ISSN : 2574-2825
Journal of Nursing and Health Studies
A Review: Pathological and Molecule-Biological Studies of Atherosclerosis
Ru Yong-xin * Liu Jing
Department of Electron Microscopy, Institute of Hematology and Blood Diseases Hospital, Peking Union College, Tianjin, 300020, China
- *Corresponding Author:
- Ru Yong-xin
Department of Electron Microscopy,
Institute of Hematology and Blood Diseases Hospital, Peking Union College, Tianjin, 300020,
China,
Tel: 8602223909061;
E-mail: ruyongxin@126.com
Received: February 18, 2022, Manuscript No. IPJNHS-22-11350; Editor assigned: February 21, 2022, PreQC No. IPJNHS-22-11350 (PQ); Reviewed: March 07, 2022, QC No. IPJNHS-22-11350; Revised: March 11, 2022, Manuscript No. IPJNHS-22-11350 (R); Published: March 18, 2022, Invoice No. IPJNHS-22-11350
Citation: Yong-xin R, Jing L (2022) A Review: Pathological and Molecule-Biological Studies of Atherosclerosis. J Nurs Health Stud Vol:7 No:1
Abstract
Atherosclerosis is a disease that arterial intima gradually are thickened and transformed into a sclerotic plaque, interfering with normal blood flow and resulting fatalities. It includes three stages, the pre stage characterized with Diffuse Intimal Thickenings (DITs) and fatty streaks, the early atherosclerotic stage with Pathological Intimal Thickening (PIT), and the late stage with fibroatheromas transformed from PIT. Every atherosclerotic stage consists distinctive morphological alterations, and biologic changes and expression of immune markers at different levels. This review summarized discoveries and achievements of microanatomy, ultrastructure, immunohistochemically staining and molecular biology in literatures about atherosclerosis. Specifically, common histological changes and pathomechanism of atherosclerosis are emphasized based on our studies in this review.
Keywords
Fibroatheromas; Pathomechanism; Endothelium Pathomechanism; Pathological intimal thickening
Introduction
Atherosclerotic cardiovascular disease is prone to ischemic myocardial infarction, stroke, which is a disease with a high mortality rate [1]. Arterial endothelial cells become vulnerable to hemodynamic factors, and dysfunction of the endothelium occurs as a sign of the initiation of atherosclerosis [2]. Conventionally, it is thought that monocytes in the blood are recruited to the dysfunctioning endothelium and differentiated into macrophages, which engulf low-Density Lipoprotein (LDL) and transform into foam cells. It is consistent with the formation of fatty streaks in intima during atherosclerosis. Chemokines secreted by the endothelial cells and macrophages stimulate sub-endothelial Vascular Smooth Muscle Cells (VSMCs), resulting its proliferation and degeneration. Along with proliferation of fibroblasts, the proliferated VSMCs synthesize ExtraCellular Matrix (ECM) and form a fibrous cap. A necrotic core is located under the fibrous cap, which is rich in lipids and cellular debris; some plaques are accompanied by calcification [3].
Physiologically, VSMCs are located in tunica media and express a series of markers with contractile function. During atherosclerosis, the contractile VSMCs are transformed into synthetic phenotype following losing of the contractile markers [4]. These transformed VSMCs are capable of secreting ECM and abundant proteoglycan. The negatively charged proteoglycan binds to the positively corresponding apolipoprotein through electrostatic interaction, resulting in lipid retention [5]. The lipoproteins retained in vessel are prone to oxidative modifications to form ox-LDL. Some VSMCs phagocytose ox-LDL and form foam cells in intima additionally. Eventually, the foam cells from macrophages and VSMCs experience apoptosis and release free cholesterol with progression of disease [6]. The synthetic VSMCs express macrophage-specific markers, with decreasing of phagocytose lipids and producing of necrotic cellular debris. It promotes the progression of atherosclerosis through an increased inflammatory response [7].
Above transformed VSMCs play various roles and drive progression of atherosclerosis by different forms. A hallmark of atherosclerosis is calcification, which is thought as calcific cells derived from VSMCs also [8].
Development of Atherosclerosis
Atherosclerosis includes three stages, pre-atherosclerosis, early and late stage (Figure 1). The pre-atherosclerosis is characterized with Diffuse Intimal Thickening (DIT), extensive accumulation of intra and extracellular lipids. The lipid-laden cells and foam cells of the neo-intima are rich vacuoles in cytoplasm [9]. DIT is resulted from VSMC proliferation and phenotype shift from initial contractile phenotype to a synthetic phenotype in intima. The synthetic VSMSs are characterized by production of proteoglycans and elastin, and expansion of rougher Endoplasmic Reticulum (rER) and mitochondria, which could participate origination of foam cells in atherosclerosis [3-10].
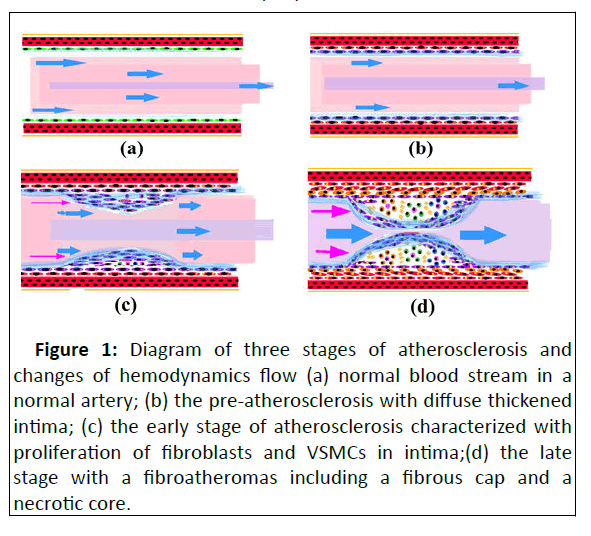
Figure 1: Diagram of three stages of atherosclerosis and changes of hemodynamics flow (a) normal blood stream in a normal artery; (b) the pre-atherosclerosis with diffuse thickened intima; (c) the early stage of atherosclerosis characterized with proliferation of fibroblasts and VSMCs in intima;(d) the late stage with a fibroatheromas including a fibrous cap and a necrotic core.
The early stage of atherosclerosis, also known as Pathological Intimal Thickening (PIT), is characterized with extracellular lipid pools, abundant VSMCs and ECM in the deep intima. The lipid pool includes plentiful free cholesterol and small portion of ECM, both promote lipoprotein retention in atherosclerotic cores. The components of ECM are mainly hyaluronic and proteoglycan. The lipoprotein undergoes oxidative modifications and plays a role in recruitment of macrophage furthermore [11-12].
The late stage of atherosclerosis gets further progression of atherosclerosis, with formation of fibroatheromas. The typical fibroatheromas contains a fibrous cap and a necrotic core. The necrotic core is formed due to aggregation of macrophages in the lipid pool, which phagocytose lipid droplets and transformed into foam cells, together with VSMC-derived foam cells due to sustained inflammatory and apoptosis [13]. Fibrosis, characterized by proliferation of fibroblastic cells and accumulation of collagen, plays an important role in arterial intimal remodeling and fibrous cap formation at late stage of atherosclerosis. The thickened fibrous cap is mainly composed of type I, type III collagen and proteoglycans. It is responsible for storing the hemorrhagic contents in the necrotic core [14].
Atherosclerosis is often complicated with intraplaque hemorrhages in atherosclerotic plaques [15]. Interbedded hemorrhages between the pseudo-media and the affected tunica media hint a reasonable mechanism of sporadic aortic aneurysms and dissections in cardiovascular diseases. The necrotic cores with a large volume always indicate a tendency of hemorrhage. These intraplaque hemorrhages and thin fibrous cap accelerate obstruction of arteries and thrombosis [16].
At late stage, the atherosclerotic plaques are also liable to neovascularization [17]. The neo-vascular and small capillaries are usually characterized with a small fissure and lumen in necrotic cores. Additionally, calcification characterized deposition of hydroxycalcium salts is a common feature during formation of atherosclerotic plaques [18].
Histological characteristics of atherosclerosis
Atherosclerotic plaques are usually cylinder-shaped and composed of three compartments: a fibrous cap on surface of atherosclerotic core and exposed to blood streams in arteries, a basal band between atherosclerotic cores and tunica media, and an atherosclerotic core located hump middle part of ASPs [19].
Fibrous caps are composed of bundles of collagen fibers and some longitudinal cells on the surface. Spindle-like foam cells are scattered among the collagen fibers. At some places, the fibrotic caps penetrate into atherosclerotic cores and become a focal fibrotic region in it [19]. Basal bands were including two parts: upper part of hyperplastic myofibroblastic cells, lower part of degenerated Vascular Smooth Muscle Cells (d-VSMCs) of affected tunica media. The both of them are separated by a thick integrate elastin layer [20].
The fibrous caps and the basal bands are merged together and become a circular fringe at periphery of the atherosclerotic plaques. The fringe contains three parts: an upper region with layers of fibroblastic and vacuolated cells among collagen fibers, an intermediate region with scattered small cells in amorphous matrix, and the basal band as those areas of bottom bands [19].
Atherosclerotic cores are characterized with focal fibrosis, accumulation of foam cells, neovascularization, hemorrhage, cholesterol deposition and calcification. collagen fibers in fibrotic areas are secreted by the synthetic VSMCs transformed from contractile VSMCs, which accelerate atherosclerosis [21]. The phenotype transformation of VSMCs was demonstrated experimentally in animal models [22-23]. Additionally, it is also thought that the synthetic-VSMCs are originated from multipotent stem cells in arterial walls and circulating mesenchymal progenitor cells [24-25]. Foam cells initially occur in sub-endothelial areas where includes lots of lipoproteins and cholesterol at early stage of atherosclerosis. Following progression of atherosclerosis, the foam cells spread and become mass aggregations in atherosclerotic cores [26].
Calcification with various sizes is usually dispersed in subregion of fibrous caps, atherosclerotic cores, and upper basal bands occasionally. It mainly includes components of calcium phosphate salts. Lesions of osseous and cartilaginous metaplasia are often located around calcified cells and materials [27].The calcified cells are associated with degeneration of SMCs, adventitial stem/progenitor cells, pericytes and myofibroblasts [28].
Ultra structural characteristics of associated cells in atherosclerosis
Transmission Electron Microscopy (TEM) have been applied in atherosclerotic study since 1980’ years, especially focused on alterations of endothelium, fibroblasts, myofibroblasts and VSMCs in atherosclerotic plaques [29-31]. The ultrastructural characteristics of the above cells during atherosclerosis listed are listed in Table 1. Additionally, ECM and materials such as collagen fiber, fibronectin and elastin are also demonstrated in atherosclerotic cores.
| Type of cells | Ultrastructural characteristics, |
|---|---|
| Endothelial cells | Abundant rER and Weibel-Palade bodies, plentiful mitochondria |
| Fibroblasts | Expanded rER, vacuoles, surrounded by collagen fibers |
| Myofibroblasts | Slender shaped, rER,periphery myofilaments,irregular lamina and fibronectin on surface, fibronexus |
| Foam cells | Full of lipid drops, lipofuscin granules |
| d/t-VSMCs | Abundant myofilaments, cytoplasmic lipid drops, lamina on the surface, few rER |
| D/t-VSMCs, degenerated and transformed VSMCs; rER, rough Endoplasmic Reticulum | |
Table 1: Ultra structural characteristics of cells in atherosclerotic plaques.
Fibroblasts and myofibroblasts are mostly located in fibrous caps, and surrounded by bundles of collagen fibers, account for stability of atherosclerotic cores on the surface. The fibroblasts are slender-shaped and contain a plenty of expended rER in cytoplasm and irregular lamina on surfaces, whereas myofibroblasts includes myofilaments with densities in cytoplasmic periphery and fibronectin fibrils on the surfaces additionally. Some of myofibroblasts are also characterized with fibronexus and foci fibronectin on the surfaces, which are defined as typical myofibroblasts as those in scar tissues and neoplastic lesions [32]. Fibroblasts and myofibroblasts are often demonstrated degenerated features of vesicles, lipid droplets and focus necrosis of cytoplasm in deep region of fibrous caps and atherosclerotic cores [33].
VSMCs are alighted mostly in basal bands and scattered atherosclerotic cores, although they are characterized with different feature. Layers of VSMC-like cells in upper regions of basal band shows a hypertrophic pattern, where the VSMCslike cells are disorganized and share intermediate phenotype of myofibroblasts and typical VSMCs. On the other hand, layers of VSMCs in deep region of basal bands are organized in elastin networks as normal tunica media patterns, which suggesting of affected tunica media of arteries. All of above cells are demonstrated degenerated and damaged features, with lipid droplets, ceroid granules, lipofuscin and dissolving appearance.
of myofilaments in cytoplasm. So, they are often named as degenerated and transformed VSMCs (d-VSMCs, t-VSMCs), the basal bands are named as a neo-intima and in some literatures [34].
Foam cells usually are aggregated atherosclerotic cores, a few of them are scattered in deep fibrous caps and upper region of basal bands adjacent to atherosclerotic cores. According to our study, typical foams include a condensed nucleus are full of lipid drops in cytoplasm. Whereas, some large cells rich in lipid droplets contain lots of vacuoles, lipofuscin and ceroid granules in cytoplasm, and caveolaes on cellular surface. These cells are roughly named as necrotic cells because it is difficult to identify their origination and entities in atherosclerosis [19].
Antigens and Molecule of Cells Associated with Atherosclerosis
In recent decades, a plenty of antigens and molecules are studied in atherosclerosis with development of biologic technics and more antibody applying in atherosclerosis study (Table 2). The roles of macrophages, VSMCs and endothelial cells are significantly investigated in these experiments.
| Detailed classification | Markers | |
|---|---|---|
| macrophages | derived macrophages | CD36, CD64, CD80, CD86,SR-A,LOX-1,MHCⅡ, |
| activated macrophages | CD68, CD11c, CD163, CD206 | |
| VSMCs | contractile phenotype | α-SMA, SMMHC, MYOCD, MYH11, ACTA2, TAGLN |
| dedifferentiated phenotype | CD68, galectin 3, ABCA1 | |
| chondrocyte-like cells | SOX9, Runx2, osteocalcin | |
| MSC-like cells | Sca1 | |
| myofibroblast-like cells | PDGFβR,ACTA2 | |
| beige adipocyte-like cells | UCP1 | |
| ECD | normal endothelium | KLF2 |
| activated endothelium | Class-ⅡMHC antigens, ELAM-1, VCAM-1, IL-8, MCP-1 |
Table 2: Markers of cells in atherosclerotic cells.
Markers of macrophage
Inflammation is thought plays an important role during atherosclerosis, which is initiated by the phagocytosis of Low- Density Lipoprotein (LDL) by monocytes. After injury of vascular endothelium, lipoproteins penetrate into subendothelial layers of arteries from plasma. The oxidized lipoproteins are phagocytosed by monocyte derived macrophages that express CD36, Scavenger Receptor A(SR-A), lectin-like receptor-1, surface markers such as MHCⅡ, CD64, CD80, and CD86 [35]. The LDL are digested by Lysosomal Acid Lipase (LAL) of endosomes/ lysosomes system of macrophages and become free cholesterol, and the macrophages transform into foam cells.
The macrophages of atherosclerotic plaques in activated state were considered as the origin of the foam cells through engulfing lipid drops. These macrophages express CD68, CD11c, CD163 and CD206 [36].
Markers of VSMCS
Another way in which foam cells are formed is due to excessive lipid loading of VSMCs from tunica media to intima. Contractile VSMCs (c-VSMCs) are strongly positive for contractile proteins of smooth muscle α-actin (α-SMA), α-tropomyosin, MYOCD and Smooth Muscle Myosin Heavy Chain (SMMHC) in tunica media [37]. On the hand, dedifferentiated VSMCs (d- VSMCs) express CD68, galectin 3 and ABCA1 under stimulation of inflammation in atherosclerotic progress. During atherosclerosis, c-VSMCs migrate into tunica intima and transfer into other phenotype-cells, such as chondrocyte-like cells, MSClike cells, myofibroblasts and beige adipocyte-like cells besides d-VSMCs [38]. These cells loss phenotype of c-VSMCs and secret various components of ECM in atherosclerotic plaques [39]. The chondrocyte -like cells are often detected by SOX9, Runt-related transcription factor2 (Runx2) and osteocalcin [40]; MSC-like by Sca1; myofibroblasts by Platelet-Derived Growth Factor Receptor-β (PDGFR-β) and alpha-actin-2; beige adipocyte-like cells by thermogenic (UCP1) [41-43].
Markers of ECD
Kruppel-Like Factor-2 (KLF2) is previously demonstrated in endothelium of human atherosclerotic specimens by method of in situ hybridization [44]. CCN1 regulates the endothelial cell phenotype by binding to receptor integrin α6β1 to activate downstream nuclear factor-К B [45]. The activated endothelial cells are also capable of expressing “endothelial activation antigens” of Class-2 MHC antigens, ELAM-1, VCAM-1, MCP-1, IL-1, IL-8, IL-8 and GM-CSF) [46]. All of the above inflammatory factors are participated in monocyte attachment and activation, and macrophage and VSMCs immigration and transformation in atherosclerosis.
Foam-cell origination of Atherosclerotic Plaques
Foam cells are a group of pathogenic cells that plays a significant role of initiating and accelerating atherosclerosis. Based on above experiments and clinical investigations, three theories about foam-cell origination are presented so far, - transformation from monocytes/macrophages, transformation from VSMCs and derived from other types of cells.
Foam-cell transformation from monocytes/ macrophages
With damaging of endothelium on various circumstances, monocytes in bloodstream are attracted to affected endothelial cells and transformed into macrophages to exert defense mechanism, i.e., phagocytosing modified lipoproteins to avoid further damage of vascular endothelium. Imbalance of lipid metabolism give rise to load lipid in macrophages excessively, so that monocytes are transformed into foam cells [47-48]. A group of proteins is essential for monocytes migrating into subendothelial region and the transformation, which includes selectin proteins on surface of endothelial cells and monocytes, Cellular Adhesion Molecules (CAMs). These macrophages derived from monocytes are characterized by a big volume and expression of CD68 [49].
Foam-cell transformation from VSMCS
VSMCs are isolated and separated by elastin laminas in tunica media of arteries physiologically, and characterized with contractile phenotype. Under pathologic conditions of hemodynamic stress and other pathogenic factors, the contractile VSMCs (c-VSMCs) are proliferated and transferred from tunica media into tunica intima. During this process, the c- VSMCs are transformed into synthetic VSMCs (s-VSMCs), which capable of synthesizing ECM and constructing a fibrous cap on surface of tunica intima. The s-VSMCs also uptake lipoproteins and cholesterol, and transform into foam cell in neo-intima that becomes an atherosclerotic cores including a plenty of lipid and cholesterol [50].
Foam-cell transformation and differentiation from other cells
Some studies and experiments have demonstrated that foam cells could be derived from Vascular Endothelial Cells (VECs) and Vascular Stem/Progenitor Cells (VSPCs) of arterial walls [51-52]. Dr. Lao and his colleagues evidenced that VECs undergoing Endothelial to Mesenchymal Transition (End MT) were a source VSMCs and foam cells in atherosclerosis [53]. A plenty of VSPCs between adventitia and tunica media is responsible for repairmen and generation of arterial wall [54]. Evelyn et al. [55]. Demonstrated a significant increase of VSPCs in the “vasculogenic zone” of atherosclerotic arteries comparing with normal vessels. The theory suggested the VSPCs were recruited and transformed into SMCs and foam cells in neo-intima during atherosclerosis [56].
Conclusion
Increasing experiments studies and clinical investigations suggest that atherosclerosis is a series of pathological processes, involving all kinds of resident cells of arteries and blood cells from periphery circulation. The pathomechanism is complicated with cell proliferation, differentiation, trans-differentiation, fatty degeneration, apoptosis and necrosis. Some alterations are compensatory and adaptive reactions, but some changes are secondary damaging. For atherosclerosis plaques are a complex tissue with hyperplasia and necrosis, the results and evidences drew from molecular and biologic studies must be carefully interpreted and concluded.
References
- Libby P, Bornfeldt KE, Tall AR (2016) Atherosclerosis: Successes, Surprises, and Future Challenges. Circ Res 118:531-543
[Crossref] [Google Scholar] [Pubmed]
- Gimbrone MA Jr, Garcia-Cardena G (2016) Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res 118(4):620-636
[Crossref] [Google Scholar] [Pubmed]
- Basatemur GL, Jorgensen HF, Clarke MCH, Bennett MR, Mallat Z, et al. (2019) Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol 16:727-744
[Crossref] [Google Scholar] [Pubmed]
- Alexander MR, Owens GK (2012) Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol 74:13-40
[Crossref] [Google Scholar] [Pubmed]
- Tran-Lundmark K, Tran PK, Paulsson-Berne G, Friden V, Soininen R, et al. (2008) Heparan sulfate in perlecan promotes mouse atherosclerosis: roles in lipid permeability, lipid retention, and smooth muscle cell proliferation. Circ Res 103:43-52
[Crossref] [Google Scholar] [Pubmed]
- Skalen K, Gustafsson M, Rydberg EK, Hulten LM, Wiklund O, et al. (2002) Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 417:750-742
[Crossref] [Google Scholar] [Pubmed]
- Gomez D, Owens GK (2012) Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res 95:156-164
[Crossref] [Google Scholar] [Pubmed]
- Durham AL, Speer MY, Scatena M, Giachelli CM, Shanahan CM, et al. (2018) Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res 114:590-600
[Crossref] [Google Scholar] [Pubmed]
- Perrotta I, Sciangula A, Perrotta E, Donato G, Cassese M, et al. (2011) Ultrastructural analysis and electron microscopic localization of Nox4 in healthy and atherosclerotic human aorta. Ultrastruct Pathol 35:1-6
[Crossref] [Google Scholar] [Pubmed]
- Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr, et al. (1994) A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis American Heart Association. Arterioscler Thromb 14:840-856
[Crossref] [Google Scholar] [Pubmed]
- Tabas I, Williams KJ, Boren J (2007) Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 116:1832-1844
[Crossref] [Google Scholar] [Pubmed]
- Langley SR, Willeit K, Didangelos A, Matic LP, Skroblin P, et al. (2017) Extracellular matrix proteomics identifies molecular signature of symptomatic carotid plaques. J Clin Invest 127:1546-1560
[Crossref] [Google Scholar] [Pubmed]
- Clarke MC, Talib S, Figg NL, Bennett MR (2010) Vascular smooth muscle cell apoptosis induces interleukin-1-directed inflammation: effects of hyperlipidemia-mediated inhibition of phagocytosis. Circ Res 106:363-372
[Crossref] [Google Scholar] [Pubmed]
- Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, et al. (2016) Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol 13:79-98
[Crossref] [Google Scholar] [Pubmed]
- Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, et al. (2005) Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 25:2054-2061
[Crossref] [Google Scholar] [Pubmed]
- Sato Y, Hatakeyama K, Yamashita A, Marutsuka K, Sumiyoshi A, et al. (2005) Proportion of fibrin and platelets differs in thrombi on ruptured and eroded coronary atherosclerotic plaques in humans. Heart 91:526-350
[Crossref] [Google Scholar] [Pubmed]
- Sluimer JC, Kolodgie FD, Bijnens AP, Maxfield K, Pacheco E, et al. (2009) Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J Am Coll Cardiol 2009 53:1517-1527
[Crossref] [Google Scholar] [Pubmed]
- Vattikuti R, Towler DA (2004) Osteogenic regulation of vascular calcification: an early perspective. Am J Physiol Endocrinol Metab 286:686-696
[Crossref] [Google Scholar] [Pubmed]
- Yong-Xin R, Xue-Bin Z, Shu-Xu D, Yongqiang Z, Ying L, et al. (2021) Histopathological and ultrastructural study of carotid atherosclerotic plaques: a study of four cases. Ultrastruct Pathol 30:1-16
[Crossref] [Google Scholar] [Pubmed]
- Song P, Zhou Y, Coughlan KA, Dai X, Xu H, et al. (2013) Adenosine monophosphate-activated protein kinase-alpha2 deficiency promotes vascular smooth muscle cell migration via S-phase kinase-associated protein 2 upregulation and E-cadherin downregulation. Arterioscler Thromb Vasc Biol 33:2800-2900
[Crossref] [Google Scholar] [Pubmed]
- Campbell JH, Campbell GR (2012) Smooth muscle phenotypic modulation--a personal experience. Arterioscler Thromb Vasc Biol 32:1784-1791
[Crossref] [Google Scholar] [Pubmed]
- Gomez D, Shankman LS, Nguyen AT, Owens GK (2013) Detection of histone modifications at specific gene loci in single cells in histological sections. Nat Methods 10:171-179
[Crossref] [Google Scholar] [Pubmed]
- Feil S, Hofmann F, Feil R (2004) SM22alpha modulates vascular smooth muscle cell phenotype during atherogenesis. Circ Res 94:863-875
[Crossref] [Google Scholar] [Pubmed]
- Tang Z, Wang A, Yuan F, Yan Z, Liu B, et al. (2012) Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun 3:875-885
[Crossref] [Google Scholar] [Pubmed]
- Bentzon JF, Falk E (2010) Circulating smooth muscle progenitor cells in atherosclerosis and plaque rupture: current perspective and methods of analysis. Vascul Pharmacol 52:11-20
[Crossref] [Google Scholar] [Pubmed]
- Kita T, Kume N, Minami M, Hayashida K, Murayama T, et al. (2001) Role of oxidized LDL in atherosclerosis. Ann N Y Acad Sci 947:199-205
[Crossref] [Google Scholar] [Pubmed]
- Bostrom KI, Yao J, Guihard PJ, Blazquez-Medela AM, Yao Y (2016) Endothelial-mesenchymal transition in atherosclerotic lesion calcification. Atherosclerosis 253:124-127
[Crossref] [Google Scholar] [Pubmed]
- Yu B, Chen Q, Le Bras A, Zhang L, Xu Q (2018) Vascular Stem/Progenitor Cell Migration and Differentiation in Atherosclerosis. Antioxid Redox Signal 29:219-235
[Crossref] [Google Scholar] [Pubmed]
- Perrotta I, Sciangula A, Concistre G, Mazzulla S, Aquila S, (2014) Internal mammary artery atherosclerosis: an ultrastructural study of two cases. Ultrastruct Pathol 38(3):199-203
[Crossref] [Google Scholar] [Pubmed]
- Birck MM, Saraste A, Hyttel P, Odermarsky M, Liuba P, et al. (2013) Endothelial cell death and intimal foam cell accumulation in the coronary artery of infected hypercholesterolemic minipigs. J Cardiovasc Transl 6:579-587
[Crossref] [Google Scholar] [Pubmed]
- Walski M, Chlopicki S, Celary-Walska R, Frontczak-Baniewicz M (2002) Ultrastructural alterations of endothelium covering advanced atherosclerotic plaque in human carotid artery visualised by scanning electron microscope. J Physiol Pharmacol 53:713-723
[Google Scholar] [Pubmed]
- Desmouliere A, Badid C, Bochaton-Piallat ML, Gabbiani G (1997) Apoptosis during wound healing, fibrocontractive diseases and vascular wall injury. Int J Biochem Cell Biol 29:19-30
[Crossref] [Google Scholar] [Pubmed]
- Baum J, Duffy HS (2011) Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol 57:376-379
[Crossref] [Google Scholar] [Pubmed]
- Saiura A, Sata M, Hirata Y, Nagai R, Makuuchi M, et al. (2001) Circulating smooth muscle progenitor cells contribute to atherosclerosis. Nat Med 7:382-383
[Crossref] [Google Scholar] [Pubmed]
- Willemsen L, de Winther MP (2020) Macrophage subsets in atherosclerosis as defined by single-cell technologies. J Pathol 250:705-714
[Crossref] [Google Scholar] [Pubmed]
- Cho KY, Miyoshi H, Kuroda S, Yasuda H, Kamiyama K, et al. (2013) The phenotype of infiltrating macrophages influences arteriosclerotic plaque vulnerability in the carotid artery. J Stroke Cerebrovasc Dis 22:910-918
[Crossref] [Google Scholar] [Pubmed]
- Bentzon JF, Weile C, Sondergaard CS, Hindkjaer J, Kassem M, et al. (2006) Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler Thromb Vasc Biol 26:2696-2702
[Crossref] [Google Scholar] [Pubmed]
- Liu M, Gomez D (2019) Smooth Muscle Cell Phenotypic Diversity. Arterioscler Thromb Vasc Biol 39:1715-23
[Crossref] [Google Scholar] [Pubmed]
- Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA, et al. (2014) Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 129:1551-1559
[Crossref] [Google Scholar] [Pubmed]
- Shroff R, Long DA, Shanahan C (2013) Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol 24:179-189
[Crossref] [Google Scholar] [Pubmed]
- Chappell J, Harman JL, Narasimhan VM, Yu H, Foote K, et al. (2016) Extensive Proliferation of a Subset of Differentiated, yet Plastic, Medial Vascular Smooth Muscle Cells Contributes to Neointimal Formation in Mouse Injury and Atherosclerosis Models. Circ Res 119:1313-1323
[Crossref] [Google Scholar] [Pubmed]
- Wang Y, Dubland JA, Allahverdian S, Asonye E, Sahin B, et al. (2019) Smooth Muscle Cells Contribute the Majority of Foam Cells in ApoE (Apolipoprotein E)-Deficient Mouse Atherosclerosis. Arterioscler Thromb Vasc Biol 39:876-887
[Crossref] [Google Scholar] [Pubmed]
- Majesky MW, Horita H, Ostriker A, Lu S, Regan JN, et al. (2017) Differentiated Smooth Muscle Cells Generate a Subpopulation of Resident Vascular Progenitor Cells in the Adventitia Regulated by Klf4. Circ Res 120:296-311
[Crossref] [Google Scholar] [Pubmed]
- Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, et al. (2005) Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol 167:609-618
[Crossref] [Google Scholar] [Pubmed]
- Hsu PL, Chen JS, Wang CY, Wu HL, Mo FE (2019) Shear-Induced CCN1 Promotes Atheroprone Endothelial Phenotypes and Atherosclerosis. Circulation 139:2877-2914
[Crossref] [Google Scholar] [Pubmed]
- Pober JS, Sessa WC (2007) Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 7:803-815
[Crossref] [Google Scholar] [Pubmed]
- Bobryshev YV, Ivanova EA, Chistiakov DA, Nikiforov NG, Orekhov AN, et al. (2016) Macrophages and Their Role in Atherosclerosis: Pathophysiology and Transcriptome Analysis. Biomed Res Int 216:958-962
[Crossref] [Google Scholar] [Pubmed]
- Crowther MA (2005) Pathogenesis of atherosclerosis. Hematology Am Soc Hematol Educ Program 2005:436-441
[Crossref] [Google Scholar] [Pubmed]
- Elliott MR, Koster KM, Murphy PS (2017) Efferocytosis Signaling in the Regulation of Macrophage Inflammatory Responses. J Immunol 198:1387-1394
[Crossref] [Google Scholar] [Pubmed]
- Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, et al. (2014) Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res 115:662-676
[Crossref] [Google Scholar] [Pubmed]
- Daub K, Langer H, Seizer P, Stellos K, May AE, et al. (2006) Platelets induce differentiation of human CD34+ progenitor cells into foam cells and endothelial cells. FASEB J 20:2559-2561
[Crossref] [Google Scholar] [Pubmed]
- Rong JX, Shapiro M, Trogan E, Fisher EA (2003) Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci U S A 100:13531-13628
[Crossref] [Google Scholar] [Pubmed]
- Lao KH, Zeng L, Xu Q (2015) Endothelial and smooth muscle cell transformation in atherosclerosis. Curr Opin Lipidol 26:449-456
[Crossref] [Google Scholar] [Pubmed]
- Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, et al. (2006) Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development 133:1543-1551
[Crossref] [Google Scholar] [Pubmed]
- Torsney E, Mandal K, Halliday A, Jahangiri M, Xu Q (2007) Characterisation of progenitor cells in human atherosclerotic vessels. Atherosclerosis 191:259-264
[Crossref] [Google Scholar] [Pubmed]
- Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, et al. (2004) Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest 113:1258-1265
[Crossref] [Google Scholar] [Pubmed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
