A Case Report on Amoxicillin/Potassium Clavulante Induced Hypersensitive Reaction
Rahul S1*, Shoaib Ali1, Kallappa C. Herakal2 and Doddayya H2
1Department of Pharmacy Practice, NET Pharmacy College, Raichur, Karnataka, India
2Department of Dermatology, Navodaya Medical College Hospital and Research Centre, Raichur, Karnataka, India
- *Corresponding Author:
- Rahul S
Department of Pharmacy Practice,
NET Pharmacy College, Raichur, Karnataka, India
Tel: +918309495395;
Email: srahul.pharmd@gmail.com
Received: May 24, 2024, Manuscript No. IPIJCR-24-19111; Editor assigned: May 27, 2024, PreQC No. IPIJCR-24-19111 (PQ); Reviewed: June 10, 2024, QC No. IPIJCR-24-19111; Revised: April 14, 2025, Manuscript No. IPIJCR-24-19111 (R); Published: April 21, 2025, DOI: 10.36648/IPIJCR.9.1.008
Citation: Rahul S, Ali S, Herakal KC, Doddayya H (2025) A Case Report on Amoxicillin/Potassium Clavulante Induced Hypersensitive Reaction. Int J
Case Rep Vol:9 No:1
Abstract
The case report addresses the use of Amoxicillin/Potassium Clavulanate, a combination antibiotic, for treating bacterial infections. It focuses on hypersensitivity reactions induced by the medication and the importance of monitoring and providing supportive therapy. It is important to highlight the necessity of performing a test dose before prescribing any antibiotic to prevent adverse reactions. The report includes a patient who experienced a hypersensitivity reaction to Amoxicillin/Potassium Clavulanate 500 mg/125 mg, leading to immediate discontinuation of the medication and the provision of supportive therapy. The results show that the patient started recovering after the intervention. The implications of this report underscore the significance of vigilance in prescribing antibiotics to prevent adverse reactions.
Keywords
Amoxicillin/Potassium Clavulanate; Antibiotic; Hypersensitivity reaction; Test dose
Abbreviations
ADR: Adverse Drug Reaction; CV: Potassium Clavulanate; OD: Omne in Die (Once daily); PO: Per Oral; Tab: Tablet
Introduction
Amoxicillin/Potassium Clavulanate is a combination antibiotic medication used for the treatment of a number of bacterial infections [1]. It consists of amoxicillin, a penicillin-type antibiotic, and potassium clavulanate, a beta-lactamase inhibitor [2,3]. Amoxicillin is a broad-spectrum β-lactam antimicrobial originally derived from penicillin. It is a bactericidal agent that targets and kills bacteria by inhibiting the biosynthesis of the peptidoglycan layer of the bacterial cell wall, which interrupts the construction of the cell wall and ultimately leads to the destruction, or lysis, of the bacteria. Clavulanic acid is a betalactamase inhibitor often used in conjunction with amoxicillin to broaden its spectrum and combat resistance. It has little to no antimicrobial activity of its own and instead works by preventing bacterial destruction of beta-lactams. Over the years, certain bacteria have evolved to develop resistance to standard β- lactam antimicrobials through the production of enzymes called β-lactamases. These enzymes target and hydrolyze the β-lactam ring, which is necessary for penicillin-like antimicrobials to work. Clavulanic acid prevents this degradation by binding and deactivating the β-lactamase, thus restoring the antimicrobial effects of amoxicillin [4]. Amoxicillin-Clavulanate is largely safe and well-tolerated in the general population, with the vast majority of adverse effects being only mild gastrointestinal symptoms. The single most common complaint is diarrhea, but others include nausea, vomiting, loose stools, and abdominal discomfort.
Dermatologic effects are less common and generally related to hypersensitivity reactions. These reactions can present anywhere on the spectrum of disease, ranging from simple pruritus or urticaria to severe and life-threatening manifestations, such as anaphylaxis, Steven-Johnson syndrome, or toxic epidermal necrosis. Patients taking amoxicillin-clavulanate require monitoring at the beginning of therapy for signs of hypersensitivity and throughout the course for signs of secondary infection, such as C. difficile colitis or candidiasis.
Case Presentatin
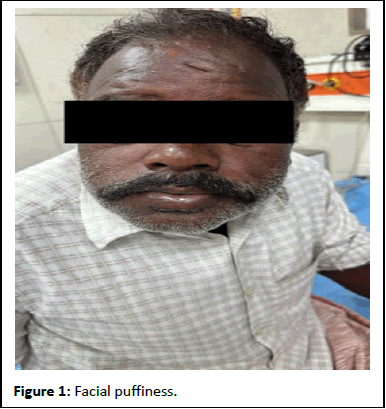
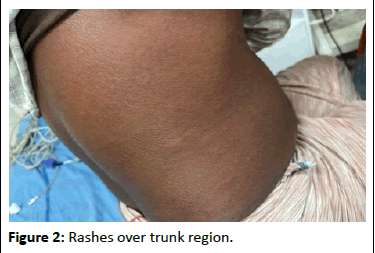
A 53-year-old was consulted with facial swelling and rashes over the trunk after self-administering Amoxicillin/Potassium Clavulanate 625, prescribed by a private practitioner for the management of wound abscesses. On evaluation, papules and plaque were noted over the abdomen and back, with swelling of the lips and face.
The drug was stopped, and he was administered Inj. Pheniramine maleate and Inj.Dexamethasone as prophylactic management of the hypersensitive reaction. Treatment started to cure the hypersensitive reaction with Bilastine 10 mg, Montelukast/Levocetrizine, and Clobetasol cream as stat medication.
With the treatment he received, the symptoms gradually showed an improving trend.
According to the Naranjo ADR probability scale (score=7), the reaction was categorized as a probable reaction to the drug. In severity, it was a major ADR. The rash was a hypersensitivity reaction (Table 1, Figures 1 and 2).
| Suspected adverse drug reaction | Naranjo’s probability assessment scale |
| Moxypra CV (Amoxicillin and Potassium Clavulanate) 625 mg PO OD | 07 (Probable) |
| Hypersensitivity reaction such as facial swelling and rashes over the back region within 1 hour of administration. |
Table 1: Naronjo’s causality assessment score.
Figure 1: Facial puffiness.
Figure 2: Rashes over trunk region.
Discussion
ADRs have been a source of concern due to being regarded as one of the primary causes of morbidity and mortality among hospitalized patients. According to numerous researches, ADRs are responsible for around 8% of all hospitalizations [5].
Amoxicillin-induced rash and facial puffiness can be attributed to various underlying mechanisms, primarily involving immunemediated responses. Amoxicillin is a beta-lactam antibiotic that can act as a hapten, forming complexes with endogenous proteins, thereby triggering an immune response. This immune reaction involves the activation of T cells and the production of antibodies, such as IgE, which leads to the release of inflammatory mediators like histamine, leukotrienes, and cytokines. The release of histamine and other inflammatory mediators induces vasodilation and increases vascular permeability, leading to erythema (redness) and edema (swelling) in the skin, manifesting as a rash. The rash is often maculopapular and may be accompanied by pruritus (itching). Similarly, histamine-induced vasodilation and increased vascular permeability can occur in the soft tissues of the face, leading to facial puffiness or edema [6]. Amoxicllin induced rash and facial puffiness can also result from a type I hypersensitivity reaction, mediated by IgE antibodies. Upon re-exposure to the drug, these antibodies bind to mast cells and basophils, triggering the release of histamine and other inflammatory mediators. Mast cell degranulation and histamine release cause vasodilation and increased vascular permeability in the skin, resulting in characteristic rash associated with allergic reactions to the drug. Mast cell degranulation and histamine release in the soft tissues of the face can lead to facial swelling or puffiness [7].
The presented case illuminates the occurrence of rashes and facial puffiness in the context of an individual undergoing management for wound abscess. The onset of rashes and facial puffiness followed the administration of Bilastine 10 mg, Montelukast/Levocetrizine, and Clobetasol cream for plaque, maculopapular rashes and facial swelling. Of particular note is the cessation of Amoxicllin/Potassium Clavulanate during this period.
The application of the Naronjo causality assessment scale, attributing a “probable” relationship (score of 7) between drug administration and rashes, facial swelling development, substantiates the association with the reaction and Amoxicillin/ Potassium clavulanate.
Therapeutically, the instituted regimen, comprising Antihistamines, and corticosteroids adheres to established protocols. Diminution of the physical manifestations, with visibly healed lesions attests to the efficacy of comprehensive therapeutic approach.
Conclusion
Amoxicillin/Potassium Clavulanate induced facial swelling and rashes occur generally within few hours or days of taking the medication with an incidence rate of 3-7%. Dechallenge resulted in normalization of the manifestations. This drug induced hypersensitivity reaction is a serious reaction but reversible if recognised early and treated accordingly. The report concludes that performing test dose before prescribing any antibiotic is necessary to prevent adverse reactions.
Acknowledgement
We gratefully thank the healthcare staff of Navodaya Medical College Hospital and Research Centre, Raichur, Karnataka, 584-103, India.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- Sinha S (2024) Amoxicillin and Clavulanate. Drugs.com
- K Health (2024) Amoxicllin-Potassium Clavulanate (Generic: Augmentin). Uses, Side effects, dosage, contraindications, warnings, interactions and more. K Pharmacy, LLC.
- Wikipedia (2024) Amoxicllin/Clavulanic-acid. Wikipedia
- GoodRx (2024) Amoxicillin/Potassium Clavulanate. GoodRx, Inc.
- Blumenthal KG, Peter JG (2019) Adverse Reactions to b-lactam antibiotics. J Allergy Clin Immunol 7:1455-1461
- Pichichero ME (2005) A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics 115:1048-1057
[Crossref] [Google Scholar] [PubMed]
- Bouvy JC, de Bruin ML, Koopmanschap MA (2015) Epidemiology of adverse drug reactions in Europe: A review of recent observational studies. Drug Saf 38:437-453
[Crossref] [Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences