ISSN : ISSN: 2576-1455
Journal of Heart and Cardiovascular Research
Adenosine Attenuates cAMP-Dependent Chloride Current In Isolated Guinea-Pig Ventricular Mycocytes
>Jiang Xu, Qi-Hua Gong, Steven J. Wieland and Amir Pelleg*
Drexel University, College of Medicine, Philadelphia, United States
- *Corresponding Author:
- Amir Pelleg
Drexel University
College of Medicine, 245 N 15th Street
NCB, MS#470, Philadelphia
PA 19102-1192, United States
Tel: 215-762-1627
Fax: 215-762-7222
E-Mail: apelleg@drexelmed.edu
Received Date: January 30, 2017; Accepted Date: February 22, 2017; Published Date: March 05, 2017
Citation: Xu J, Gong QH, Wieland SJ, et al. Adenosine Attenuates cAMP-Dependent Chloride Current In Isolated Guinea-Pig Ventricular Mycocytes. J Heart Cardiovasc Res. 2017, 1:1.
Abstract
The hypothesis that adenosine can attenuate cAMP-dependent chloride current (ICl) was tested in isolated single guinea-pig ventricular myocytes. Using the patch clamp technique, whole cell ICl was quantified during 220 msec voltage steps in the range of -100 mV to +50 mV from a holding potential of -30 mV under conditions of equal intra- and extra-cellular chloride concentrations and minimized Na+, Ca2+ and K+ currents. The addition of either isoproterenol (ISO) or forskolin (both 1 μM) enhanced transmembrane current from 120 ± 15 pA to 353 ± 50 pA, and from 110 ± 15 pA to 606 ± 133 pA, respectively (each, p<0.05). The reversal potential of this current shifted to ~ + 30 mV upon substitution of NaCl with Na-glutamate indicating that the current was ICl. Adenosine (Ado, 100μM) attenuated the ISOand forskolin-enhanced currents to 198 ± 55pA and 377 ± 74 pA, respectively (each, p<0.05). The A1AdoR agonist N6-cyclopentyladenosine (CPA, 5 μM) exerted similar action, and the A1AdoR antagonist 8-cyclopentyltheophylline (CPT, 5 μM) partially reversed it. Pre-treatment with pertussis toxin (PTX; 1 μg/ml for 3 hrs.) also abolished the effect of Ado. It was concluded that (i) A1AdoR and PTX-sensitive G protein(s) mediated the effect of Ado on cAMP-dependent ICl, (ii) the activation of adenylyl cyclase with forskolin modulates the action of CPT on A1AdoR-mediated agonists actions, and (iii) This additional manifestation of the anti-adrenergic action of Ado in the mammalian ventricular myocardium, could play a role in its cardio-protective effect under pathophysiologic conditions.
Keywords
A1 adenosine receptor; Pertussis toxin; G proteins; Catecholamines
Introduction
A cyclic adenosine 5’-monophosphate (cAMP)-activated Cl− current in rabbit and guinea pig heart was discovered in 1989 [1,2]. Since then, multiple chloride channels have been identified in cardiac cells [3]. These include CFTR, ClC-2, ClC-3, CLCA, Bestrophin and Ano1 (12). The β-adrenergic-agonists activated channel, which is regulated by cAMP-dependent protein kinase A (PKA) phosphorylation conducts Cl- dependent outward rectifying current (ICl) [4]. The cystic fibrosis transmembrane conductance regulator (CFTR), which is a member of the adenosine triphosphate-binding cassette (ABC) transporter superfamily, may be responsible for some of the Cl− current activated by PKA [3,5]. Catecholamines, which stimulate adenylyl cyclase and thereby increase the intracellular level of cAMP, are important regulators of cardiac function; thus, it has been hypothesized that ICl plays an important role in cardiac electrophysiology, in particular during increased sympathetic input to the heart [6,7].
That extracellular adenosine (Ado) can regulate ionic currents in the mammalian heart is well established [8,9]. The effects of Ado on two ionic currents, i.e., the L type Ca2+ current (ICa) and hyperpolarization activated inward pacemaker current (If) are mediated, in part, by the anti-adrenergic action of the nucleoside [9]. Specifically, the enhancement of ICa and If by catecholamines is attenuated by Ado acting on A1Ado receptor (A1AdoR), and is mediated by a pertussis toxin (PTX)-sensitive GTP binding protein Gi and inhibitory effect on adenylyl cyclase [9]. More than two decades ago, a preliminary report (abstract) have shown for the first time that Ado can also inhibit isoproterenol-induced ICl in mammalian ventricular myocytes [10]. In addition, the antiadrenergic action of Ado can be the manifestation of inhibition of norepinephrine release from sympathetic nerve endings in the heart [11].
Thus, the present study was aimed at testing the hypothesis that adenosine can attenuate catecholamines/cAMP-dependent ICl in ventricular myocytes by activating A1AdoR and a PTX-sensitive G protein(s). The present data support this hypothesis and suggest that the attenuation of ICl by Ado is another important manifestation of the antiadrenergic action of the nucleoside on the guinea pig ventricular myocardium.
Material and Methods
The present studies have been approved by the institutional ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Materials
The modified Krebs-Henseleit (K-H) solution contained (mM): 127 NaCl, 4.6 KCl, 2 CaCI2, 1.1 MgSO4, 2 sodium pyruvate, 10 glucose, 10 creatine, 20 taurine, 5 ribose, 0.01 adenine, 0.1 allopurinol, and 5 HEPES. The composition of the Ca2+- free solution was the same as that of the K-H solution except for the deletion of CaCl2 as extracellular solution. pH was adjusted to 7.4 with NaOH and the osmolarity was 310 mOsmol. The intracellular solution consisted of (mM): 120 CsCl, 20 NaCl, 2 EGTA, 1 Mg-ATP and 10 HEPES. pH was adjusted to 7.2 with CsOH and the osmolarity was 305 mOsmol. The following chemicals were used: Adenosine, N6- cyclopentyladenosine (CPA), a selective adenosine A₠receptor agonist, 8-cyclopentyltheophylline (CPT), a potent and selective antagonist for the adenosine receptors, isoproterenol (ISO), forskolin (stock solution 23.3 mM in DMSO), Na-glutamate, a replacement of NaCl, nifedipine, to block Ca2+ currents, BaCl2, to block inward rectifying K+ current, TTX, to minimize rapid Na+ activation, bovine serum albumin, dispase (Boehringer Mannheim, Indianapolis, IN), collagenase (Worthington Biochemical Co., Freehold, N.J.) , trypsin, and PTX (Calbiochem Corp. San Diego, CA). Unless otherwise specified, all compounds were from Sigma-Aldrich, Inc. (St Louis, MO).
Myocyte isolation
Single guinea-pig cardiac myocytes were isolated using an established method [12,13]. Briefly, hearts were isolated from anesthetized (sodium pentobarbital, 30 mg/kg, i.v.) guinea-pigs pretreated with heparin (200 U/kg), and perfused retrogradely via the aorta with a warm (35°C) oxygenated buffer solution for 10 min followed by perfusion with Ca2+- free buffer solution for 20 min during the last 15 min of that period the following substances were added to the perfusate: collagenase (0.3 mg/ ml), dispase (0.04 mg/ml), trypsin (0.04 mg/ml) and albumin (2 mg/ml). Single cells were obtained by dispersion of small tissue specimens excised from the hearts.
Whole-cell recording
The whole cell voltage patch clamp technique was used to record Cl- currents [14,15] from guinea pig cardiac myocytes. An Axopatch 200A amplifier (Axon Instruments) and a PC running pClamp 8 were used to apply pulse protocols and record data. The holding potential was -30mV or -40 mV with identical results to inactivate Na+ channels and was consecutively stepped from –100 mV up to +50 mV in 10 mV increments for 220 ms. Patch micropipettes were fabricated from borosilicate glass (Kimex 51, KimbleGlass, Inc.) on a vertical pipet puller (Narishige, Amityville, NY) and fire-polished to a final bath resistance of 2-4 MΩ. The interior of the pipette is filled with an intracellular solution for whole-cell recording. A chlorided silver wire was placed in contact with this solution to connect to the amplifier. The micropipette was pressed against a cell membrane and suction is applied to form a high resistance seal between the glass and the cell membrane. After formation of a gigaseal, the cell membrane patch was ruptured to provide an access to the intracellular space of the cell. Stimuli were used via a multi-barrel pipet connected to a Pico-spritzer unit. Cells were continuously superfused with K-H solution containing different chemical compounds as mentioned above after mounting in a recording chamber. The recording chamber volume was 0.2 ml and it was superfused at a rate of 1.0 ml/min. Cl- current was induced by either isoproterenol (ISO; 0.1μM) or forskolin (1 μM). Ado (100 μM) and 5 μM CPA and 5μM CPT were used as agonists and antagonist at A1AdoR, respectively. Experiments were conducted at room temperature (23°C).
PTX treatment
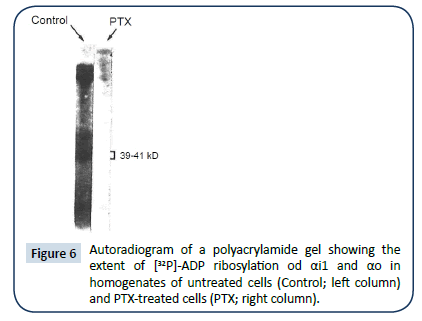
To determine the role of PTX sensitive G protein(s) in the actions of Ado, isolated ventricular myocytes were treated with PTX (1 μg/ml) for three hours prior to experimentation. The efficacy of PTX in inactivating Gi/Go was determined using [32P]NAD+ as a substrate for the PTX-catalyzed [32P]ADP-ribosylation of the α-subunit of Gi and Go as described below.
PTX-induced G-protein inactivation
Control and PTX-treated cell membranes (180 μg protein) were incubated for 30 min at 30°C in a solution containing ATP (1 mM), GTP (0.1 mM), MgCl2 (5 mM), EDTA (1 mM), DTT (1 mM), thymidine (10 mM), NAD+ (5 μM) including [32P]NAD+ to yield specific activity of 3x104 cpm/pmol, PTX (0.2 μg), HEPES (pH=8, 10 mM), BSA (5 μg) and SDS (0.025%). The assay mixture volume was 100 μl. Reactions were stopped by the addition of 2% SDS in 10 mM Tris, pH=7.5, EDTA (1 mM), NaCl (0.9%) (TEN), followed by boiling for two minutes and subsequent addition of an equal volume of TEN containing NP40 (5%). Samples were incubated overnight at 4°C with or without the anti-Giα/Goα antibody 10C1 (2 μg/sample) a monoclonal antibody. Antibody-bound Gα subunits were precipitated using pansorbin and pellets were washed and resuspended by boiling in SDS-PAGE sample buffer. Samples were separated by SDS-PAGE on 12% minigels, which were dried and exposed to radiographic film. The 10C1 antibody is a derived monoclonal antibody against recombinant Gi2α, which reacts with Gi1-3α and Goα subunits. In all cases, sample loads contained equal amounts of protein as well as equivalent levels of Gα immunoreactivity.
Data acquisition and analyses
Currents were measured during 220 msec voltage steps from either -30 mV or -40 mV holding potentials to -100 mV up to +50 mV using an amplifier with 100 MΩ headstage controlled by software (Axon Instruments) at a sampling rate of 10 kHz. Current vs. voltage plots were obtained using currents measured at each step. Data across all cells are presented as ICl at -100 mV membrane potential. Statistical significance at the level of p<0.05 was determined using ANOVA and Student’s t test corrected for multiple measurements.
Results
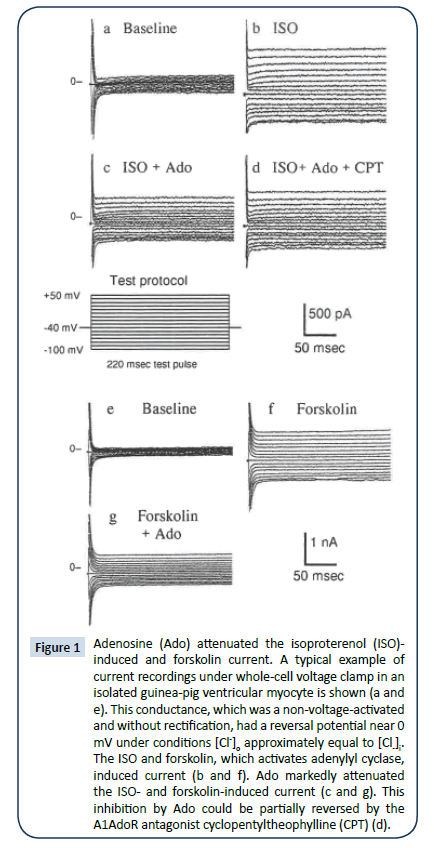
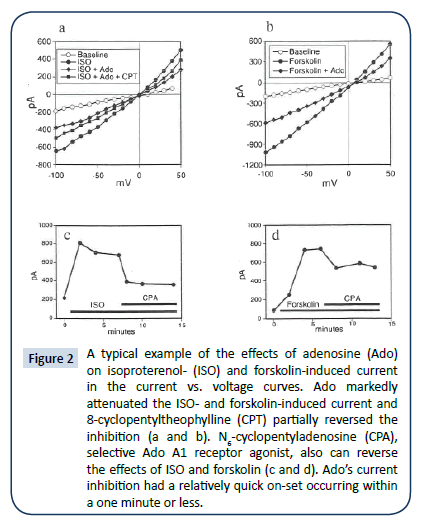
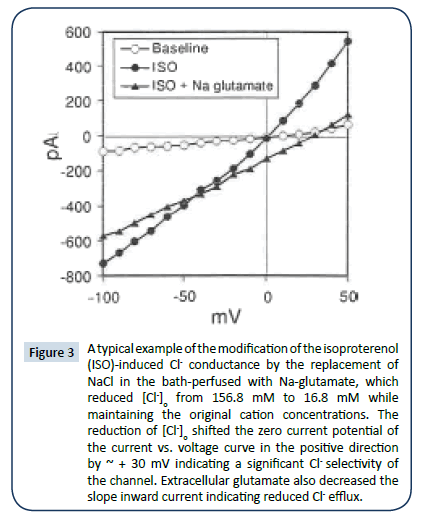
A typical example of current recordings under whole-cell voltage clamp in an isolated guinea-pig ventricular myocyte is shown in (Figure 1a-d). This conductance, which was a non-voltageactivated and without rectification, had a reversal potential near 0 mV when [Cl-]o was approximately equal to [Cl-]i. Both Ado (Figure 1c and 2a) and A1AdoR agonist CPA (Figure 2c) markedly attenuated the ISO-induced current; this inhibition was partially reversed by the A1AdoR antagonist CPT (Figure 1d and 2a). The induction of anion conductance by ISO was mimicked by forskolin, which activates adenylyl cyclase. Ado also inhibited the forskolin-induced current (Figure 1g and 2b). Forskolin carrier alone had no effect (data not shown). CPA, a selective adenosine A₠receptor agonist, also can reverse the effects of ISO and forskolin (Figure 2c and 2d). The effects of Ado on ISO and forskolin are shown in the current vs. voltage curves in Figures 2a and 2b. Ado’s current inhibition had a relatively quick on-set occurring within one minute or less. Further characterization of ISO-induced conductance indicated that it was similar to cAMPactivated- conductance described previously [16]. Replacement of NaCl in the bath perfused with Na-glutamate, reduced [Cl-]o from 156.8 mM to 16.8 mM while maintaining the original cation concentrations. The reduction of [Cl-]i shifted the zero current potential of the I/V curve in the positive direction by ~30 mV indicating a significant Cl- selectivity of the channel (Figure 3). Similar results were obtained in additional four myocytes. Extracellular glutamate also decreased the slope inward current indicating reduced Cl- efflux (Figure 3). Similar reduction of cAMP-activated conductance has been reported when aspartate replaced extracellular Cl- [1,2,17]. Thus, the substitution of [Cl-]o with nonpermeant glutamate or aspartate seems also to produce a partial block of Cl- flux through the chloride channel, which is manifested as reduced inward current.
Figure 1: Adenosine (Ado) attenuated the isoproterenol (ISO)- induced and forskolin current. A typical example of current recordings under whole-cell voltage clamp in an isolated guinea-pig ventricular myocyte is shown (a and e). This conductance, which was a non-voltage-activated and without rectification, had a reversal potential near 0 mV under conditions [Cl-]o approximately equal to [Cl-]i. The ISO and forskolin, which activates adenylyl cyclase, induced current (b and f). Ado markedly attenuated the ISO- and forskolin-induced current (c and g). This inhibition by Ado could be partially reversed by the A1AdoR antagonist cyclopentyltheophylline (CPT) (d).
Figure 2: A typical example of the effects of adenosine (Ado) on isoproterenol- (ISO) and forskolin-induced current in the current vs. voltage curves. Ado markedly attenuated the ISO- and forskolin-induced current and 8-cyclopentyltheophylline (CPT) partially reversed the inhibition (a and b). N6-cyclopentyladenosine (CPA), selective Ado A1 receptor agonist, also can reverse the effects of ISO and forskolin (c and d). Ado’s current inhibition had a relatively quick on-set occurring within a one minute or less.
Figure 3: A typical example of the modification of the isoproterenol (ISO)-induced Cl- conductance by the replacement of NaCl in the bath-perfused with Na-glutamate, which reduced [Cl-]o from 156.8 mM to 16.8 mM while maintaining the original cation concentrations. The reduction of [Cl-]o shifted the zero current potential of the current vs. voltage curve in the positive direction by ~ + 30 mV indicating a significant Cl- selectivity of the channel. Extracellular glutamate also decreased the slope inward current indicating reduced Cl- efflux.
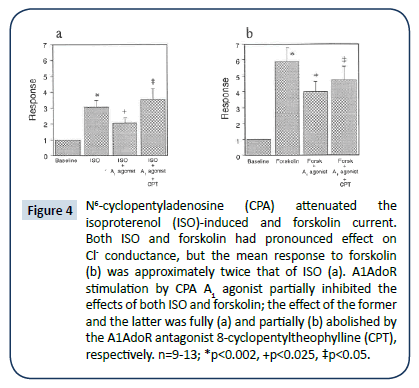
Although Iso-induced and forskolin-induced ICl they were qualitatively similar they differed quantitatively. Both ISO and forskolin had a pronounced effect on Cl- conductance (Figure 1-2), but the mean response to forskolin was approximately twice that of ISO. ISO and forskolin (each, 1 μM) enhanced transmembrane current from 120 ± 15 pA to 353 ± 50 pA (Figure 4a) and from 10 ± 15 pA to 606 ± 133 pA (Figure 4b) (each, p<0.05), respectively. A1AdoR stimulation by either Ado or CPA partially inhibited the effects of both ISO and forskolin. The A1AdoR agonist’s inhibition of the response to ISO was fully abolished by the A1AdoR antagonist CPT, and its inhibition of the response to forskolin was markedly reduced by CPT (Figure 4a and 4b).
Figure 4: N6-cyclopentyladenosine (CPA) attenuated the isoproterenol (ISO)-induced and forskolin current. Both ISO and forskolin had pronounced effect on Cl- conductance, but the mean response to forskolin (b) was approximately twice that of ISO (a). A1AdoR stimulation by CPA A1 agonist partially inhibited the effects of both ISO and forskolin; the effect of the former and the latter was fully (a) and partially (b) abolished by the A1AdoR antagonist 8-cyclopentyltheophylline (CPT), respectively. n=9-13; *p<0.002, +p<0.025, ‡p<0.05.
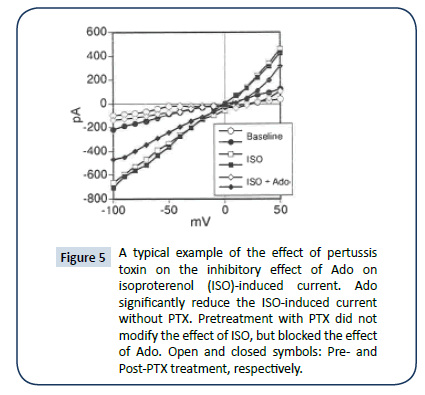
Pretreatment with PTX (1 μg/ml; for 3 hrs) did not modify the effect of ISO, but blocked the effect of Ado (Figure 5, filled symbols=PTX treated). Assays of membranes of PTX-treated myocytes indicated efficient ribosylation of candidate G proteins thereby, removing them from the pool of proteins available for radioactive ribosylation (Figure 6).
Figure 5: A typical example of the effect of pertussis toxin on the inhibitory effect of Ado on isoproterenol (ISO)-induced current. Ado significantly reduce the ISO-induced current without PTX. Pretreatment with PTX did not modify the effect of ISO, but blocked the effect of Ado. Open and closed symbols: Pre- and Post-PTX treatment, respectively.
Discussion
The present study confirm the preliminary report regarding Ado’s inhibition of ISO-induced ICl [10]. In addition, the following new results were obtained in single isolated guinea-pig ventricular myocytes: (i) Ado also attenuated either also forskolin-induced ICl, (ii) CPA, an A1AdoR agonist had a similar effect, (iii) CPT, an A1AdoR antagonist attenuated the effects of both Ado and CPA on ISO-induced ICl and to a lesser extent their effects on forskolininduced ICl, and (iv) the effects on Ado and CPA were mediated by a PTX sensitive G protein(s).
These data demonstrate for the first time that the activation of A1AdoR and an associated PTX sensitive G protein mediate not only the negative dromotropic action of Ado [18], but also its inhibitory effect on catecholamine/cAMP-dependent ICl in the guinea-pig ventricular myocardium. Thus, the anti-adrenergic action of Ado is manifested not only in the attenuation of ISO-induced ICa and If [9] but also ICl. The present results are in congruence with the demonstration of ISO/cAMP dependent ICl in ventricular myocytes [6,7] and confirm Ado’s reversal of ICl activation by histamine and ISO [7,17].
In the mammalian heart, Ad exerts direct and indirect effects; the latter is manifested in the attenuation of β-adrenoceptordependent effects of catecholamines [19]. In the atria, sinus node and AV node, Ado exerts both types of actions, while in the ventricle only the indirect anti- β-adrenergic action, i.e., the attenuation of the biochemical, inotropic and electrophysiologic effects of catecholamines [20,21,22]. An exception is the direct action of Ado on basal ICa in the ferret ventricular myocardium [23]. The effect of Ado on ISO-induced ICl is in agreement with its effects on ISO-induced ICa and If and delayed afterdepolarizations [24,25]. Moreover, adenosine antagonized catecholamineinduced electrophysiologic effects in human His-Purkinje system in vivo [26].
Several mechanisms are involved in the anti- β-adrenergic action of Ado including (i) inhibition of adenylyl cyclase [27-29,30], (ii) modulation of β-adrenoceptor signal transduction [30,31] and (iii) dephosphorylation of intracellular proteins independent of cAMP [32]. The present observation regarding Ado’s equal effectiveness in reducing ISO- and forskolin-induced ICl argues against the involvement of the aforementioned second mechanism. Several studies have indicated that the inhibitory action of Ado on adenylyl cyclase and the anti- β-adrenergic actions of Ado are mediated by A1AdoR and a PTX-sensitive G protein [32-36]. The present data are in agreement with these previous observations and argue against the involvement of A2AdoR in the anti-β- adrenergic actions of Ado. In cardiac myocytes, acetylcholine (Ach) and carbachol decrease ISO- or forskolin-induced ICl via the activation of Gi [16]. In view of the similarity between the actions of Ado and Ach in the mammalian heart [9] and the present observation with PTX, it is highly likely that the inhibition of ICl by Ado was mediated by Gi.
It should be note that A1AdoR mediates multiple actions of Ado including negative chronotropic and dromotropic effects, reduction of atrial contractility, attenuation of the stimulatory actions of catecholamines on beta-adrenergic receptors, reduction of lipolysis in adipose tissue, reduction of urine formation, and inhibition of neurotransmitter release [37]. However, these actions are associated with a variable receptor reserve profiles, which determine the hierarchy of the manifestation of these effects caused by a given concentration of Ado [37]. Specifically, A1AdoR is coupled more efficiently to an inhibition of ISO-induced ICa than to the activation of IK [38].
In the present study, Ado inhibited the action of forskolin on ICl. This is in agreement with the well-established stimulatory effect of forskolin on adenylyl cyclase resulting in increased intracellular levels of cAMP [39]. In addition, these data are in agreement with the previously reported attenuation by Ado of forskolin-induced If [9]. CPT antagonized the depressant effect of Ado on forskolin-induced ICl, however, this antagonism was less pronounced than that observed with ISO-induced ICl. There is no immediate explanation for this difference; it could be speculated that forskolin interferes with the actions of CPT directly or indirectly. Indeed, interaction between forskolin and A2AdoR signal transduction has been reported [40].
In summary, the present data indicate the Ado suppresses catecholamine/cAMP-dependent chloride conductance in guinea-pig ventricular myocytes and that this action is mediated by A1AdoR and a PTX sensitive G protein(s). Multiple chloride channels modulated by various signals including stretch, calcium, and phosphorylation have been characterized in human myocardium [3,41-47]. However, cAMP-dependent chloride conductance has not been demonstrated in human myocardium heretofore. Thus, the clinical significance of the present characterization of another aspect of the anti-adrenergic action of Ado as another aspect If Ado’s cardio-protective mechanism remains to be determined [48,49].
References
- Babinski A, Nairn AC, Greengard P, Gadsby DC (1989) Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes Nature 340: 718-721.
- Harvey RD, Hume JR (1989) Autonomic regulation of chloride current in the heart. Science 244: 983-985.
- Duan D (2009) Phenomics of cardiac chloride channels: the systematic study of chloride channel function in the heart. J Physiol 587: 2163-2177.
- Hume JR, Duan D, Collier ML, Yamazaki J, Horowitz B (2000) Anion transport in heart. Physiol Rev 80: 31-81.
- Hart P, Warth JD, Levesque PC, Collier ML, Geary Y, et al. (1996) Cystic fibrosis gene encodes a cAMP-dependent chloride channel in heart. ProcNatlAcadSci 93: 6343-6348.
- Ackerman MJ, Clapham DE (1993) Cardiac chloride channels. Trends Cardiovasc Med 3: 23-28.
- Hume JR, Harvey RD (1991) Chloride conductance pathways in heart. Am J Physiol 261: C399-412.
- Burnstock G, Pelleg A (2015) Cardiac purinergic signalling in health and disease. Purinergic Signal 11: 1-46.
- Pelleg A, Belardinelli L (1993) Cardiac electrophysiology of adenosine and adenosine 5’-triphosphat: basic and clinical aspects. Cardiovasc Res 27: 54-61.
- Song Y, Thedford S, Lerman BB, Belardinelli L (1992) Adenosine-sensitive afterdepolarizations and triggered activity in guinea-pig ventricular myocytes. Cir Res 70: 743-753.
- Bott-Flugel L, Bernshausen A, Schneider H, Luppa P, Zimmermann K, et al. (2011) Selective attenuation of norepinephrine release and stress-induced heart rate increase by partial adenosine A1 agonism. PLoS ONE 6: e18048.
- Piper HM (1982) Culturing of calcium stable adult cardiac myocytes. J Mol Cell Cardiol 14: 397-412.
- Warth JD, Collier ML, Hart P, Geary Y, Gelband CH, et al. (1996) CFTR chloride channels in human and simian heart. Cardiovasc Res 31: 615-624.
- Wieland SJ, Fletcher JE, Gong QH (1992) Differential modulation of a sodium conductance in skeletal muscle by extracellular fatty acids. Am J Physiol 263: C308-312.
- Xu J, Tong H, Wang L, Hurt CM, Pelleg A (1993) Endogenous adenosine, A1-adenosine receptor and Pertussis toxin sensitive guanine nucleotide binding-protein mediate hypoxia-induced atrio-ventricular nodal conduction block in the guinea-pig heart in vivo. Cardiovasc Res 27: 134-140.
- Hamill OP, Marty A, Neher E, Sakmann B (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell free membrane patches. Pflug Arch 391: 85-100.
- Gadsby DC, Nagal G, Hwang TC (1995) The CFTR chloride channel of mammalian heart. Annu Rev Physiol 57: 387-416.
- Rankin AC, Sitsapesan R, Kane KA (1990) Antagonism of adenosine and ATP of an isoprenaline-induced background current in guinea-pig ventricular myocytes. J Mol Cell Cardiol 22: 1371-1378.
- Harvey RD, Hume JR (1990) Histamine activates the chloride current in cardiac ventricular myocytes. J CradiovascElectrophysiol 1: 309-317.
- Dobson JG, Fenton RA, Romano FD (1987) The antiadrenergic actions of adenosine in the heart. In Gerlach E and Becker BF (eds) “Topics and Perspectives in Adenosine Research”, Springer-Verlag, Berhn/Heidelberg, pp 356-358.
- Endoh M, Yamashita S (1980) Adenosine antagonizes the positive inotropic action mediated via β-, but not α-adrenoceptors in the rabbit papillary muscle. Eur J Pharmacol 65: 445-448.
- Schrader J, Baumann G, Gerlach E (1977) Adenosine as an inhibitor of myocardial effects of catechoalmines. Pflug Arch 372: 29-35.
- Pelleg A (1987) Cardiac electrophysiology and pharmacology of adenosine and ATP: Modulation by the autonomic nervous system. ClinPharmacol 27: 366-372.
- Belardinelli L, Giles WR, West A (1988) Ionic mechanisms of adenosine actions in pacemaker cells from rabbit heart. J Physiol 405: 615-633.
- Qu Y, Campbell DL, Whorton AR, Strauss HC (1993) Modulation of basal L-type ICa by adenosine in isolated ferret right ventricular myocytes. J Physiol 471: 269-293.
- Lerman BB, Wesley RC, DiMarco JP, Haines DE, Belardinelli L (1988) Antiadrenergic effects of adenosine on his-purkinje automaticity evidence for accentuated antagonism. J ClinInvet 82: 2127-2135.
- Hazeki O, Katada T, Kurose H, Ui M (1983) Effects of adenosine on cyclic AMP accumulation in cardiac and other cells and its modification by islet-activating protein. In Daly JW, Kuroda Y, Phillis JN, Shimizu H, Ui M, (eds) “Physiology and Pharmacology of Adenosine Derivatives”, Raven Press, New York, pp.41-49.
- Hosey MM, McMahon KK, Green RD (1984) Inhibitory adenosine receptors in the heart: characterization by ligand binding studies and effects on beta-adrenergic receptor stimulated adenylatecyclase and membrane protein phosphorylation. J Mol Cell Cardiol 16: 931-942.
- LaMonica DA, Frohloff N, Dobson JG (1985) Adenosine inhibition of catecholamine-stimulated cardiac membrane adenylatecyclase. Am J Physiol 248: H737-744.
- Linden J, Hollen CE, Patel L (1985) The mechanism by which adenosine and cholinergic agents reduce contractility in rat myocardium. Correlation with cyclic adenosine monophosphate and receptor densities. Circ Res 56: 728-735.
- Romano FD, Fenton RA, Dobson JG (1988) The adenosine R agonist phenylisopropyl-adenosine, reduces isoproterenol affinity binding to the β-adrenergic receptor of rat myocardial membranes. Second MessengPhosphoprot 12: 29-43.
- Romano FD, MacDonald SG, Dobson JG (1989) Adenosine receptor coupling to adenylatecyclase of rat ventricular myocyte membranes. Am J Physiol 257: H1088-1095.
- Endoh M, Maruyama M, Taira N (1983) Modification by islet-activating protein of direct and indirect inhibitory actions of adenosine on rat atrial contraction in relation to cyclic nucleotide metabolism. J CariovascPharmacol 5: 131-142.
- Gupta RC, Neumann J, Durant P, Watanabe AM (1993) A1-adenosine receptor-mediated inhibition of isoproterenol-stimulated protein phosphorylation in ventricular myocytes. Circ Res 72: 65-74.
- Henrich M, Piper HM, Schrader J (1987) Evidence for adenylatecyclase-coupled A1-adenosine receptors on ventricularcardiomyocytes from adult rat and dog heart. Life Sci 41: 2381-2388.
- Hopwood AM, Harding SE, Harris P (1987) Pertussis toxin reduces the antiadrenergic effect of 2-chloroadenosine on papillary muscle and direct negative inotropic effect of 2-chloroadenosine on atrium. Eur J Pharmacol 141: 423-428.
- Brown LA, Humphrey SM, Harding SE (1990) The anti-adrenergic effect of adenosine and its blockade by pertussis toxin: a comparative study in myocytes isolated from guinea-pig, rat and failing human heart. Br J Pharmacol 101: 484-488.
- Dhalla AK, Shryock JC, Shreeniwas R, Belardinelli L (2003) Pharmacology and therapeutic applications of A1 adenosine receptor ligands. Curr Top Med Chem 3: 369-385.
- Tytgat J (1994) How to isolate cardiac myocytes. Cardiovasc Res 28: 280-283.
- Seamon KB, Padgett W, Daly JW (1981) Forskolin: unique diterpene activator of adenylatecyclase in membranes and intact cells. Proc Nat AcadSci 78: 3363-3367.
- Correia-de-Sa P, Ribeiro JA (1993) Facilitation of [3H]-Ach release by forskolin depends on A2-adenosine receptor activation. NeurosciLett 151: 21-24.
- Duan DY, Liu LL, Bozeat N, Huang ZM, Xiang SY, et al. (2005) Functional role of anion channels in cardiac diseases ActaPharmacol Sin 26:265-278.
- Duan D (2013) Phenomics of cardiac chloride channels. ComprPhysiol 3: 667-692.
- Li GR, Feng J, Wang Z, Nattel S (1996) Transmembrane chloride currents in human atrial myocytes. Am J Physiol 270: C500-507.
- Oz MC, Sorota S (1995) Forskolin stimulates swelling-induced chloride sensitive current, not cardiac cystic fibrosis transmembrane-conductance regulator current, in human cardiac myocytes. Circ Res 76: 1063–1070.
- Sakai R, Hagiwara N, Kasanuki H, Hosoda S (1995) Chloride conductance in human atrial cells. J Mol Cell Cardiol 27: 2403-2408.
- Wieland SJ, Chou RH, Gong QH (1990) Macrophage colony stimulating factor (CSF-1) modulates a differentiation-specific inward rectifying potassium current in human leukemic (HL-60) cells. J Cell Physiol 142: 643-651.
- Pelleg A, Hurt CM, Michelson EL (1990) Cardiac effects of adenosine and adenosine 5’-triphosphate. Ann NY AcadSci 603: 19-30.
- Srinivas M, Shryock JC, Dennis DM, Baker SP, Belardinelli L (1997) Differential A1 adenosine receptor reserve for two actions of adenosine on guinea pig atrial myocytes. MolPharmacol 52: 683-691.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences