ISSN : ISSN: 2576-1455
Journal of Heart and Cardiovascular Research
Mechanisms of Sodium Hypochlorite-Induced Contraction of Rat Aorta: Potential Importance in Atherogenesis and Apoptotic Phenomena in Cardiovascular Diseases
Jian-Ping Liu1, Wenyan Li1, Bella T Altura1,3-5 and Burton M Altura1-5*
1Department of Physiology and Pharmacology, The State University of New York Downstate Medical Center, Brooklyn, NY, USA
2Department of Medicine, The State University of New York Downstate Medical Center, Brooklyn, NY, USA
3The Center for Cardiovascular and Muscle Research, The State University of New York Downstate Medical Center, Brooklyn, NY, USA
4School of Graduate Studies in Molecular and Cellular Science, The State University of New York, Downstate Medical Center, Brooklyn, NY, USA
5Bio-Defense Systems Inc., Rockville Center, NY, USA
- *Corresponding Author:
- Altura BM
Department of Physiology and Pharmacology
The State University of New York
Downstate Medical Center
Brooklyn, New York, USA
Tel: +718-270-2194
E-mail: baltura@downstate.edu
Received date: 06 June 2017; Accepted date: 01 August 2017; Published date: 7 August 2017
Citation: Liu JP, Li W, Altura BT, Altura BM (2017) Mechanisms of Sodium Hypochlorite Induced Contraction of Rat Aorta:Potential Importance in Atherogenesis and Apoptotic Phenomena in Cardiovascular Diseases. J Heart Cardiovasc Res. Vol. 1 No. 2: 107.
Copyright: © 2017 Liu JP, et al. This is an open-access article distributed under the terms of the creative Commons attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
The present study was designed to investigate the effects of sodium hypochlorite (NaOCl) on isolated rat aortic rings with and without endothelium. In the absence of any vasoactive agent, NaOCl alone elicited an endothelium-independent contraction in rat aortic rings in a concentration-dependent manner. NaOCl-induced contractions of intact rat aortic rings appeared, however, to be slightly stronger than those on denuded rat aortic rings. Our results demonstrate a stronger contractile effect of NaOCl on aortic segments of rats with increasing age and weight. The contractile responses to NaOCl were neither reversible nor reproducible in the same ring; even small concentrations of NaOCl resulted in tachyphylaxis. Removal of extracellular calcium ions (Ca2+) or buffering intracellular Ca2+ with 10 μM acetyl methyl ester of bis (o-aminophenoxy) ethane-N,N,N′,N′,-tetraacetic acid (BAPTA-AM) significantly attenuated the contractile actions of NaOCl. The presence of 1 μM staurosporine, 1 μM bisindolylmaleimide I, 1 μM Gö697{12-(2-Cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo{2,3-a] pyrrolo {3,4-c} carbazole, inhibitors of protein kinase C (PKC), 2 μM PD-098059 {2-(2-Amino-3-methoxyphenyl)-4H-1-benzopyran-4-one, inhibitor of extracellular signal-regulated kinases)}, 10 μM genistein (inhibitor of tyrosine kinase), and 1 μM wortmannin {an antagonist of phosphatidylinositol 3-kinases (PI3Ks)} completely inhibited the contractions induced by NaOCl. Several specific antagonists of possible endogenously- formed vasoconstrictors did not inhibit or attenuate NaOCl-induced contractions. Our new results suggest that NaOCl-triggered contractions on rat aortic rings are Ca2+-dependent. Several intracellular signal transduction systems seem to play roles in NaOCl-induced vasoconstriction of rat aortic rings.
Keywords
Sodium hypochlorite (NaOCl); Aortic ring; [Ca2+]i; Vascular smooth muscle; Signal transduction pathways; Aging
Introduction
Hypochlorite (OCl-) is one of the most aggressive oxidants of reactive oxygen species which can be produced by the myeloperoxidase (MPO)-H2O2-chloride system. During phagocytosis, neutrophils undergo a burst of respiration in which oxygen is reduced to superoxide anion (O2−), which dismutates to form H2O2. MPO is discharged from the cytoplasmic granules into the phagosome following particle ingestion. It is thought to utilize H2O2 to oxidize halides, which then react with and kill ingested microbes. The initial product of the MPO-H2O2-chloride system is hypochlorous acid, and the subsequent formation of chlorine, chloramines, hydroxyl radicals, singlet oxygen, and ozone have been proposed [1-4].
These same toxic agents can be released to the outside of cells, where they may attack normal tissues and cells, and thus contribute to the pathogenesis of disease [2,5]. It is known that hypochlorite plays important roles in both normal and pathological conditions. Reactive oxygen species are continuously generated in vivo, but an excessive level of these species is considered to be responsible for several pathophysiological processes, partly by affecting vascular tone and vessel wall interactions with leukocytes [6]. Under pathological conditions, hypochlorite may be formed at relatively high concentrations, and it has been documented that myeloperoxidase is a component of human atherosclerotic lesions [1]. The MPO-H2O2 system can cause chlorination of phospholipids [4].
A large body of studies on blood vessels has shown that reactive oxygen species can modulate vascular tone directly or indirectly leading to vasodilation or vasoconstriction [7-13]. Individual reactive oxygen species may exert different and sometimes complex actions on the tone of blood vessels via different mechanisms [8,10,12,14]. It has been suggested that hypochlorite can produce both relaxant and contractile actions in sheep isolated pulmonary artery rings, which may be dependent upon concentration [15]. However, as for the contractile action of hypochlorite, the available information is relatively scarce and imprecise.
Up to now, the exact mechanism(s) by which NaOCl exerts its vascular effects still remain to be elucidated. It is possible that the NaOCl-induced vascular actions are mediated via second messenger systems. Previous studies from our lab have shown that protein kinase C, mitogen-activated protein kinase, and nonreceptor tyrosine kinases play essential roles in H2O2-, hydroxyl radical- and peroxynitrite-triggered signal transduction pathways in vascular smooth muscle cells [8,9,12,16,17]. It is, however, unclear whether NaOCl acts on vascular tone via similar or different mechanisms. Therefore, the current study was undertaken to investigate the effects of NaOCl on isolated ring segments of rat aorta and to gain insight into the underlying mechanisms of its action, with special attention given to potential signal transduction mediators generated by NaOCl.
Materials and Methods
This is a descriptive study with retrospective data analysis.
General procedures
Male adult Wistar rats (200 g-470 g) were sacrificed by stunning and subsequent decapitation. The aortas were isolated according to previously established methods. The vessels for the ring segments were carefully excised, cleaned, and the tissues cut into 3- to 4 mm segments. For intact tissue preparation, extreme care was taken to avoid damage of endothelial cells. For denuded arteries, the intima of the vessels were removed by gently rubbing against the teeth of a pair of forceps [18]. The tissues were placed in normal Krebs- Ringer bicarbonate (KR buffer) solution containing (in mM): NaCl 118, KCl 4.7, KH2PO4 1.2, MgSO4 1.2, CaCl2 2.5, dextrose 10 and NaHCO3 25 [19,20]. The segments were mounted on stainless-steel pins under 2 g resting isometric tension in organ baths, attached to force transducers (Grass Model FT 03) and connected to Grass Model 7 polygraphs. The organ baths, containing KR buffer, were gassed continuously with 95% O2 and 5% CO2 and warmed to 37°C (pH 7.4). Tissues were allowed to equilibrate for at least 90 min before data collection. Incubation media were routinely changed every 15 min as a precaution against interfering metabolites [21]. At the beginning of an experiment, rings were exposed for 30-45 min to 80 mM KCl and this was repeated every 30-45 min, until responses were stable (two to three times). Successful removal of endothelium was assessed by showing that acetylcholine (10-8 to 10-6 M) failed to relax the denuded segments. Segments precontracted by 5×10-7 M phenylephrine did relax 90% in response to acetylcholine in endothelium-intact segments [22,23]. Responses to NaOCl and other drugs were expressed as a percentage of the stable level of contraction induced by 80 mM KCl. All of the experiments and protocols in this study were approved by the Animal Use and Care Committee at our institution.
Extracellular calcium (Ca2+) and intracellular Ca2+ experiments
For the extracellular Ca2+-free experiments, the rat aortic ring segments were equilibrated in Ca2+-free KR buffer containing 0.2 mM EGTA for at least 90 min before initiation of the experiments [24]. For intracellular Ca2+-buffered experiments, the acetyl methyl ester of bis (o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid (BAPTA-AM) (a membrane permeable Ca2+ chelator) was added to the bath medium, at a final bath concentration of 10 μM BAPTA-AM [24]. After obtaining stable conditions for the ring segments, experiments were begun [15,12,24].
Reagents
The following pharmacological agents were purchased from Sigma (St. Louis, MO): NaOCl, bisindolylmaleimide I HCl, acetylcholine HCl, phenylephrine HCl, N,N-tetraacetic acid (EGTA), indomethacin, propranolol HCl, naloxone HCl, atropine sulfate, H2O2, phentolamine methanesulfonate, staurosporine, and genistein. 1, 2-Bis-(O-aminophenoxy) ethane-N, N, N', N'- tetraacetic acid acetoxymethyl ester (BAPTA/AM) was purchased from Molecular Probes (Eugene, OR). Dimethyl sulfoxide (DMSO), Gö6976 (12-(2-Cyanoethyl)-6,7,12,13- tetrahydro-13-methyl-5-oxo-5H-indolo{2,3-a}pyrrolo{3,4-c} carbazole), PD-098059 {2-(2-Amino-3-methoxyphenyl)-4H-1- benzopyran-4-one} and wortmannin were purchased from CALBIOCHEM (La Jolla, CA, USA). All other organic and inorganic chemicals were obtained from Fisher Scientific (Fair Lawn, NJ, USA) and were of the highest purity.
Results
Rat age, body weight and NaOCl-induced vasoconstriction of intact aortic rings
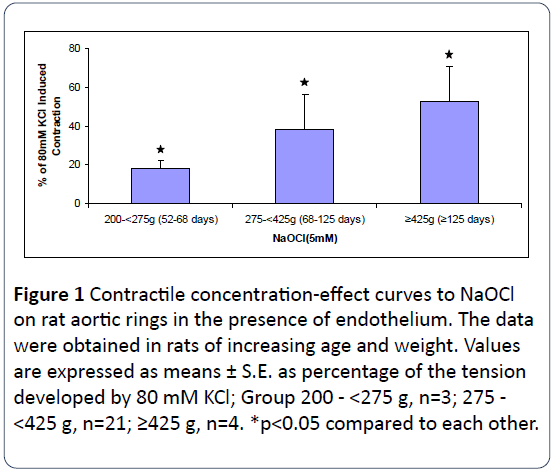
To examine for a possible role of age and body weight in NaOCl-induced vasoconstriction of intact aortic rings, experiments were carried out in rats of different ages and weights. As shown in Figure 1, we observed a stronger contractile effect of NaOCl on aortic segments with increasing age and weight of the rats. These results suggest that the contractile responses of the vessels to NaOCl are associated with both age and body weight. This was not observed for KClinduced contractions.
Figure 1: Contractile concentration-effect curves to NaOCl on rat aortic rings in the presence of endothelium. The data were obtained in rats of increasing age and weight. Values are expressed as means ± S.E. as percentage of the tension developed by 80 mM KCl; Group 200 - <275 g, n=3; 275 - <425 g, n=21; ≥425 g, n=4. *p<0.05 compared to each other.
Contractile effects of NaOCl on rat aortic rings
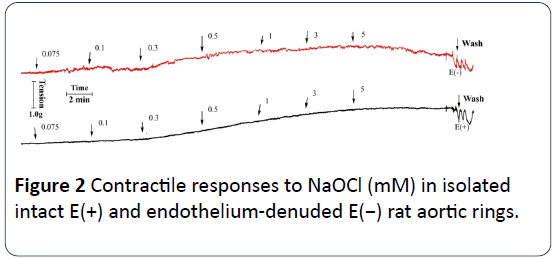
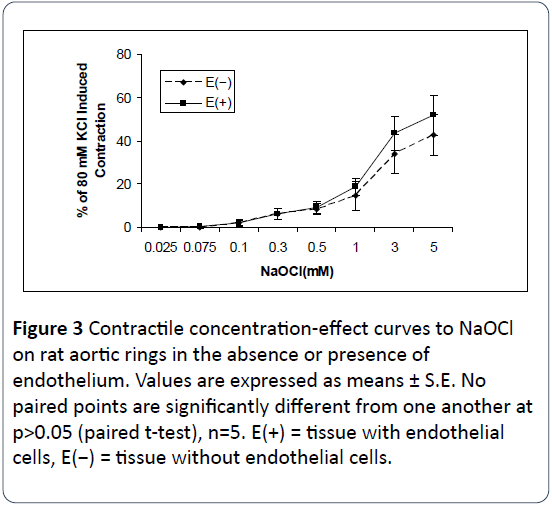
In the absence of any vasoactive agent, NaOCl alone induced contractile effects on rat aortic rings. Figure 2 shows a typical tracing of the vasoconstrictor effects of NaOCl on rat aortic rings with and without endothelium. As shown in Figures 2 and 3, NaOCl elicits concentration-dependent contractions of most rat aortic rings at concentrations of from 75 μM to 5 mM. However, reproducible threshold contractile responses were obtained up to 100 μM NaOCl. In addition, a comparison of the concentration-response curves with and without endothelium demonstrates that most of the contractile effects exerted by NaOCl on rat aortic rings are endothelium-independent (Figure 3). NaOCl-induced contractions of intact rat aortic rings appeared to be slightly stronger than those on denuded rat aortic rings at the highest concentration, but no paired points are significantly different from one another. It should be noted, here, that the vasoconstrictor effects of NaOCl on rat aortic rings were neither reversible nor reproducible on the same ring. Although not shown, preliminary experiments using the trypan blue exclusion assays indicate that contractions of hypochlorite greater than 100μM cause cytotoxicity to primary aortic smooth muscle cells in culture.
Figure 3: Contractile concentration-effect curves to NaOCl on rat aortic rings in the absence or presence of endothelium. Values are expressed as means ± S.E. No paired points are significantly different from one another at p>0.05 (paired t-test), n=5. E(+) = tissue with endothelial cells, E(−) = tissue without endothelial cells.
Effects of extra-and intracellular calcium on NaOCl-induced vasoconstriction of intact rat aortic rings
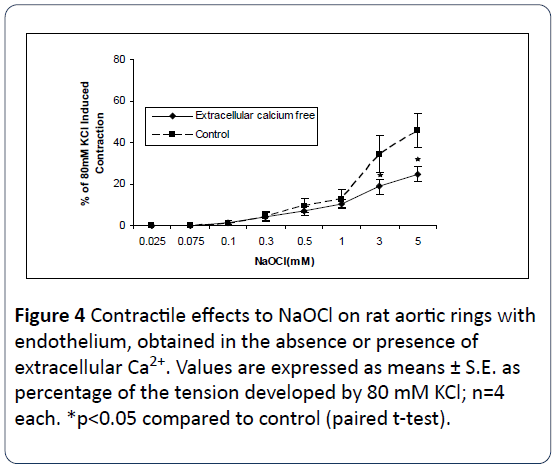
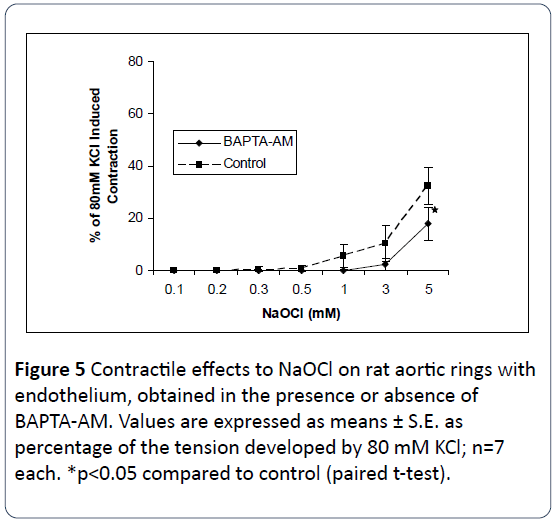
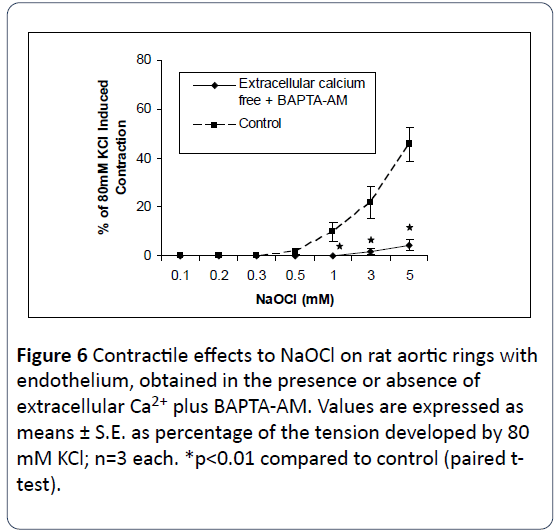
The relationship between NaOCl-induced contraction and extra- and intracellular Ca2+ was next examined in this study. As shown in Figures 4 and 5 removal of either extracellular Ca2+ from the bathing medium, or use of buffered intracellular Ca2+ by preincubation of the tissues in 10 μM BAPTA-AM, results in significant attenuation of NaOCl-induced contractions. As shown in Figure 6, incubation in [Ca2+]o-free medium together with use of BAPTA-AM, produced significant and almost complete inhibition of the contractile effects of NaOCl. These results suggest the NaOCl-induced contractions on rat aortic rings are dependent upon both extracellular- and intracellular calcium.
Effects of protein kinase C (PKC) antagonists on NaOCl-induced vasoconstriction of intact rat aortic rings
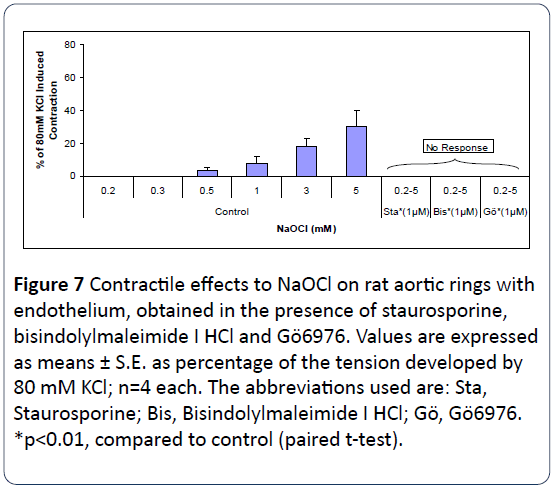
Figure 7 shows that pretreatment of intact rat aortic rings with staurosporine, bisindolylmaleimide I HCl (both potent antagonists of PKC), or Gö6976 (a PKCα-and PKCβ1-selective antagonist), for 20 min, completely inhibited NaOCl-induced vasoconstrictions. These three PKC antagonists were all dissolved in DMSO. The DSMO concentrations applied in these experiments were <1 μM for staurosporine and bisindolylmaleimide I HCl, and around 7.5 μM for Gö6976. In control experiments, equivalent amounts of DMSO were employed and exerted no effects on NaOCl-induced vasoconstriction (n=4 each, data not shown).
Figure 7: Contractile effects to NaOCl on rat aortic rings with endothelium, obtained in the presence of staurosporine, bisindolylmaleimide I HCl and Gö6976. Values are expressed as means ± S.E. as percentage of the tension developed by 80 mM KCl; n=4 each. The abbreviations used are: Sta, Staurosporine; Bis, Bisindolylmaleimide I HCl; Gö, Gö6976. *p<0.01, compared to control (paired t-test).
Effects of PD-098059, genistein and wortmannin on NaOCl-induced vasoconstriction of intact rat aortic rings
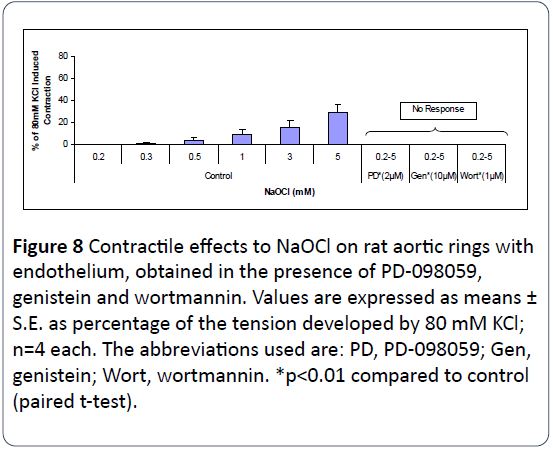
Figure 8 illustrates that pretreatment of intact rat aortic rings with PD-098059 (an antagonist of mitogen-activated protein kinase), genistein (an antagonist of protein tyrosine kinase) or wortmannin {an antagonist of phosphatidylinositol 3-kinases (PI3Ks)} for 20 min completely inhibited NaOClinduced vasoconstrictions of rat aortic rings. The DSMO concentrations applied in these experiments were <1 μM. In control experiments, equivalent amounts of DMSO were employed and exerted no effects on NaOCl-induced vasoconstriction (n=4 each, data not shown).
Figure 8: Contractile effects to NaOCl on rat aortic rings with endothelium, obtained in the presence of PD-098059, genistein and wortmannin. Values are expressed as means ± S.E. as percentage of the tension developed by 80 mM KCl; n=4 each. The abbreviations used are: PD, PD-098059; Gen, genistein; Wort, wortmannin. *p<0.01 compared to control (paired t-test).
Variety of pharmacologic antagonists fail to attenuate or inhibit the contractile action of NaOCl on rat aortic rings
Incubation of intact rat aortic rings with a wide variety of specific amine, opiate and prostanoid antagonists (i.e., 10-6 M diphenhydramine, 10-5 M cimetidine, 10-6 M phentolamine, 10-6 M methysergide, 10-5 M propranolol, 10-6 M atropine, 10-5 M naloxone and, 10-5 M indomethacin) failed to either attenuate or interfere with contractions induced by NaOCl (data not shown, n=4-6 each).
Discussion
Reactive oxygen species are known to be involved in the progression of various cardiovascular diseases. One source of reactive oxygen species is activated neutrophils, which can release superoxide anion radicals and hydrogen peroxide by membrane-bound NADPH oxidases. These reactive oxygen species not only destroy bacteria, but may also affect mammalian tissues. In addition, hydrogen peroxide serves as a substrate for myeloperoxidase, an enzyme that is released by activated neutrophils during inflammatory processes, as seen, for instance, in reperfusion injury and atherosclerosis [24]. Myeloperoxidase catalyzes the oxidation of chloride by hydrogen peroxide, yielding hypochlorite, an extremely potent oxidant [24], and the local concentration of HOCl potentially exceeds 100 μM/l under pathological conditions in vivo [16,25-27].
The hypochlorite concentrations in our studies could be considered as biologically/pathologically-relevant to some diseases such as atherosclerosis and inflammation in vivo.
The present study demonstrates that NaOCl produces vasoconstriction of isolated rat aortic rings in a concentrationdependent manner. The fact that almost full contractile actions on the rat aortic rings are observed, after removal of the endothelium, indicates that most, if not all, of the NaOClinduced contractile are independent of endothelial cellderived mediators. Our findings indicate that NaOCl exerts a stronger contractile action on aortic segments obtained from older and heavier rats. Such data suggests that aortic smooth muscle cells become more and more sensitive to the contractile (and possibly) destructive actions of OCl- with age. This could support the notion that the aging process predisposes the vasculature to pathological actions of oxidants, such as hypertension, atherogenesis, heart diseases, inflammatory conditions, and strokes. The current work also demonstrates that the NaOCl-produced contractions are not reproducible and elicit tachyphylaxis in any one ring (with or without endothelium). What is of singular interest, here, is that even challenge sometimes with threshold levels of NaOCl results in tachyphylaxis. This indicates that the contractile actions of NaOCl are not reversible and probably lead to permanent vascular damage at low levels of hypochlorite. This vascular damage may, in part, be due to apoptotic phenomena as preliminary studies in our lab indicate that concentrations of NaOCl >100 μM cause apoptosis (e.g., activation of several caspase enzymes) in primary cultured rat aortic smooth muscle cells and cerebral vascular smooth muscle cells, similar to that we reported for peroxynitrite, H2O2 and hydroxyl radicals [17,18]. This is currently under investigation in our lab.
At present, the underlying mechanisms whereby NaOCl triggers vasoconstriction are poorly understood. The current work was designed to better define the effects of NaOCl on rat aortic rings and to probe the relationships between contractility of vessels induced by generation of OCl-, Ca2+ and possible signaling pathways. It was also of interest to design the current study in a way to be able to compare potential mechanisms of action of NaOCl, H2O2 and hydroxyl radicals on rat aortic smooth muscle.
It has been well documented that cytosolic calcium increases during contraction of vascular smooth muscles either as a consequence of influx of Ca2+ from the extracellular space or release of Ca2+ from intracellular stores. Conversely, relaxation is usually accompanied by a decrease in the cytosolic Ca2+ level in smooth muscle cells [28]. In this study, removal of extracellular Ca2+ as well as buffering intracellular Ca2+ with the membrane-permeable Ca2+ chelator BAPTA-AM both inhibited most of the contractile actions of NaOCl on rat aortic rings. This suggests that the passage of extracellular calcium ions across the vascular smooth membranes as well as a release of intracellular free calcium ions from intracellular stores probably play essential roles in NaOCl-induced contractile effects. This is in agreement with a previous conclusion that hydroxyl radicals can enhance the voltagedependent influx of Ca2+ in rabbit lingual smooth muscle cells [29] and compares favorably to the actions of H2O2 on rat aortic rings [12] and cerebral arterial smooth muscle cells [14] as well as hydroxyl radicals in rat aortic rings [16].
Multiple cellular signal pathways are known to participate in mechanisms of vasoconstriction, such as activation of protein kinase C (PKC), protein tyrosine kinase, mitogen-activated protein kinase (MAPK), and PI3Ks [28]. Vascular smooth muscle cells after exposure to NaOCl may result in release of a variety of vasoactive substances that in turn modulate vascular tone. To assess the involvement of such possible substances in NaOCl-induced contraction, several specific pharmacological inhibitors as well signaling pathway inhibitors were employed in the present study. A variety of pharamacologic amine antagonists, an opiate antagonist and a Cox-I inhibitor failed to inhibit the contractile action of NaOCl, suggesting that endogenous formation or release of catecholamines, histamine, serotonin, acetylcholine, opiates, or prostanoids do not play any role in NaOCl-induced contractile actions.
PKC, a family of Ca2+-sensitive and Ca2+-insensitive phospholipid-dependent protein kinases, that are present in high concentrations in vascular smooth muscle, have been shown to play important roles in cellular signal transduction [20,28,30,31]. However, up until the present study, no experimental evidence has appeared on the possible role of PKC in NaOCl-induced contractile effects on rat aortic rings. In the present work, NaOCl-induced vasoconstrictions were found to be completely inhibited in the presence of two potent PKC antagonists (bisindolylmaleimide I and staurosporine) and one selective PKCα and PKCâ1 antagonist (Gö6976). This suggests that PKC isoforms, probably PKCα (possibly PKCβ1 as well) may be a pivotal signaling mechanism by which NaOCl acts on the aortic smooth muscle cells to promote vasoconstriction. This is, thus, similar to that found for H2O2 in rat aortic rings and cerebral arteries [12,14] and hydroxyl radicals in rat aortic rings [16].
It has been reported that PI3Ks are a group of enzymes that act directly to provide biochemical links between a novel phosphatidylinositol pathway and nonreceptor tyrosine kinases that appear to modulate Ca2+ channels through activity of these PI3Ks [32]. Up to now, there has been no information regarding PI3Ks and NaOCl-induced vasoconstriction on any vessel type. In this paper, we report that one potent antagonist of PI3Ks (wortmannin) completely attenuated NaOCl-induced contractions, suggesting the possible involvement of products of PI3Ks in such responses. Recently, H2O2-induced contractions [12,14,33] and hydroxyl radical-induced contractions [16] were found to be somewhat dependent on PI3Ks as well.
Previous studies have shown that protein tyrosine phosphorylation is an essential component in signal transduction pathways in smooth muscle cells [34,35]. Our present data also demonstrates that genistein, an inhibitor of protein tyrosine kinase [35], which is an enzyme for catalyzing protein tyrosine phosphorylation, completely suppressed the NaOCl-induced vasoconstriction, which indicates the likely involvement of protein tyrosine phosphorylation in contractions caused by NaOCl. This conclusion is supported by other investigators who have demonstrated that oxidative stress can promote protein tyrosine phosphorylation in smooth muscle cells [12,13,16,36].
Several studies have been reported which indicate MAPK responds to diverse stimuli, including reactive oxygen species, and can transduce signals from the cell membrane to the nucleus [13,36-41]. Activation of MAPK plays an important role in modulating vascular tone [13,41]. In this investigation, the specific MAPK antagonist, PD-098059 [37-43], significantly prevented NaOCl-induced contractions in rat aortic rings. This suggests that activation of MAPK is probably an important pathway in NaOCl-mediated action on rat aortic smooth muscle cells.
Conclusion
In summary, the data from the present investigation suggest that NaOCl induces contractile actions on rat aortic rings in concentrations of from 75 μM to 5 mM, but not relaxation on rat aortic rings under resting tension. These NaOCl-induced contractions are mediated via the activation of several wellknown signal transduction pathways, and elevation of intracellular free Ca2+ levels, and are independent of the endothelium. Production of, and contact, of at least rat aorta with this most highly-reactive oxygen species, even in low concentration, appears to result in irreversible damage of the aortic smooth muscle cells. Such findings are thus quite different from those observed for H2O2, O2.−, or peroxynitrite [8,10,12,14,37-43] but resemble those obtained for hydroxyl radicals [16] and requires further investigation. Lastly, if our new findings hold for the peripheral microvasculature, they could be suggestive of a very important role for generation of hypochlorite in regulation of the microvasculature and its pathophysiology, particularly in inflammatory processes, atherogenesis, coronary heart diseases and the aging process.
Acknowledgement
The work described herein was supported in part by a NIH Research Grants (National Heart, Lung and Blood Institute; National Institute on Alcoholism and Alcohol Abuse. National Institute on Mental Health) to B.M.A. and B.T.A.
References
- Daugherty A, Dunn JL, Raten DL, Heinecke JW (1994) Myeloperoxidase, a Catalyst for Lipoprotein Oxidation, is Expressed in Human Atherosclerotic Lesions. J Clin Invest 94: 437-444.
- Klebanoff SJ (2005) Myeloperoxidase: Friend and Foe. J Leukoc Biol 77: 598-625.
- Schultz J, Kaminker K (1962) Myeloperoxidase of the Leukocyte of Normal Human Blood. i. Content and Localization. Arch Biochem Biophys 96: 465-467.
- Winterbourn CC, Vandenberg JJM, Roitman E, Kuypers FA (1992) Chlorohydrin Formation from Unsaturated Fatty-acids Reacted with Hypochlorous ACID. Arch Biochem Biophys 296: 547-555.
- Reeves EP, Nagl M, Godovac-Zimmermann J, Segal AW (2003) Reassessment of the Microbicidal Activity of Reactive Oxygen Species and Hypochlorous Acid with Reference to the Phagocytic Vacuole of the Neutrophil Granulocyte. J Med Microbiol 52: 643-651.
- Fattman CL, Schaefer LM, Oury TD (2003) Extracellular Superoxide Dismutase in Biology and Medicine. Free Radic Biol Med 35: 236-256.
- Karas C (1999) Increased Activity of H2O2 in Aorta Isolated from Chronically Streptozotocin-Diabetic Rats: Effects of Antioxidant Enzymes and Enzymes Inhibitors. Free Radic Biol Med 27: 16-27.
- Li J, Li W, Altura BT, Altura BM (2004a) Peroxynitrite- Induced Relaxation in Isolated Canine Cerebral Arteries and Mechanisms Of Action. Toxicol Appl Pharmacol 196: 176-182.
- Li J, Li W, Altura BT, Altura BM (2005) Peroxynitrite- Induced Relaxation in Isolated Rat Aortic Rings and Mechanisms of Action. Toxicol Appl Pharmacol 209: 269-276.
- Rubanyi JR (1988) Vascular Effects of Oxygen-Derived Free Radicals. Free Radic Biol Med 4: 107-120.
- Silveir LR, Pereira-Da-Silva L, Juel C, Hellsten Y (2003) Formation of Hydrogen Peroxide and Nitric Oxide in Rat Skeletal Muscle Cells During Contractions. Free Radic Biol Med 35: 455-464.
- Yang ZW, Zheng T, Zhang A, Altura BT, Altura BM (1998) Mechanisms of Hydrogen Peroxide-Induced Contraction of Rat Aorta. Eur J Pharmacol 344: 169-181.
- Yang ZW, Wang J, Zheng T, Altura BT, Altura BM (2001b) Ethanol-Induced Contractions in Cerebral Arteries: Role Of Tyrosine and Mitogen-Activated Protein Kinases. Stroke 32: 249-257.
- Yang ZW, Zheng T, Wang J, Zhang A, Altura BT, et al. (1999) Hydrogen Peroxide Induces Contraction AND Raises [ca2+]i in Canine Cerebral Arterial Smooth Muscle: Participation of Cellular Signaling Pathways. Naunyn-Schmiedeberg's Arch Pharmacol 360: 646-653.
- Whitney G, Throckmorton G, Isales C, Takuwa Y, Yeh J, et al. (1995) Kinase Activation and Smooth Muscle Contraction in the Presence and Absence of Calcium. J Vasc Surg 22: 37-44.
- Li J, Li W, Altura BT, Altura BM (2004b) Mechanisms of Hydroxyl Radical-Induced Contraction of Rat Aorta. Eur J Pharmacol 499: 171-178.
- Jenner AM, Ruiz JE, Dunster C, Halliwell B, Mann GE, et al. (2002) Vitamin C Protects Against Hypochlorous Acid-Induced Glutathione Depletion and Dna Base and Protein Damage in Human Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol 22: 574-580.
- Zhang AM, Cheng TP-O, Altura BM (1992b) Magnesium Regulates Intracellular Free Ionized Calcium Concentration and Cell Geometry in Vascular Smooth Muscle Cells. Biochim Biophys Acta 1134: 25-29.
- Altura BT, Altura BM (1980) Withdrawal of Magnesium Causes Vasospasm While Elevated Magnesium Produces Relaxation of Tone in Cerebral Arteries. Neurosci Lett 20: 323-327.
- Zhang AM, Altura BT, Altura BM (1992a) Endothelial-Dependent Sexual Dimorphism in Vascular Smooth Muscle: Role of Mg2+ and Na+. Br J Pharmacol 105: 305-310.
- Altura BM, Altura BT (1970) Differential Effects of Substrate Depletion on Drug-Induced Contractions of Rabbit Aorta. Am J Physiol 219: 1698-1705.
- Turan NN (2000) Hypochlorous Acid-induced Responses in Sheep Isolated Pulmonary Artery Rings. Pharmacol Res 41: 589-596.
- Zheng T, Li W, Zhang A, Altura BT, Altura BM (1998) Alpha-tocopherol Prevents Ethanol-Induced Elevation of [Ca2+]i in Cultured Canine Cerebral Vascular Smooth Muscle Cells. Neurosci Lett 245: 17-20.
- Sand C, Peters SL, Pfaffendorf M, van Zweiten PA (2003) Effects of Hypochlorite and Hydrogen Peroxide on Cardiac Autonomic Receptors and Vascular Endothelial Function. Clin Exp Pharmacol Physiol 30: 249-253.
- Baker MS, Rice Evans CA, Burdon RH (1992) Free Radical Damage and Its Control. Elsevier, New York; pp. 301-317.
- Fukai K, Kaneda M, Takahashi E, Washio M, Doi K (1994) Protective Effects of Sulfhydryl Compounds on HOCl-induced Intracellular Calcium Increase in Single Rat Ventricular Myocytes. J Mol Cell Cardiol 26: 455-461.
- Schraufstatter IU, Browne K, Harrisa A, Hyslop PA, Jackson JH, et al. (1990) Mechanisms of Hypochlorite Injury of Target Cells. J Clin Invest 85: 554-562.
- Horowitz A, Menice CB, Laporte R, Morgan KG (1996) Mechanisms of Smooth Muscle Contraction. Physiol Rev 76: 967-1003.
- Sasaki H, Okabe E (1993) Modification By Hydroxyl Radicals of Functional Reactivity in Rabbit Lingual Artery. Jpn J Pharmacol 62: 305-314.
- Kariya K, Takai Y (1987) Distinct Functions Of Down-Regulation-Sensitive and-Resistant Types of Protein Kinase C in Rabbit Aortic Smooth Muscle Cells. FEBS Lett 219: 119-124.
- Blair LA, Marshall J (1997) IGF-1 Modulates N and L Calcium Channels in a PI 3-Kinase-Dependent Manner. Neuron 19: 421-9.
- Yang ZW, Wang J, Zheng T, Altura BT, Altura BM (2001a) Importance of PKC and PI3Ks in Ethanol-Induced Contraction of Cerebral Arterial Smooth Muscle. Am J Physiol Heart Circ Physiol 280: H2144-H2152.
- Albrecht DE, Tidball JG (1997) Platelet-Derived Growth Factor-Stimulated Secretion of Basement Membrane Proteins by Skeletal Muscle Occurs by Tyrosine Kinase-Dependent and -Independent Pathways. J Biol Chem 272: 2236-2244.
- Malarkey K, Belham CM, Paul A, Graham A, McLees A, et al. (1995) The Regulation of Tyrosine Kinase Signalling Pathways by Growth Factor and G-Protein-Coupled Receptors. Biochem J 309: 361-375.
- Baas AS, Berk BC (1995) Differential Activation of Mitogen-Activated Protein Kinases by H2O2 And O2− in Vascular Smooth Muscle Cells. Circ Res 77: 29-36.
- Blumer KJ, Johnson GL, Lange Carter CA (1994) Mammalian Mitogen-Activated Protein Kinase Kinase Kinase (MEKK) can Function in a Yeast Mitogen-Activated Protein Kinase Pathway Downstream of Protein Kinase C. Proc Natl Acad Sci U.S.A 91: 4925-9.
- Li J, Li W, Su J, Liu W, Altura BT, et al. (2003) Hydrogen Peroxide Induces Apoptosis in Cerebral Vascular Smooth Muscle Cells: Possible Relation to Neurodegenerative Diseases And Strokes. Brain Res Bull 62: 101-6.
- Post GR, Brown JH (1996) G Protein-Coupled Receptors and Signaling Pathways Regulating Growth Responses. FASEB J 10: 741-9.
- Touyz RM, Yao G (2003) Modulation of Vascular Smooth Muscle Cell Growth by Magnesium-Role of Mitogen-Activated Protein Kinases. J Cell Physiol 197: 326-335.
- Li M, Mossman BT, Kolpa E, Timblin CR, Shukla A, et al. (2003) Age-related Differences in MAP Kinase Activity in VSMC in Response to Glucose or TNF-alpha. J Cell Physiol 197: 418-425.
- Yang ZW, Wang J, Zheng T, Altura BT, Altura BM (2000) Low [Mg2+]o Induces Contraction and [Ca2+]i Rises in Cerebral Arteries: Roles of Tyrosine and Mitogen-Activated Protein Kinases. Am J Physiol Heart Circ Physiol 279: H185-H194.
- Rao GN (1997) Protein Tyrosine Kinase Activity is Required for Oxidant-Induced Extracellular Signal-Regulated Protein Kinase Activation and c-Fos and c-Jun Expression. Cell Signal 9: 181-7.
- Shen JZ, Zheng XF, Kwan CY (2000) Differential Contractile Actions of Reactive Oxygen Species on Rat Aorta: Selective Activation of ATP Receptor by H2O2. Life Sci 66: PL291-296.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences