Zika-Induced Microcephaly and Neurodevelopment
Reproduction and Developmental Sciences DBMS, Queen’s University Kingston, Canada
- *Corresponding Author:
- Dr. Sidra Shafique, PhD
Reproduction and Developmental Sciences DBMS
Queen’s University Kingston, Canada
Tel: 16474033520
E-mail: s.shafique@queensu.ca
Received date: December 28, 2017; Accepted date: January 06, 2018; Published date: January 15, 2018
Citation: Shafique S (2018) Zika-Induced Microcephaly and Neurodevelopment. Res J Congenit Disease Vol. 1 No. 1:3.
Copyright: © 2018 Shafique S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Zika virus [ZV] infection in pregnancy causes microcephaly in the developing fetus. The cause and effect relationship is well established through human case studies and experimental models. The fetal neural tissue development is markedly disrupted by the ZV with the most significant effect on the developing grey matter of the brain tissue via the infection of neural progenitor cells and widespread apoptosis. ZV infects undifferentiated precursor neuronal cells that are rapidly undergoing mitosis, thus making it highly likely to perturb mitotic spindle and centromere related genes and proteins. The infected cells fail to differentiate into functional neurons and undergo apoptosis. Reviewing the literature informs that ZV infects the undifferentiated neural stem cells and the cells expressing the AXL receptors. The focused gene expression studies in ZV infected cells may help to identify the perturbed genes and the mechanisms of apoptosis of the selected cell population as well as the pathology of microcephaly itself.
The purpose of this review is to understand the most vulnerable cell type susceptible to ZV infection in context with the changes in human ZV-induced microcephalic brain tissue morphology, mouse model and in vitro spheroids and organoids studies. Here we discuss the human brain development and correlate it with the experimental evidence of the ZV effects on the neuronal development in vivo and in vitro.
Keywords
Zika virus; Infection; Neural stem cells
Structure and Transmission of Zika Virus
Zika virus was found in the forests of Uganda for the first time, in 1947, and remained without any major outbreaks until 2015-2016 epidemic of Brazil [1,2]. Otherwise, the prevalence of ZV has also been documented in China, South Asia and Africa [3]. Zika virus [ZV] is a flavivirus related to the Family of Flaviviridae and Genus of Flavivirus, Arboviruses [4]. Through various phylogenetic studies, the diverse strains of ZV have been identified including Asian and African lineages [5]. Asian lineage strain was predominantly involved in 2007 Yap island sporadic epidemics and 2013-2014 French Polynesia spread while the African lineage strain is the one identified in Americas [5,6]. The unique ability of Zika to survive at higher temperatures as compared to other flaviviruses and its spread by the mosquito vector, Anopheles Aegypti have played the key role in its rapid spread in Brazil and South America [7].
ZV has a similar structure as of Dengue [DENV] and West Nile [WNV] viruses of Flaviviridae Family [4]. It consists of positive sense RNA genome core surrounded by a capsid and an outermost shell consisting of the envelop proteins which act as antigens to stimulate immune respone by the host [8]. The virus infects the cells and its RNA genome is translated into a long polyprotein strand containing three structural and seven nonstructural proteins [8]. The outer envelope proteins (E), precursor membrane (prM) and capsid (C) proteins are the structural proteins. The E protein is antigenic and generates the immune response by the host immune system [9]. The nonstructural proteins are mostly involved with immune evasion, replication and infectivity of the virus [10].
The spectrum of ZV infection symptoms include asymptomatic infection, rash and fever and in complicated cases, the neurological condition, Guillain-Barre (GB) syndrome [11,12]. The virus infects the male reproductive organs, is present in the semen and may result in male infertility via infecting the spermatogonia [13]. The infected semen is proposed to cause sexual transmission of ZV [13]. By now, it has been identified as a teratogenic virus being vertically transmitted from infected mother to developing fetus through the placenta [14]. In cases of pregnancies complicated with ZV infection, the fetal complications may include fetal loss, growth retardation and microcephaly [15]. Thus, the ZV transmission and teratogenicity have strong devastating effects on the health of future human generations.
ZV has been related to a full range of pathological conditions in the developing fetus referred to as Congenital Zika Syndrome (CZS). These abnormalities include microcephaly, decreased brain tissue, damage to the back of the eye, joints with limited movement and increased muscle tone [16]. Microcephalic babies are identified having head circumference less than 32 cm or below the third centile [17,18]. The association between Zika Virus (ZV) and the microcephaly has been established with the evidence of 20-fold increase in incidence of microcephaly during 2015-2016 ZV outbreak in Brazil and the presence of ZV in the affected fetal brain [19]. As a whole, all signs and symptoms of CZV stem from the damage to the developing brain tissue in the fetus. Out of all CZV abnormalities, the microcephaly has been most extensively studied till date and the cause-effect relationship has been effectively established between ZV and the microcephaly through case reports and mouse model experiments [19-21].
Neurodevelopment and Microcephaly
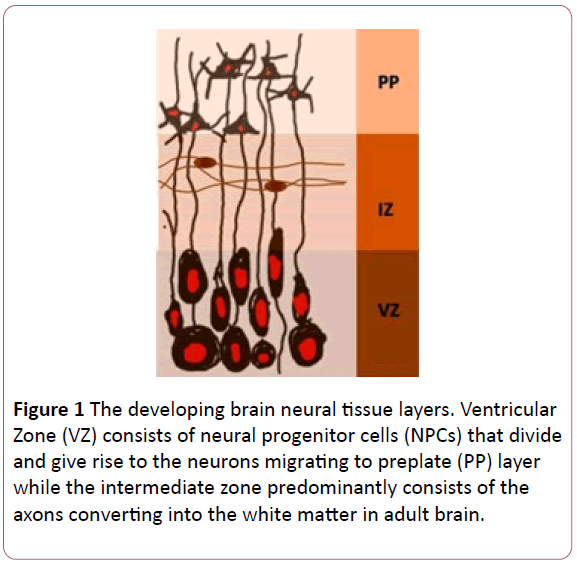
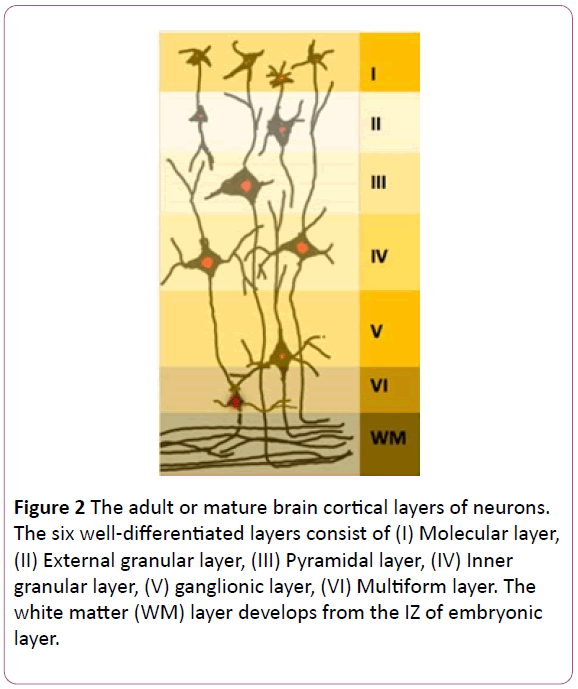
The embryonic period in humans extends upto eighth gestational week. The primitive structure of developing brain is established by the end of the embryonic period in human development. The first definitive neural structure, the neural tube, develops between Embryonic Day 20 to 27. During the process of neural tube development, the neural progenitor cells are differentiated from ectoderm, lined up in the midline and form the neural plate. The neural plate contains neural progenitor cells that line the developing neural tube. The anterior or rostral end of neural tube develops into brain while the posterior or caudal end of neural tube forms spinal cord. The neural progenitor cells lining the neural tube are called ventricular zone [VZ] neurons which divide through symmetrical division increasing the population of neural progenitor cells themselves. The differentiating cells migrate to the developing neural layers intermediate zone [IZ] and preplate [PP] (Figure 1). The neural progenitor cells in VZ are the precursors of all the six adult cortical layers (Figure 2). During embryonic development, the neurons undergo extensive synaptogenesis and synaptic pruning phases ultimately forming the set number of neurons and synapses at the time of birth. The gross morphological changes in the developing fetal brain reflect the underlying cellular proliferation and differentiation. In summary, the normal brain development depends on fine orchestration among cell division, differentiation and organization into definitive cortical layers. The gross fetal brain size grows in proportion to the size of the mitotic and differentiating cell population [22].
Figure 1: The developing brain neural tissue layers. Ventricular Zone (VZ) consists of neural progenitor cells (NPCs) that divide and give rise to the neurons migrating to preplate (PP) layer while the intermediate zone predominantly consists of the axons converting into the white matter in adult brain.
Figure 2: The adult or mature brain cortical layers of neurons. The six well-differentiated layers consist of (I) Molecular layer, (II) External granular layer, (III) Pyramidal layer, (IV) Inner granular layer, (V) ganglionic layer, (VI) Multiform layer. The white matter (WM) layer develops from the IZ of embryonic layer.
Microcephaly or the small brain is characterized by reduced brain tissue that can be primary and secondary as of its causation [18]. Primary microcephaly is defined as the morphological condition of brain characterized by the reduction in the brain tissue which is not related to any congenital syndrome [23]. Primary microcephaly is a multifactorial birth defect and the causes may include genetic, TORCH infections and idiopathic. Genetic perturbations may involve mitotic spindle and centrosomal abnormalities and impaired DNA repair mechanisms [23]. The mitotic spindle formation and function of the centrosome is highly orchestrated during cell division process in general, and within the developing neural tissue in particular, therefore, mutated centrosome-related activity results in abnormal cell proliferation [24]. The high rate of cell division during development demands efficient DNA repair mechanisms. Genetic mutations in the DNA double-stranded breaks’ repairing proteins is one of the underlying mechanisms of microcephaly. As all these mechanisms during cell division are inter-dependent, one perturbed gene may result in multiple downstream effects [23]. Cell cycle regulatory proteins and altered cilia function seemed to be involved too [23]. TORCH infections are a group of infections caused by Treponema Pallidum, Toxoplasmosis, Rubella, Cytomegalovirus and Herpes Simplex [25].
Whatever the underlying cause may be, the microcephaly is the result of low number of differentiated cortical neurons from the neural progenitor cells [26]. Due to the widespread damage to the developing cortical tissue, the microcephalic babies have abnormal brain functions of all the brain components including cognitive, motor, visual and hearing [24].
Morphology of ZV-Induced Microcephaly in Human Pregnancy
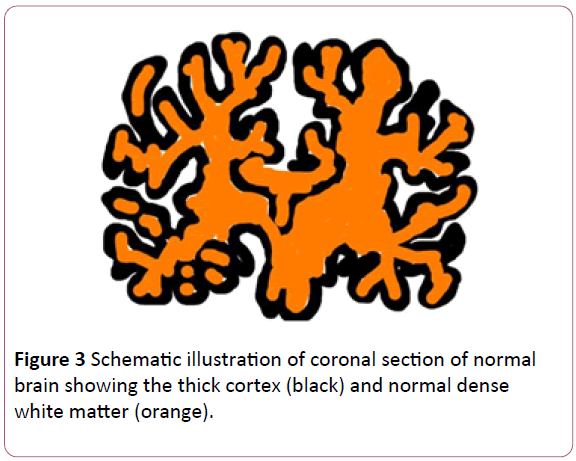
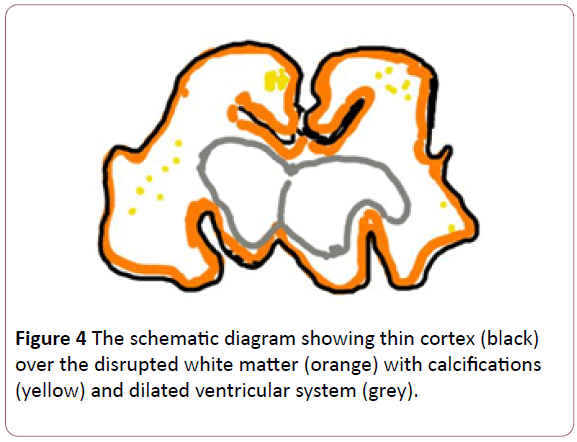
Here we discuss the morphological changes reported in the cases of ZV-induced microcephalic fetal brain tissue. Mlakar et al. (2016) reported a case of microcephaly where maternal ZV infection was established in the thirteenth week of pregnancy. The fetus developed intrauterine growth retardation and decreased fetal movements by the end of 32 weeks. The pregnancy was terminated on patient’s request. The gross pathological findings of fetal brain included almost complete absence of cortical gyri, sporadic foci of calcification and dilated lateral ventricles distinguished from normal brain (Figures 3 and 4). The cerebellum and brain stem was under-developed and the brain weight, in total, was 4 SD below average. Histological examination revealed the clumped highly stained bodies seemed to be of damaged neuronal cell bodies, axons and dendrites. Interestingly, the white cortical matter in frontal, parietal and occipital lobe was unaffected, the cerebral nuclei were well-developed with normal cellular histology. ZV RNA of Bahia Brazil strain was isolated from the fetal brain tissue with the evidence of viral particles on electron microscopy [19].
Figure 3: Schematic illustration of coronal section of normal brain showing the thick cortex (black) and normal dense white matter (orange).
In a similar case of ZV-induced microcephaly, reported by Driggers et al. (2016) the brain weight was below normal with well-differentiated basal ganglia and well-developed occipital lobe. The histopathology indicated thin parietal cortex with focal apoptotic areas and deficient subventricular zone cellular volume. The ZV RNA was isolated from fetal spleen, membranes, placenta and umbilical cord in addition to brain. According to this study, the ZV propagated only in the cell lines incubated with brain tissue harboring ZV. Furthermore, the ZV propagation was many-fold in neuroblastoma cell-lines as compared to Vero cells in vitro [21].
Role of Neural Progenitor Cells in In Vitro Experiments
Evidence suggests the high ZV infectivity and propagation in the neural precursor cell lines in in vitro studies. Garcez, et al. (2016), studied the ZV effects on human iPS-derived Neural Stem cells (iPS-NSCs), neurospheres and brain organoids. ZV infection and cell death was significant in iPS-NSCs. The significant growth reduction in neurospheres and brain organoids was observed. Considering neurospheres and brain organoids as models of early embryonic development, the reduced growth of NSCs in both models indicated that the ZV infection of NSCs in early fetal development results in severe neural damage [27].
In a similar study, Tang et al. (2016) compared the ZV infectivity among human iPS-Neural Stem Cell-derived Neural Progenitor Cells (hNPCs), human iPSCs, human embryonic stem cells (hESC) and fully differentiated neurons. Out of the various neural cell lines, the well-differentiated neurons were least infected. Human embryonic kidney cell line HEK293T were even less susceptible to ZV infection as compared to human neural lineage [28]. This indicates the presence of a novel factor in the precursor neural progenitor cells being essential for ZV survival and propagation.
ZV infects neuroglia as well and the susceptibility of astrocytes, oligodendrocyte precursor cells and microglia may explain the presence of calcifications and foci of the stromal damage in ZV-induced microcephaly [29]. In NPCs, ZV globally dysregulates the gene expression especially of apoptosis related pathway and upregulates the AXL genes [20] AXL are the candidate receptors for ZV as the blockade of extracellular domain of AXL receptors remarkably reduces the ZV infectivity in a glial cell line that explains the high susceptibility of the neuroglia populations expressing AXL receptors to ZV infection [29].
ZV replicates efficiently causing microcephaly, cortical thinning, inhibiting the NPCs growth and the cell death in a direct ZV-inoculated embryonic mouse model [20]. The focus of further investigating ZV pathogenesis may be narrowed down to NPCs and embryonic mouse model.
Conclusion
The evidence suggests the diverse pathogenesis and morphological changes in ZV-induced microcephalic fetal brain tissue caused by its infection of immune cells, neuroglia and NPCs. Among the many cell types, the NPCs damage comes up as one of the major mechanisms causing microcephaly. ZV infects undifferentiated precursor neuronal cells that are rapidly undergoing mitosis, therefore, the identification of disrupted genes in NPCs may be a step forward towards the therapeutic development. Microcephaly is the teratological complication of ZV that will continue to affect future human generations warranting urgent need of understanding the mechanism of ZVinduced damage to NPCs and provide a robust knowledge base to develop effective therapeutics.
References
- Hennessey M, Fischer M, Staples JE (2016) Zika Virus Spreads to New Areas - Region of the Americas. MMWR Morb Mortal Wkly Rep 65: 55-58.
- Malone RW, Homan J, Callahan MV, Glasspool-Malone J, Damodaran L, et al. (2016) Zika virus: medical countermeasure development challenges. PLoS Negl Trop Dis 10: e0004530.
- Li J, Xiong Y, Wu W, Liu X, Qu J, et al. (2016) Zika Virus in a Traveler Returning to China from Caracas,Venezuela. Emerg Infect Dis 22: 22.
- Faye O, Freire CC, Iamarino A, Faye O, de Oliveira JV, et al. (2014) Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis 8: e2636.
- Enfissi A, Codrington J, Roosblad J, Kazanji M, Rousset D (2016) Zika virus genome from the Americas. Lancet 387: 227-228.
- Faria NR, Azevedo RS, Kraemer MU, Souza R, Cunha MS, et al. (2016) Zika virus in the Americas: Early epidemiological and genetic findings. Science 352: 345–349.
- Kostyuchenko VA, Lim EX, Zhang S, Fibriansah G, Nig TS, et al. (2016) Structure of the thermally stable Zika virus. Nature 533: 425-428.
- Shi Y, Gao GF (2017) Structural Biology of the Zika Virus. Trends Biochem Sci 42: 443-456.
- Fernandez-Garcia MD, Mazzon M, Jacobs M, Amara A (2009) Pathogenesis of flavivirus infections: using and abusing the host cell. Cell host & microbe 5: 318-328.
- Avirutnan P, Fuchs A, Hauhart RE, Somnuke P, Youn S, et al. (2010) Antagonism of the complement component C4 by flavivirus nonstructural protein NS1. J Exp Med 207: 793-806.
- Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, et al. (2016) Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165: 1081-1091.
- Oehler E, Watrin L, Larre P, Leparc-Go I, Lastere S, et al. (2014) Zika virus infection complicated by Guillain-Barre syndrome--case report, French olynesia. Euro Surveill 19: 20720.
- Ma W, Li S, Ma S, Jia L, Zhang F, et al. (2016) Zika Virus Causes Testis Damage and Leads to Male Infertility in Mice. Cell 167: 1511-1524.
- Kam YW, Lee CYP, Teo TH, Howland SW, Amrun SN, et al. (2017) Cross-reactive dengue human monoclonal antibody prevents severe pathologies and death from Zika virus infections. JCI insight 2: 8.
- Ventura CV, Maia M, Ventura BV, Linden VVD, Araújo EB, et al. (2016) Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arquivos brasileiros de oftalmologia 79: 1-3.
- No authors (2017) Microcephaly & Other Birth Defects. Centers for Disease Control and Prevention.
- Freitas BDP, Dias JRDO, Sacramento GAPJ, Ko AI, Maia M, et al. (2016) Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil. JAMA ophthalmology 134: 529-535.
- Hagen M, Pivarcsi M, Liebe J, Bernuth H, Didonato N, et al. (2014) Diagnostic approach to microcephaly in childhood: a two-center study and review of the literature. Dev Med Child Neurol 56: 732-741.
- Mlakar J, Korva M, Tul N, Popović M, Poljšak P, et al. (2016) Zika virus associated with microcephaly. N Engl J Med 374: 951-958.
- Li C, Xu D, Ye Q, Hong S, Jiang Y, et al. (2016) Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell stem cell 19: 120-126.
- Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jääskeläinen AJ, et al. (2016) Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med 374: 2142-2151.
- Stiles J, Jernigan TL (2010) The basics of brain development. Neuropsychol Rev 20: 327-348.
- Alcantara D, O'driscoll M (2014) Congenital microcephaly. In American Journal of Medical Genetics Part C: Seminars in Medical Genetics 166:124-139.
- Gilmore EC, Walsh CA (2013) Genetic causes of microcephaly and lessons for neuronal development. Wiley Interdisciplinary Reviews: Developmental Biology 2: 461-478.
- Neu N, Duchon J, Zachariah P (2015) TORCH infections. Clinics in perinatology 42: 77-103.
- Woods CG, Bond J, Enard W (2005) Autosomal recessive primary microcephaly (MCPH): a review of clinical, molecular, and evolutionary findings. Am J Hum Genet 76: 717-728.
- Garcez PP, Loiola EC, da Costa RM, Higa LM, Trindade P, et al. (2016) Zika virus impairs growth in human neurospheres and brain organoids. Science 352: 816-818.
- Tang H, Hammack C, Ogden SC, Wen Z, Qian X, et al. (2016) Zika virus infects human cortical neural progenitors and attenuates their growth. Cell stem cell 18: 587-590.
- Retallack H, Di Lullo E, Arias C, Knopp K A, Laurie MT, et al. (2016) Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc Natl Acad Sci USA 113:14408-14413.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences