ISSN : 2393-8854
Global Journal of Research and Review
A Brief Review on Antitubercular Activity of Pharmacological Active Some Triazole Analogues
Department of Pharmacy, Guru Ram Das (PG) IMT, Dehradun, (Uttarakhand), India
Abstract
Triazole is a five member heterocyclic nucleus has attracted a wide consideration in exploration for the new remedial agents. This nucleus acts very important role in biological fields for various biologically active molecules. The chemistry of triazole compounds has expected considerable interest due to their synthetic and effective biological properties likes analgesic, antinflammatory, antioxidant, analeptic, sedatives, antianxiety, antimicrobials, anticonvulsant, anticancer, and other biological activities. There are various known drugs in market containing the triazole moiety likes voriconazole, triazolam, fluconazole, intraconazole, furacylin, alprazolam, etizolam etc. This review supplied a concise outline on triazole compounds as an antitubercular molecule. Because present antitubercular drugs are more toxic and multiple drug therapy is required. So it is significant to find out the triazole analogues with excellent antitubercular activity.
Keywords
Antitubercular agents, Triazole derivatives, Pharmacological activities.
INTRODUCTION
Nitrogen enclosing heterocyclic compounds has been draw growing attention because of their usefulness in various types of applications. Due to the search for innovative biological active compounds is one of the most demanding tasks to the researchers. The nitrogen containing heterocyclic compounds have been attracted to numerous chemical scientists. The triazole moiety is one of the most significant five member heterocycle compound which is a quality of natural and synthetic compounds. Triazole and its various derivatives have a extensive range of purposes. They are principally surrounded by the type of compounds used such as antimicrobial, antifungal, antiviral, antiinflammatory, analgesic, antiepileptic, antihypertensive, antimalarial, antioxidants, antihistaminic, antianxiety, antidepressant, and antitubercular agents etc [1-5]. Triazole derivatives are also used as optical corrosion inhibitors, brightening agents, and as additives with a variety of other purposes. Various pigments and dye stuffs have this heterocyclic nucleus. The significances of triazole derivatives have good position in the field heterocyclic chemistry, due to its various types of biological activities. This nitrogen containing heterocyclic compounds are found in abundance in most of the medicinal compounds. The triazole has established substantial awareness due to their synthetic and effective biological significance. There are two probable isomers of triazole depending on the location of nitrogen atom in the moiety [6-12]. Triazole derivatives have drawn huge attention to chemists due to its broad diversity of activities, low toxicity and high-quality pharmacokinetic and pharmacodynamic outlines.
Chemistry
Triazole is also recognized as pyrrodiazole. It is an organic heterocyclic compounds having a five membered diunsaturated ring structure. It composed of three nitrogen atoms and two carbon atoms at non-adjacent positions in their ring structure. The simplest structure of the triazole family is triazole itself. Triazole is a crystalline solid, white to pale yellow colour, weakly basic compound, characteristic odour. It is soluble in water and alcohol and melting point (MP) 120°C and boiling point (BP) at 260°C. It occur as a pair of chemically isomeric compounds 1, 2, 3-triazole (1) and 1, 2, 4-triazole (2) with molecular weight of 69.06 and molecular formula C2H3N3 [12-15].
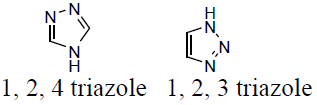
Pharmacological actions
The triazole compounds are versatile and have been characteristics in various clinically used drugs. The most appropriate studies have exposed that triazole derivatives have extensive spectrum pharmacological behaviors such as antimicrobial, anti-inflammatory, analgesic, anti convulsant, antimalarial, antiviral, antiproliferative, anticancer and various other pharmacological activities [15-20]. Nowadays research is concentrated towards the introduction of new and safe therapeutic agents for clinical importance. The achievement of imidazole moiety as an essential moiety of various medicinal agents guided to introduction of the triazole compounds. The triazole compounds are said to be the isosters of imidazole compounds in which the carbon atom of imidazole is isosterically substituted by nitrogen atom.
Antitubercular activity
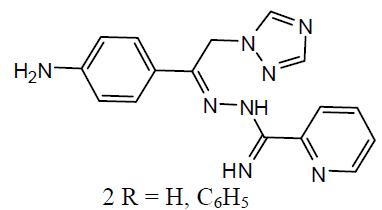
Tuberculosis (TB) is an infection disease and caused by a Gram positive bacteria called Mycobacterium tuberculosis. The M. tuberculosis frequently attacks the lungs, but they can also harm other parts of the body [21]. Some 1, 2, 4-triazole analogues (1) were reported as anti-TB agents [22]. The N`-[1-aryl-2-(1H-imidazol-1-yl and 1H-1, 2, 4-triazol-1-yl)-ethylidisne]-pyridine-2 carbo -xamidrazone derivatives (2) and evaluated their anti-TB activity [23].
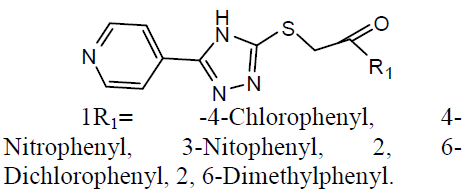
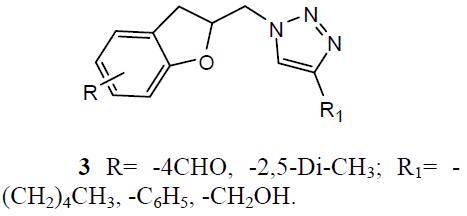
Different 1, 4-Disubstituted-1, 2, 3- triazoles (3) has been developed and screened for anti-TB activity against M. tuberculosis H37Rv and exhibited anti-TB activities with MIC ranging from 12.5 to 3.12 ug/ml [24]. Newly 1, 2, 4 triazoles analogs has been synthesized and carried in vitro anti-TB activity against M. tuberculosis H37Rv strain. Compound 3-(3-pyridyl)-5- (4-methylphenyl)-4-(N-4-chloro-1, 3-benzo thiazol-2-amino)-4H-1, 2, 4 triazole (4) was exhibited improved antitubercular activity than reference drug rifampicin [25].
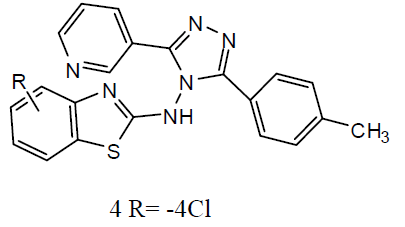
A series of 2-substituted-5- [isopropyl thiazole] clubbed 1, 2, 4-triazole and 1, 3, 4-oxadiazole were evaluated for their anti-TB activity against M. tuberculosis H37Rv strain by broth dilution assay method, compound 4-(2-chlorobenzylidene amino)-5-(4-isopropylthiazol-2-yl)-4H-1, 2, 4-triazole-3-thiol (5) were exhibited considerable anti-TB activity [26] . A series of N-{4- [(4-amino-5-sulfanyl-4H-1, 2, 4-triazol-3- yl)methyl]-1, 3-thiazol-2-yl}-2-substitutedamides (6) were also exhibited [27] preliminary in vitro antibacterial activity against Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and Salmonella typhus and were also evaluated as anti-TB activity against M. tuberculosis H37 Rv strain by broth micro dilution assay method. The antibacterial statistics of the tested compounds showed that most of the synthesized compounds were showed better antibacterial activity against different bacteria strains and compared to reference drugs. The in vitro anti-TB activity also reported that the tested compounds against M. tuberculosis strain H37 Rv showed moderate to better activity. The thiazolyltriazole derivatives (7) were investigated of their anti-TB and antimicrobial activities [28]. Various compounds have shown promising anti-tubercular activity while others were inactive.
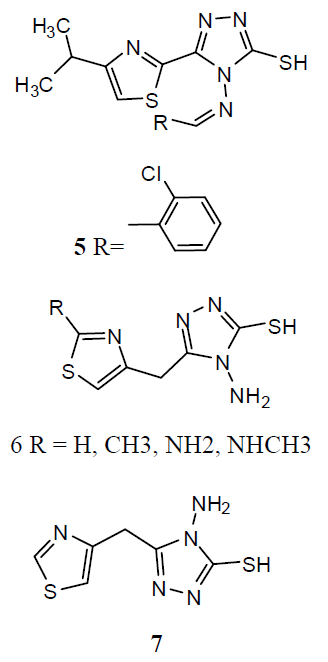
Various derivatives of substituted 1, 2, 4-triazol-(3-yl) benzene-1, 2, 3-triol derivative (8) and screened them for antitubercular activity. It was found that compounds 8a and b possess comparable activity with that of standard drug Rifampicin against M. tuberculosis. The remaining compounds were found less active than reference drug [29]. The thiazolyltriazole analogues (9) were developing new molecules with enhanced effectiveness for treating M. tuberculosis H37Rv strain infections and with decreased drug resistance. They also investigated them for their anti-TB activities. Numerous compounds have shown promising activity against Mtb. The 1, 2, 4-triazole substitutes are showing antitubercular effects such as α- [5-(2-furyl)-1, 2, 4-triazoles-3ylthio] acehydrazide (10) and other related compounds were also showing antitubercular activities. Mannich-bases of substituted triazoles are also exhibited good antibacterial activities [30].
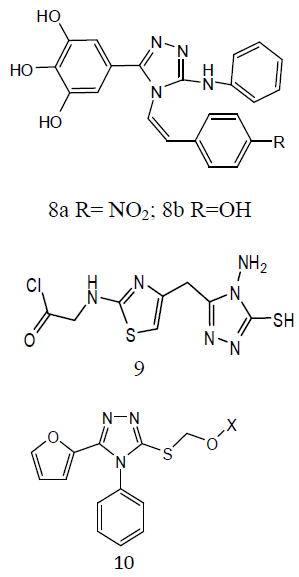
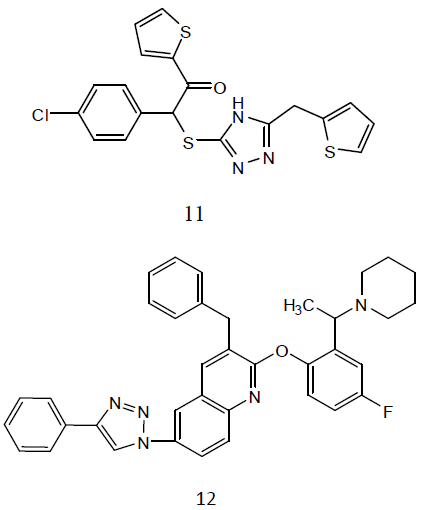
Some 3-alkylsulphanyl-1, 2, 4-triazole derivatives (11) and evaluated them for anti- TB activity. Antitubercular effects of the compounds was evaluated against M. tuberculosis H27Rv at 6.25 μg/mL and the evaluated compounds exhibited significant inhibition ranging from 58-84 ℅ [31]. A series of quinoline derivatives possessing triazolo (12) ureido and thioureido substituents and evaluated their anti-TB properties. Three compounds inhibited M. tuberculosis H37Rv up to 96%, 98% and 94% respectively, at concentration of 6.25μg/ml. the minimum inhibitory concentration (MIC) value is 3.125μg/ml was observed for two of the tested compounds while for one compound was found to be MIC value 6.25 μg /ml [32].
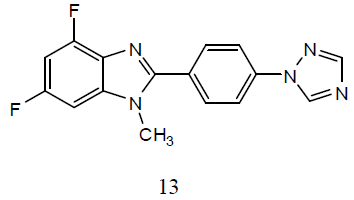
A series of 2-[4-(1H-[1, 2, 4]-triazol- 1-yl)phenyl]-1-substituted-4,6-difluoro- 1Hbenzo[d] imidazole derivatives (13) for their preliminary in-vitro antibacterial activity against P. aeruginosa, E. coli, S. aureus, and S. typhus and then these compounds were screened for their anti-TB activity against M. tuberculosis H37Rv strain. The antibacterial data suggested that the analogues with electronegative substituents emerged as the most promising antimicrobials. A few of the selected analogues are under further evaluation for secondary anti-TB screening, as they have shown better activity when compared to rifampin [33].
DISCUSSION
Triazole ring can be used as an attractive pharmacophore to produce innovative functional drug molecules, providing a convenient and efficient pathway to build up a variety of bioactive and useful molecules. The triazole ring is also an important isostere of imidazole, oxazole, pyrazole, thiazole, amide moiety in designing various types of new drug molecules. A large number of triazole derivatives have been extensively prepared and investigated for their biological activities, which is one of the most active areas in the researches and developments of new drugs [34-38]. Triazole nucleus have been included into a ample array of therapeutically attractive drug applicants including anti-inflammatory, anticancer, antimicrobial, anti fungal, central nervous system (CNS) stimulants, sedatives, antianxiety, antidepressant, antiviral, and other related biological activities. Particularly, triazole compounds as antifungal drugs have been playing a quite important role in the treatment of fungous infection. Triazole analouges with strong pharmacological activities, low toxicities or adverse effects, less drug resistances, high bioavailability, good pharmacokinetics and drug-targeting, diversity of drug administration, broad spectrum, better curative effect have been frequently becoming clinical drugs or candidates for the management of diverse types of diseases [39-42]. All these showed extensive potential of triazole-based compounds as therapeutic agents.
Table 1: Physical properties of 1, 2, 3-triazole and 1, 2, 4-triazole
| S. No. | 1, 2, 3-triazole ring moiety | 1, 2, 4-triazole moiety |
|---|---|---|
| 1 | Molecular formula-C2H3N3 | Molecular formula-C2H3N3 |
| 2 | Molar mass 69.0654 | Molar mass-69.0654 |
| 3 | Boiling point 203°C | Boiling point-260 |
| 4 | Melting point 23-25°C | Melting point-120-121°C |
| 5 | Density 1.192 g/cm-3 | Density - 1.394 g/cm-3 |
| 6 | Appearance colourless liquid | Appearance white solid |
| 7 | Solubility in water very soluble | Solubility in water very soluble |
| 8 | Alkalinity (pKb) 9.4 | Alkalinity (pKb) 10.3 |
| 9 | Acidity (pKa) 1.2 | Acidity (pKa) 2.2 |
| 10 | Vapour Pressure 0.4 mmhg (at 25°C) | Vapour Pressure 0.02 mmhg (at 25°C) |
CONCLUSION
Triazole analogues have paying attention in the fields of chemical, medicine and agrochemical research area, due to it’s exclusively structures and chemical properties. Triazole and its analogues belong to a class of remarkably active compounds having different types of pharmacological properties. Triazole compounds have finalized much significance as they have also been explored for their diverse biological activities. Different new and potent compounds will prepared to explore more effective and potent molecule by substitution of different atoms or groups on triazole ring with different pharmacological activities.
REFERENCES
- Bay HA, Quaddouri B, Guaadaoui A, Touzani R, Benchat N, Hamal A, Taleb M, Bellaoui, M, Kadiri SE, Synthesis and Biological Activity of New Triazole Compounds, Drug Design Discovery, 7, 2010, 41-45.
- Brodie, M.J, Rosenfeld, W.E, Vazquez, B, Sachdeo, R, Perdomo, C, Mann, A, Arroyo, S, Rufinamide for the adjunctive treatment of partial seizures in adults and adolescents: a randomized placebo-controlled trial. Epilepsia, 2009, 50 (8), 1899–909.
- Chen J, Sun XY, Chai KY, Lee JS, Song MS, Quana ZS, Synthesis and anticonvulsant evaluation of 4-(4- alkoxylphenyl)-3-ethyl-4H-1, 2, 4-triazoles as open-chain analogues of 7-alkoxyl-4,5- dihydro[1, 2, 4]triazolo[4, 3-a]quinolines, Bioorg & Med Chem, 15, 2007, 6775-6781.
- Demaray, J.A, Thuener, J.E, Dawson MN, Sucheck SJ, Synthesis of triazole- oxazolidinones via a one-pot reaction and evaluation of their antimicrobial activity. Bioorg Med Chem Lett, 2008, 18, 4868-48.
- Demirbas A, Sahin D, Demirbas N, Karaoglu SA, Synthesis of some new 1, 3, 4-thiadiazol-2-ylmethyl-1, 2, 4-triazole derivatives and investigation of their antimicrobial activities, Eur J Med Chem, 44, 2009, 2896-2903.
- Demirbas, N, Synthesis and antimicrobial activities of some new 1-(5-phenyl amino- [1, 3, 4] thiadiazol-2-yl) methyl-5-oxo-[1, 2, 4] triazole and 1-(4-phenyl-5-thioxo-[1, 2, 4] triazol-3-yl) methyl-5-oxo- [1, 2, 4] triazole derivatives. Eur J of Med Chem, 2004, 39, 793–804.
- Ebdrup, S, Sorensen, L.G, Olsen, O.H, Jacobsen, P, Synthesis and structure-activity relationship for a novel class of potent and selective carbamoyl-triazole based inhibitors of hormone sensitive lipase. J Med Chem, 2004, 47, 400–410.
- Khalil N.SAM, Efficient synthesis, structure, and antimicrobial activity of some novel N- and S-β-dglucosides of 5-pyridin- 3-yl-1, 2, 4-triazoles, Carbohydrate Res, 341, 2006, 2187-2199.
- Siddiqui N, Ahsan W, Alam MS, Ali R, Jain S, Azad B, Akhtar J. Triazoles: as potential bioactive agents. Inter J Pharm Sci Rev& Res, 8(1), 2011; 161-169.
- Amir, M, Kumar, H, Javed, S.A, Khan, S.A, 1, 3, 4-Oxadiazole/ thiadiazole and 1,2,4- triazole derivatives of biphenyl- 4-yloxy acetic acid: Synthesis and preliminary evaluation of biological properties. Eur J Med Chem, 2008, 43, 2688-2698.
- Wang X, Wan K, Zhou C, Synthesis of novel sulfanilamidederived 1, 2, 3-triazoles and their evaluation for antibacterial and antifungal activities, Eur J Med Chem, 45, 2010, 4631-4639.
- Kokil, R.G, Synthesis and In Vitro Evaluation of Novel 1, 2, 4-Triazole Derivatives as Antifungal Agents. Letters in drug Design & Discovery, 2010, 7, 46-49.
- Havaldar, F.H, Patil, A.R, Syntheses of 1, 2, 4 Triazole Derivatives and their Biological Activity. Eur J Chem, 2008, 5, 347-354.
- Yan S, Liu Y, Chen Y, Liu L, Lin J, An efficient one-pot synthesis of heterocycle- fused 1,2,3-triazole derivatives as anti- cancer agents, Bioorg & Med Chem Lett, 20, 2010, 5225–5228.
- Siddiqui, A. A, Arora, Amit, Synthesis of some 1, 2, 4-triazoles as potential antifungal agents. Indian journal of chemistry, 2005, 44 B, 838.
- Ibrahim DA, Synthesis and biological evaluation of 3, 6- disubstituted [1, 2, 4] triazole [3, 4-b] [1, 3, 4] thiadiazole derivatives as a novel class of potential anti- tumor agents, Eur J Med Chem, 44, 2009, 2776-2781.
- Jadhav, G.R, Shaikh, M.U, Kale, R.P, Shiradkar, M.R, Gill, C.H, SAR study of clubbed [1, 2, 4]-triazolyl with fluorobenzimidazoles as antimicrobial and antituberculosis agents. Eur J Med Chem, 2009, 44, 2930–2935.
- Jin HG, Sun XY, Chai KY, Piao HR, Quan ZS, Anticonvulsant and toxicity evaluation of some 7-alkoxy-4,5-dihydro-[1, 2, 4] triazolo[4, 3-a]quinoline-1(2H)-ones, Bioorg & Med Chem, 14, 2006, 6868-6873.
- Jordao, A.K, Afonso, P.P, Ferreira, V.F, De Souza, M.C, Almeida, M.C, Beltrame, C.O, Paiva, D.P, Wardell, S.M, Wardell, J.L, Tiekink, E.R, Damaso, C.R, Cunha, AC. Antiviral evaluation of Namino- 1,2,3- triazoles against Cantagalo virus replication in cell culture. Eur J Med Chem, 2009, 44, 3777–3783.
- Narayana B, Raj KKV, Ashalatha BV, Kumari NS, Synthesis of some new substituted triazolo [4, 3-a] [1, 4] benzodiazepine derivatives as potent anticonvulsants, Eur J Med Chem, 41, 2006, 417-22.
- Kaplancikli, T.A, Turan-Zitouni, G, Chevallet, P. Synthesis and antituberculosis activity of new 3-alkylsulfanyl-1, 2, 4- triazole derivatives. J Enzyme Inhib Med Chem, 2005, 20,179–182.
- Mali RK, Toraskar MP, Mali KK, Naik PP and Shirodkar PY, Synthesis of Some Antifungal and Anti-tubercular-1,2,4- Triazole Analogues, Inter J Chem Tech Res,1 (2), 2009, 168-173.
- Mamolo MG, Zampieri D, Falagiani V, Vio L, Fermeglia M, Ferrone M, Pricl S, Banfi E and Scialino G, antifungal and antimycobacterial activity of new N`-[1- aryl-2-(1Himidazol-1-yl and 1H-1, 2, 4- triazol-1-yl)-ethylidene]-pyridine-2-carboxa- midrazone derivatives: a combined experimental and computational approach. Arkivoc, 5, 2004, pp. 231-250.
- Tripathi RP, Yadav AK, Ajay A, Bisht SS, Chaturvedi V, Sinha SK, Application of Huisgen (3+2) cycloaddition reaction: Synthesis of 1-(2,3-dihydrobenzofuran-2- ylmethyl [1,2,3]-triazoles and their antitubercular evaluations, Eur J Med Chem, 45, 2010, 142–148.
- Patel NB, Khan IH, Rajani SD, Pharmacological evaluation and characterizations of newly synthesized 1,2,4-triazoles, Euro J Med Chem, 45, 2010, 4293-4299.
- Kumar GVS, Rajendraprasad Y, Mallikarjuna BP, Chandrashekar SM, Kistayya, C, Synthesis of some novel 2- substituted-5-[isopropylthiazole] clubbed 1, 2, 4-triazole and 1, 3, 4-oxadiazoles as potential antimicrobial and anti- tubercularagents, Eur J Med Chem, 45, 2010, 2063–2074.
- Shiradkar M, Kumar GVS, Dasari V, Tatikonda S, Akula KC, Shah R, Clubbed triazoles: A novel approach to antitubercular drugs, Eur J Med Chem, 42, 2007, 807-816.
- Shiradkar MR, Murahari KK, Gangadasu HR, Suresh T, Kalyan CA, Panchal D, Kaur R, Burange P, Ghogare J, Mokale V, Raut M, Synthesis of new S-derivatives of clubbed triazolyl thiazole as anti- Mycobacterium tuberculosis agents, Bioorg & Med Chem Lett, 15, 2007, 3997-4008.
- Sun XY, Hua C, Deng XO, Wei CX, Sun ZG, Quan ZS. Synthesis and anti- inflammatory activity evaluation of some novel 6-alkoxy (phenoxy)-[1, 2, 4] triazole [3, 4- a] phthalazine-3-amine derivatives, Eur J Med Chem, 2010, 45, 4807-4812.
- Polya, J.B, Comprehensive Heterocyclic Chemistry, 1984: vol 5, Pergamon Press, P- 733.
- Kaplancikli, T.A, Turan-Zitouni, G, Chevallet, P. Synthesis and antituberculosis activity of new 3-alkylsulfanyl-1, 2, 4- triazole derivatives. J Enzyme Inhib Med Chem, 2005, 20,179–182.
- Upadhayaya, R.S, Kulkarni, G.M, Vasireddy, N.R, Vandavasi, J.K, Dixit, S.S, Sharma, V, Chattopadhyaya. J, Design, synthesis and biological evaluation of novel triazole, urea and thiourea derivatives of quinoline against Mycobacterium tuberculosis. Bioorg Med Chem, 2009, 17, 4681–4692.
- Jadhav, G.R, Shaikh, M.U, Kale, R.P, Shiradkar, M.R, Gill, C.H, SAR study of clubbed [1, 2, 4]-triazolyl with fluorobenzimidazoles as antimicrobial and antituberculosis agents. Eur J Med Chem, 2009, 44, 2930–2935.
- Shiradkar, M, Kumar, Suresh, Dasari, V, Tatikonda, S, Akula, K.C, Shah, R, Clubbed triazoles: a novel approach to antitubercular drugs. Eur J Med Chem, 2007, 42, 807–816.
- Sun, X. Y, Jin, Y. Z, Li, F. N, Li, G, Chai, K.Y, and Quan, Z. S, Synthesis of 8-alkoxy- 4,5-dihydro-[1, 2, 4] triazole [4, 3-a] quinoline-1-ones and evaluation of their anticonvulsant properties. Arch Pharm Res, 2006, 29, 1080–1085.
- Tehranchian S, Akbarzadeh T, Fazeli MR, Jamalifar H, Shafiee A, Synthesis and antibacterial activity of 1-[1,2,4-triazol-3-yl] and 1-[1,3,4-thiadiazol-2-yl]-3-methylthio- 6,7-dihydrobenzo[c]thiophen-4(5H) ones, Bioorg & Med Chem Lett, 15, 2005, 1023- 1025.
- Vishnumurthy, K.A,, Satyendra RV, Vagdevi HM, Rajesh KP, Manjunatha H, Shruthi A, Synthesis, in vitro antioxidant, anthelmintic and molecular docking studies of novel dichloro substituted benzoxazole- triazolo-thione derivatives. Eur J Med Chem, 2011, 46, 3078–3084.
- Vlahakis JZ, Lazar C, Crandall IE, Szarek, WA, Anti-Plasmodium activity of imidazolium and triazolium salts, Bioorg & Med Chem Lett, 18, 2010, 6184–6196.
- Wang, Z, Wu, B, Kuhen, K.L, Bursulaya, B, Nguyen, T.N, Nguyen, D.G, He Y, Synthesis and biological evaluations of sulfanyltriazoles as novel HIV-1 non- nucleoside reverse transcriptase inhibitors. Bioorg Med Chem Lett, 2006, 16, 4174– 4177.
- Xie ZF, Chai KY, Piao HR, Kwak KC, Quan ZS, 2005, Synthesis and anticonvulsant activity of 7-alkoxyl-4,5- dihydro-[1, 2, 4] triazolo[4, 3-a] quinolines, Bioorg & Med Chem Lett, 15, 4803-4805.
- Zhang J, Redman N, Litke AP, Zeng J, Zhan J, Chan KY, Chang, CT, Synthesis and antibacterial activity study of a novel class of cationic anthraquinone analogs, Bioorg & Med Chem, 19, 2011, 498–503.
- Zhu, Y, Olson, S.H, Graham, D, Phenylcyclobutyl triazoles as selective inhibitors of 11β- hydroxysteroid dehydrogenase type. Bioorg Med Chem Lett, 2008, 18, 3412–3416.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
