazi Al-Hariri hospital for surgical specialties, private histopathology laboratories in Baghdad and center of blood diseases.
References
- Mandage R, Telford M, Rodríguez JA, Farré X, Layouni H, et al. (2017) Genetic factors affecting EBV copy number in lymphoblastoid cell lines derived from the 1000 Genome Project samples. PLoS One 12: 1-19.
- Young LS, Rickinson AB (2004) Epstein - Barr virus: 40 Years On. Nature Reviews 4: 757-768.
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348: 1625-1638.
- Zappacosta R, Colasante A, Viola P, Antuono TD, Lattanzio G, et al. (2013) Chromogenic In Situ Hybridization and p16/Ki67 Dual Staining on Formalin-Fixed Paraffin-Embedded Cervical Specimens: Correlation with HPV-DNA Test, E6/E7 Mrna Tes and Potential Clinical Applications. BioMed Research International.
- Ali SHM, Al-Alwany SHM (2013) Molecular Localization of Epstein Barr Virus and Rb Tumor Suppressor Gene Expression in Tissues from Prostatic Adenocarcinoma and Benign Prostatic Hyperplasia. Journal of Natural science Research 3: 70-76.
- Komisaruk BR, Wise N, Frangos E, Liu WC, Allen K, et al. (2011) Women's Clitoris, Vagina, and Cervix Mapped on the Sensory Cortex: fMRI Evidence, Surprise finding in response to nipple stimulation. J Sex Med 8: 2822-2830.
- Kleer CG, Tseng MD, Gutsch DE, Rochford RA, Wu Z, et al. (2002) Detection of Epstein-Barr virus in rapidly growing fibro adenomas of the breast in immunosuppressed hosts. Mod Pathos 15: 759-764.
- Robert JN (2006) Epidemiology and Biostatistics Secrets. Mosby Elsevier, Philadelphia, USA.
- Shi Y, Peng SL, Yang LF, Chen X, Tao YG, et al. (2016) Co-infection of Epstein-Barr virus and human papillomavirus in human tumorigenesis. Chin J Cancer 35: 1186-1191.
- Witaker NJ, Gleen WK, Sahrudin A, Orde MM, Delprado W, et al.(2013): Human papillomavirus and Epstein Barr virus in prostate cancer: Koilocytes indicate potential oncogenic influences of human papillomavirus in prostate cancer. Prostate 73(3): 236-241.
- Ammatuna P, Giovannelli L, Giambelluca D, Mancuso S, Rubino E, et al. (2000) Presence of human papillomavirus and Epstein-Barr virus in the cervix of women infected with the human immunodeficiency virus. J Med Virol 62: 410-415.
- Joshi D, Quadri M, Gangane N, Joshi R, Gangane N (2009) Association of Epstein Barr Virus Infection (EBV) with Breast Cancer in Rural Indian Women. POLS 4: 342-348.
- Plaza G, Manzanal AI, Fogué L, Santón A, Carlos J, et al. ( 2002) Association of Epstein-Barr virus and nasopharyngeal carcinoma in Caucasian patients. Ann Otol Rhinol Laryngol 111: 210-216.
- Li J, Zhang XS, Xie D, Deng HX, Gao YF, et al. (2007) Expression of Immune-Related Molecules in Primary EBV Positive Chinese Nasopharyngeal Carcinoma Associated with Latent Membrane Protein 1 (LMP1) Expression. Cancer Biol Ther 6: 1997-2004.
- Shao JY, Ernberg I, Biberfeld P, Heiden T, Zeng YX, et al. (2004) Epstein - Barr virus LMP1 Status in Relation to Apoptosis, P53 Expression and Leucocyte Infiltration in Nasopharyngeal Carcinoma. Anticancer Res 24: 2309-2318.
- Khabaz MN (2013) Association of Epstein-Barr virus infection and breast carcinoma. Arch Med Sci 9: 745-751.
- Zekri ARN, Bahnassay AA, Mohamed WS, El-Kassem FA, El-khalidi SJ (2012) Epstein-Barr virus and breast cancer: Epidemiological and Molecular study on Egyptian and Iraqi women. J Egypt Natl Cancer Inst 24: 123-131.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences