Vitamin-D An Immune Modulator and Growth Inhibitor of Mycobacterium Tuberculosis H37Rv
Preeti Soni, Shivangi and Laxman S Meena*
CSIR-Institute of Genomics and Integrative Biology, India
- *Corresponding Author:
- Laxman S Meena
CSIR-Institute of Genomics and Integrative Biology
India
Tel: 011-27666156
Fax: 011-27667471
E-mail: meena@igib.res.in, laxmansm72@yahoo.com
Received date: June 27, 2018; Accepted date: August 10, 2018; Published date: August 20, 2018
Citation: Soni P, Shivangi, Meena LS (2018) Vitamin D-An Immune Modulator and Growth Inhibitor of Mycobacterium Tuberculosis H37Rv. J Mol Biol Biotech Vol.3: No.1:2.
Abstract
Tuberculosis (TB), one of the threatening diseases and is still is one of the major causes of death in country, since millions of people die each year from this sickness. TB occurred in any part of body such as bone, the central nervous system, and many other organ systems. It is primarily a pulmonary disease that started by the deposition of Mycobacterium tuberculosis (M. tuberculosis), as aerosol particles, onto lung alveolar surfaces. The progression of the disease has several outcomes, depending largely on the response by the host immune system. The efficiency of this response is affected by intrinsic factors (genetics) as well as extrinsic factors such as nutritional and physiological state of the host. Studies showed that how vitamin D deficiency may directly linked to impairment in the regulation of immune system and significance of its immunomodulatory actions and its control on TB. Increased risk of TB has been linked to low level of vitamin D in human body. Many observations suggested that vitamin D can act as an immunomodulator which modulates function and by means of various cellular and molecular mechanisms, it regulates human immune system. The general aim of this article will be to provide a viewpoint on the potential benefits of vitamin D and its role in prevention and treatment of TB.
Keywords
Mycobacterium tuberculosis; Vitamin D; Nitric oxide; Regulatory T cell; Phagosome
Abbreviation
M. tuberculosis: Mycobacterium tuberculosis H37Rv; TB: Tuberculosis; DR-TB: Drug-resistant TB; MDR-TB: Multidrugresistant TB; DOTS: Directly observed treatment short course; BCG: Bacillus Calmette-Guerin; HCAP: Human cationic antimicrobial protein; TGN: Trans Golgi Network; LAM: Lipoarabinomannan; VDD: Vitamin D deficiency; TH: T helper cells; IFγ: Interferon γ; DBP-MAF: Vitamin D binding Protein - Macrophage Activating Factor; TNFα: Tumour necrosis factor α; MHC: Major histocompatibility complex; TLR: Interleukin (IL), Toll- like receptors; HIV/AIDS: Human immunodeficiency virus infection and acquired immune deficiency syndrome; MS: Multiple sclerosis; RA: Rheumatoid Arthritis; DM: Diabetes Mellitus; SLE: Systemic Lupus Erythematosus.
Introduction
Tuberculosis (TB) has become mainly cause of human deaths now a days and this airborne infectious disease is caused primarily by pulmonic pathogen Mycobacterium tuberculosis (M. tuberculosis) which causes this disease in any part of body [1]. TB propagation is said to be the major cause of illness and consequently death worldwide [2]. TB has been ranked 9th worldwide as a lethal disease caused from a single infectious agent, ranking above human immunodeficiency virus infection and acquired immune deficiency syndrome (HIV/AIDS). In 2017, there were more than 1 million TB deaths among HIV people and among these 90% were adults, 65% were male, 10% were people living with HIV (74% in Africa) and 56% were in five countries including India. Drug-resistant TB (DR-TB) is a rising threat, approximately 600 000 new cases were found with resistance to rifampicin (the most effective first-line drug) out of which more than half had multidrugresistant TB (MDR-TB) [3]. Almost half (48%) of these cases were in India only. Globally, the TB death rate is decreasing at about 3% per year. TB incidence is gradually dropping at about 1.5-2% per year and 14-16% of TB cases die from the disease; by 2020, these figures need to improve to 5-6% per year and 9.5-10%, respectively, to touch the first target of the ‘End TB Strategy’ [4].
Despite of various vaccines against TB such as Bacillus Calmette-Guerin (BCG) and directly observed treatment, shortcourse (DOTS) had been generated [5]. TB patients are increasing day by day. Various attempts have been done in combating this disease worldwide and among these cures, vitamin D is more prominent [6]. Various factors that may possibly affect the occurrence and development of TB has been reported, one of them is Vitamin D Deficiency (VDD) [7,8]. Researchers have found that vitamin D is essential for activating our immune defense system and that with insufficient intake of the vitamin D, the killer cells of the immune system for example T cells and other cells will not be able to respond to and combat serious infections in the body as it has been found that T cells first search for vitamin D for their activation and if they cannot find enough of it the condition will inhibit the process of activation [9]. This manuscript provides the essential aspects of vitamin D, which are crucial for inhibiting growth of M. tuberculosis and affects its survival inside the host cell.
Vitamin D deficiency related to disease development
There are various evidences that prove linkage between vitamin D deficiency & autoimmune diseases and to facilitate progression of existing autoimmune disease such as multiple sclerosis (MS), rheumatoid arthritis (RA), diabetes mellitus (DM), inflammatory bowel disease and systemic lupus erythematosus (SLE) [10] .Also with decreased in utero exposure to vitamin D and islet cell autoimmunity. Lower in utero exposure evaluated by a lower intake of vitamin D by women in pregnancy causing abnormality in foetus [11]. Studies showed that group progressed to a definitive disease, patients or diseased person has been found with significantly lower level of vitamin D than healthy or a disease free person. In other diseases also, similar correlation have been observed between disease activity and severity and low levels of vitamin D [12]. The possible reason for vitamin D deficiency might be lack in Vitamin D Acquisition mechanism, Vitamin D Deficiency induced by infection or it could be due to Vitamin D Deficiency induced by drug [13].
Vitamin D synthesis mechanism
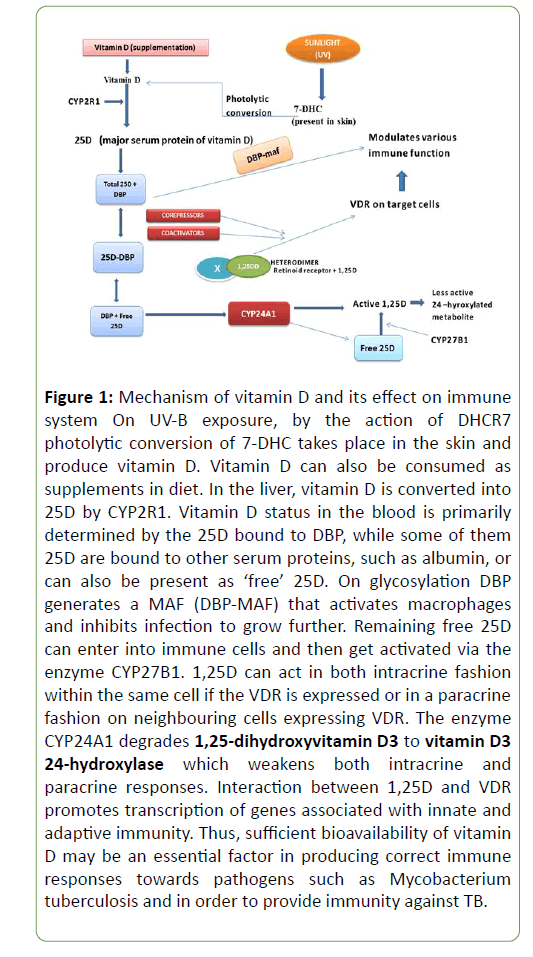
As shown in Figure 1, in human the main source of vitamin D is sunlight. By the action of sunlight the 7-DHC present in skin gets converted into vitamin D (cholecalciferol; a sterol essential for deposition of calcium in bones with the help of sunlight) through photolytic reaction. Vitamin D may also be consumed through dietetic supplements. In liver, the vitamin D is first hydroxylated to form 25-OH vitamin D3 (25D) [14]. Though it is inactive but is a major serum of vitamin D. In kidney, this inactive serum converted into active 1,25 dehydroxy vitamin D (1,25D) via enzyme calcidiol by 1-α- hydroxylase (CYP27B1). Further this active 1, 25 D metabolized to the inactive 1,24,25 vitamin D by 24-hydroxylase (CYP24). 1,25 D acts on the intestine and stimulates calcium reabsorption, and upon bone, it promotes osteoblast differentiation and matrix calcification. 1,25D reaches to the tissues and binds to the vitamin D receptor (VDR) [15] that includes forming a heterodimer with the retinoid X receptor (RXR). It further interacts with co-activators and co-repressors that translocate the 1, 25D-VDR-RXR heterodimer to the nucleus where it binds to vitamin D responsive elements (VDRE) in the promoter region and prompts expression of vitamin D [16].
Figure 1: Mechanism of vitamin D and its effect on immune system On UV-B exposure, by the action of DHCR7 photolytic conversion of 7-DHC takes place in the skin and produce vitamin D. Vitamin D can also be consumed as supplements in diet. In the liver, vitamin D is converted into 25D by CYP2R1. Vitamin D status in the blood is primarily determined by the 25D bound to DBP, while some of them 25D are bound to other serum proteins, such as albumin, or can also be present as ‘free’ 25D. On glycosylation DBP generates a MAF (DBP-MAF) that activates macrophages and inhibits infection to grow further. Remaining free 25D can enter into immune cells and then get activated via the enzyme CYP27B1. 1,25D can act in both intracrine fashion within the same cell if the VDR is expressed or in a paracrine fashion on neighbouring cells expressing VDR. The enzyme CYP24A1 degrades 1,25-dihydroxyvitamin D3 to vitamin D3 24-hydroxylase which weakens both intracrine and paracrine responses. Interaction between 1,25D and VDR promotes transcription of genes associated with innate and adaptive immunity. Thus, sufficient bioavailability of vitamin D may be an essential factor in producing correct immune responses towards pathogens such as Mycobacterium tuberculosis and in order to provide immunity against TB.
Vitamin D and the immune system
Immune system provide protection from disease causing pathogen, inhibit their growth inside the body and boost up the body to produce specific immune response against the invading species in order to promote self- tolerance. Vitamin D is an important regulator of immune system that inhibits growth of various pathogens. By each passing years studies on consequence of vitamin D deficiency on immune system have been more classified and it has been found that vitamin D deficient person is more prone to infection [17]. Vitamin D acts as a modulator for human immune system by various cellular and molecular mechanisms it carried out [18]. It performs various important roles in addition to maintaining calcium homeostatis and promoting bone health [19].
Influence of Vitamin D to innate immunity of the host
Vitamin D modulates immune responses via both innate as well as adaptive immunity. Vitamin D3 is a steroid hormone that plays a critical role in modulating and functioning of various immunogenic responses. Studies proved that M. tuberculosis shows reduced proliferation in macrophages that were treated with 1,25D and this effect was enhanced by addition of the cytokine, usually IFN-γ which stimulates the activity of macrophage CYP27B1 [20]. Interactions between intracellular receptor protein and vitamin D receptor (VDR) produce cellular responses to 1, 25-D3 which acts as a ligand dependent transcription factor. During this VDR-mediated genomic signalling, the vitamin D3–VDR complex gets activated and heterodimerizes with the retinoid X receptor and formed complex [21]. This heterodimer complex then binds to the vitamin D response element (VDRE) consensus sequence, which is located in the upstream of the 1, 25-D3- activated genes and then directly regulates the transcription of antimicrobial peptide cathelicidin [22]. Many factors contribute to the vitamin D3-induced antimicrobial effects against mycobacteria such as transcriptional activation of the antimicrobial peptide cathelicidin mediated through the binding of the vitamin D3–VDR complex to VDRE sites on the cathelicidin promoter and also non genomic regulatory mechanisms such as PI3K signalling pathway. It has been found that M. tuberculosis inhibits PI3K trafficking pathway by its cell wall contents such as Lipoarabinomannan (LAM) which is a large molecule of glycosylated phosphatidylinositol composed of domains that include a glycosylphosphatidyl anchor which attaches the molecule to the cell wall, a D-mannan core and a terminal D-arabinan as a carbohydrate skeleton. The LAM present in the wall of M. tuberculosis contains product that blocks the delivery of Cathepsins and Proton pump from Trans Golgi Network (TGN) to phagosome and thereby the recruitment of PI3K complex which is required for phagosome maturation. LAM also blocks the fusion of endosome with phagosome and prevents killing of bacteria. It has been observed that treatment with 1, 25-D3 persuades the formation of a VDR–phosphatidylinositol 3-kinase (PI3K) complex and its activation in monocytes. Phagosome maturation followed by phagolysosomal fusion through PI3K pathway kills bacteria and helps to inhibit M. tuberculosis survival in host tissue and induce antimicrobial activity [23] as shown in Figure 2.
Action of Vitamin D by Autophagy, ROS, NO & Antimycobacterial peptide
Autophagy is the destruction of damaged or redundant cellular components also non-functional proteins occurring in vacuoles within the cell and has also been found to link with immune processes and TB disease. The basic principle that autophagy works on is associated with developed mycobactericidal activity i.e. the ability to eliminate intracellular virulent M. tuberculosis by the host cell through the maturation of mycobacterial phagosomes and its further fusion with lysosome to form phagolysosomes. Also, recent progresses have revealed mechanisms of regulation of autophagic process in innate immune responses towards mycobacteria [24]. Autophagy is a defence mechanism inhibiting M. tuberculosis survival in infected macrophages. It has been showed that 1, 25 D promotes autophagy in monocytes, which inhibits autophagosome formation that suppress antibacterial activity [25]. Also the antimicrobial peptide shows great response towards killing of M. tuberculosis and its survival in human body. The only member of the cathelicidin family that has been identified in human body is HCAP-e18/LL-37. As proved earlier, the binding of the vitamin D3–VDR complex to VDRE sites present on the cathelicidin promoter results in activation of cathelicidin which triggers and initiates antimicrobial activity against intracellular infection caused by M. tuberculosis in body and in addition cathelicidins also serves multiple roles as moderators of inflammatory responses, chemoattractants, binding and defusing bacterial cell wall (LPS), and promoting reformation of epithelial cells over a wound for healing. Innate antibacterial mechanisms also include the generation of reactive oxygen species (ROS), and monocytes treated with 1,25D showed increased ROS generation against M. tuberculosis consequently decreases its survivability in host body. Another ROS, nitric oxide (NO) has an important key role in the eradication of bacteria from human body [26]. Studies also showed linkage between vitamin D prominence and its NO-mediated killing of mycobacteria and absence of the enzyme that synthesizes NO has showed poor response to infection irrespective of vitamin D prominence [27]. Similar vitamin D-mediated NO mechanism in humans is less clear, but NO production stimulated by 1,25D has been shown to be sufficient to suppress M. tuberculosis proliferation in host tissues [28].
Vitamin D and adaptive immunity
Although innate immunity has been proved to be more responsive in killing M. tuberculosis but sometimes host body also requires adaptive immunity to eradicate pathogens completely. Immune response generated by adaptive immunity may influence by innate induced factors to facilitate progression of immune responses for existing disease and it also gets modulated by the action vitamin D which utilizes the ability of T-cell cytokines to produce antibacterial responses, [29]. Vitamin D inhibits the T and B cell proliferation, differentiation and immunoglobulin secretion [30]. TH cells, with 1, 25(OH) 2D exerting effects on T-cell proliferation and cytokine production. Studies also confirmed that, Vitamin D has potential to inhibit T cell proliferation. Antigen activates the naïve TH cells which differentiate into further TH1 & TH2 subgroups with distinct cytokines supports both cell mediated as well as humoral immunity. Vitamin D has found to inhibit TH1 (IFNϒ, TNF) as they can alter the monocyte differentiation into DCs that helps the bacteria to escape from killing whereas promotes TH2 formation (anti-inflammatory cytokines) as it promotes the expression of antigen binding to MHC class I molecules. This shift in the phenotype from TH1 to TH2 is responsible for suppressing TB infection and other autoimmune diseases [31]. Due to this, there is decrease in production of inflammatory cytokines (IL-17, IL-21) which otherwise causes tissue damage and increase in production of anti-inflammatory cytokines such as IL-10. It also inhibits production of monocytes of inflammatory cytokines such as IL-1, IL-6, IL-8, IL-12 and TNFα. It also inhibits DC differentiation and maturation as mature DC produce immune response while presenting antigen to T-cell whereas immature DC produce tolerance which is important in context of autoimmunity and abolition of self-tolerance. Due to physiological cell death and tissue turnover number of self-antigens increases which are then presented by immature DCs with decreased expression of MHC class II molecules & IL-12 to maintain self-tolerance. It has been found that treatment with 1,25D shows increase in S this tolerance of immature DC hence increases the production of chemokines and to promote suppressor T-cell function that decreases bacterial survivability. This shows the versatility of vitamin D in regulation of DC function and differentiation [29,32].
It has been observed that the antibacterial activity of vitamin D cannot completely determined by pathogen–TLR interaction but may also involve number of cytokines associated with adaptive immunity such as T lymphocyte (Tcell) cytokine, IFN-γ are the inducer of monocyte CYP27B1 activity which enhances intracrine antibacterial responses to 25D [33]. Other cytokine such as IL-1 also shows similar effect. In contrast to this another cytokine, IL-4, which shows suppressive response, promotes the catabolic vitamin D enzyme CYP24A1 and reduces the induction of LL37 by vitamin D [34]. Many T-cell cytokines are themselves able to apply powerful antibacterial effects for example; IL-1β promotes phagosome maturation and autophagy, while IL-4 inhibits starvation and IFN-γ-induced autophagy [35]. Studies have proved that 1,25D shows dual nature. Depending on the contained expression of VDR 1,25D can act in a paracrine fashion on neighbouring cells, or in an intracrine fashion on the same cell [36]. In this manuscript authors exploits various ways of vitamin D to boost immunity and treatment of TB as shown in Figure 2.
Figure 2: Schematic diagram showing vitamin D synthesis and its effects on the survival of M. tuberculosis. Vitamin D can be acquired directly from exposure of sunlight and can also be consumed as daily diet supplement. Vitamin D is a steroid hormone can act as an immunomodulator for human immune system by various cellular and molecular mechanisms it carried out. Vitamin D modulates immune responses via both innate as well as adaptive immunity. Vitamin D uses various innate immunity factors such as the antimicrobial peptide i.e. cathelicidins which effects the survivability of M. tuberculosis in host body, Autophagy- a self killing mechanism , nitrogen oxide (NO) and reactive oxygen species(ROS) has also been proved to be effective against infection caused by M. tuberculosis. Host body also requires adaptive immunity to eradicate pathogens completely. Adaptive immune response may influence by innate induced factors to assist development of immune responses from host immunity for existing disease and utilizes the ability of T-cell cytokines, interleukins, TNF, chemokines, T helper and T regulatory cells to produce antibacterial responses for killing of this bacterium.
Conclusion and Future Perspective
Vitamin D deficiency is associated with various health conditions ranging from soft bones and skeletal deformities to cancer and person suffering from VDD is more susceptible to TB. Recent studies have clearly shown that vitamin D is a diverse regulator of both innate and adaptive immune responses. The current link between vitamin D and TB is mainly based on bacterial killing through combined innate and adaptive immune responses but there are many other aspects of this disease where vitamin D is least effective. Although no current data have been reported for this aspect but this is likely to be an important feature for future studies. With the discovery of antimicrobial peptide gene regulation by the vitamin D pathway a different concern in regarding its impact on the immune system has arisen. Significant progress has been observed in vitamin D3-mediated innate immunity and autophagy which upon activation contributes to antimycobacterial responses through phagosomal maturation. The current article showed that linkage between VDD and most of the prominent diseases such as AIDS, Diabetes, complications in pregnancy and many more but the concern is that this list is increasing continuously. Also relation of vitamin D with respiratory disorders has arisen as a new area of interest. Epidemiologic studies showed that innate immune responses by vitamin D may not only restricted to bacterial infections but also to other infections such as cold n flu, influenza etc. The use of vitamin D as a preventive drug for influenza has shown great effect in preventing of illness and reduction of asthma like disease. Although the mechanism is yet not clear but has broad implications for influenza research. Clinical relevance showed that vitamin D3-induced antituberculosis therapy produces effects that act as supplementation on TB treatment and essential for future therapeutic modalities. The therapeutic use of vitamin D to boost immunity is an exciting possibility from future perspective.
Acknowledgement
The authors acknowledge economic sustain from the project GAP0145 of the Department of Science and Technology (SERB). We also acknowledge the support from scientific and administrative staff of CSIR-IGIB.
Conflict of Interest
None
References
- Meena LS, Rajni (2010) Survival mechanisms of pathogenic Mycobacterium tuberculosis H37Rv. FEBS J 277: 2416-2427.
- Jo EK (2010) Innate immunity to mycobacteria: vitamin D and autophagy. Cellular Microbiology 12: 1026-1035.
- World Health Organization, Global Tuberculosis Report 2014 (WHO, 2014).
- World Health Organization, Global Tuberculosis Report 2017 (WHO, 2017).
- Rajni, Meena LS (2010) Guanosine triphosphatases as novel therapeutic targets in tuberculosis. Int J Infect Dis 14: 682-687.
- DeLuca HF. (2004) Overview of general physiologic features and functions of vitamin D. Am j Clin Nutr 80: 1689S–1696S.
- Kumari P, Meena LS (2014) Factors affecting susceptibility to Mycobacterium tuberculosis: A close view of immunological defence mechanism.SAppl Biochem Biotechnol 174: 2663-2673.
- Huang SJ, Wang XH, liu ZD, Cao WL, Han Y, et al. (2017) Vitamin D deficiency and the risk of tuberculosis: a meta-analysis. Drug Des Devel Ther 11: 91–102.
- Essen MRV, Kongsbak M, Schjerling P, Olgaard K, Odum N, et al. (2010) Vitamin D controls T cell antigen receptor signalling and activation of human T cells. Nat Immunol 11: 344–349.
- Adorini L (2005) Intervention in autoimmunity: the potential of vitamin D receptor agonists. Cell Immunol 233: 115-124.
- Fronczak CM, Baron E, Chase HP, Ross C, Brady HL, et al. (2003) In utero dietary exposures and risk of islet autoimmunity in children. Diabetes Care 26: 3237-3242.
- Patel S, Farragher T, Berry J, Bunn D, Silman A, et al. (2007) Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis Rheum 56: 2143–2149.
- Coussens AK., Martineau AR, Wilkinson RJ (2014) Anti-Inflammatory and Antimicrobial Actions of Vitamin D in Combating TB/HIV. Scientifica (Cairo).
- Bikle DD, Siiteri PK, Ryzen E, Haddad JG (1985) Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab 61: 969–975.
- MacDonald PN, Baudino TA, Tokumaru H, Dowd DR, Zhang C (2001) Vitamin D receptor and nuclear receptor coactivators: crucial interactions in vitamin D-mediated transcription. Steroids 66: 171–176 .
- Dusso A, Brown A, Slatopolsky E (1994) Extrarenal production of calcitriol. Semin Nephrol 2: 144–155.
- Zehnder D, Bland R, Williams MC, Mcninch RW, Howie AJ, et al. (2001) External Expression of 25-Hydroxyvitamin D3-1a-Hydroxylase.J ClinEndocrinol Metab 86: 888-894.
- Chun RF, Adams JS, Hewison M (2011) Immunomodulation by vitamin D: implications for TB. Expert Rev Clin Pharmacol 4: 583–591.
- Aranow C (2011) Vitamin D and the Immune System. J Investig Med 59: 881–886.
- Adams JS, Gacad MA (1985) Characterization of 1 α-hydroxylation of vitamin D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. J Exp Med 161: 755–765.
- Pike JW, Meyer MB, Watanuki M, Kim S, Zella LA, et al. (2007) Perspectives on mechanisms of gene regulation by 1,25-dihydroxyvitamin D3 and its receptor. J Steroid Biochem Mol Biol 103: 389–395.
- Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, et al. (2001) Extrarenal expression of 25-hydroxyvitamin D (3)-1 α-hydroxylase. J Clin Endocrinol Metab 86: 888–894.
- Ullrich HJ, Beatty WL, Russell DG (1999) Direct delivery of procathepsin D to phagosomes: implications for phagosome biogenesis and parasitism by Mycobacterium. Eur J Cell Biol 78: 739-748.
- Vergne I, Chua J, Deretic V (2003) Mycobacterium tuberculosis Phagosome Maturation Arrest: Selective Targeting of PI3P-Dependent Membrane Trafficking. Traffic 4: 600–606.
- Jo EK (2010) Innate immunity to mycobacteria: vitamin D and autophagy. Cell Microbiol 12: 1026-1035.
- Sly LM, Nauseef WM, Reiner NE (2001) 1α, 25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J Biol Chem 276: 35482-35493.
- Waters WR, Palmer MV, Nonnecke BJ, Whipple DL, Horst RL (2004) Mycobacterium bovis infection of vitamin D-deficient NOS2-/- mice. Microb Pathog 36:11-17.
- Rockett KA, Brookes R, Udalova I, Vidal V, Hill AV, et al. (1998) 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect Immun 66: 5314– 5321.
- Hewison M (2011) Vitamin D and Innate and Adaptive Immunity. Vitam Horm 86: 23-62.
- Bhalla AK, Amento EP, Serog B, Glimcher LH (1984) 1, 25-Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol 133: 1748–1754.
- Salgame P (2005) Host innate and Th1 responses and the bacterial factors that control Mycobacterium tuberculosis infection. Curr Opin Immunol 17: 374–380.
- Szeles L, Keresztes G, Torocsik D , Balajthy Z , Krenacs L, et al. (2009) 1,25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J Immunol 182: 2074-2083.
- Koeffler HP, Reichel H, Bishop JE, Norman AW (1985) γ-interferon stimulates production of 1,25-dihydroxyvitamin D3 by normal human macrophages. Biochem Biophys Res Commun 127: 596-603.
- Edfeldt K, Liu PT, Chun R, Fabri M, Schenk M, et al. (2010).T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci U S A 107: 22593-22598.
- Harris J, Haro SAD, Master SS, Keane J, Roberts EA, et al. (2007) T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity 27: 505–517.
- Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C (2010) Vitamin D: modulator of the immune system.
CSIR-Institute of Genomics and Integrative Biology, India
- *Corresponding Author:
- Laxman S Meena
CSIR-Institute of Genomics and Integrative Biology
India
Tel: 011-27666156
Fax: 011-27667471
E-mail: meena@igib.res.in, laxmansm72@yahoo.com
Received date: June 27, 2018; Accepted date: August 10, 2018; Published date: August 20, 2018
Citation: Soni P, Shivangi, Meena LS (2018) Vitamin D-An Immune Modulator and Growth Inhibitor of Mycobacterium Tuberculosis H37Rv. J Mol Biol Biotech Vol.3: No.1:2.
Keywords
Mycobacterium tuberculosis; Vitamin D; Nitric oxide; Regulatory T cell; Phagosome
Abbreviation
M. tuberculosis: Mycobacterium tuberculosis H37Rv; TB: Tuberculosis; DR-TB: Drug-resistant TB; MDR-TB: Multidrugresistant TB; DOTS: Directly observed treatment short course; BCG: Bacillus Calmette-Guerin; HCAP: Human cationic antimicrobial protein; TGN: Trans Golgi Network; LAM: Lipoarabinomannan; VDD: Vitamin D deficiency; TH: T helper cells; IFγ: Interferon γ; DBP-MAF: Vitamin D binding Protein - Macrophage Activating Factor; TNFα: Tumour necrosis factor α; MHC: Major histocompatibility complex; TLR: Interleukin (IL), Toll- like receptors; HIV/AIDS: Human immunodeficiency virus infection and acquired immune deficiency syndrome; MS: Multiple sclerosis; RA: Rheumatoid Arthritis; DM: Diabetes Mellitus; SLE: Systemic Lupus Erythematosus.
Introduction
Tuberculosis (TB) has become mainly cause of human deaths now a days and this airborne infectious disease is caused primarily by pulmonic pathogen Mycobacterium tuberculosis (M. tuberculosis) which causes this disease in any part of body [1]. TB propagation is said to be the major cause of illness and consequently death worldwide [2]. TB has been ranked 9th worldwide as a lethal disease caused from a single infectious agent, ranking above human immunodeficiency virus infection and acquired immune deficiency syndrome (HIV/AIDS). In 2017, there were more than 1 million TB deaths among HIV people and among these 90% were adults, 65% were male, 10% were people living with HIV (74% in Africa) and 56% were in five countries including India. Drug-resistant TB (DR-TB) is a rising threat, approximately 600 000 new cases were found with resistance to rifampicin (the most effective first-line drug) out of which more than half had multidrugresistant TB (MDR-TB) [3]. Almost half (48%) of these cases were in India only. Globally, the TB death rate is decreasing at about 3% per year. TB incidence is gradually dropping at about 1.5-2% per year and 14-16% of TB cases die from the disease; by 2020, these figures need to improve to 5-6% per year and 9.5-10%, respectively, to touch the first target of the ‘End TB Strategy’ [4].
Despite of various vaccines against TB such as Bacillus Calmette-Guerin (BCG) and directly observed treatment, shortcourse (DOTS) had been generated [5]. TB patients are increasing day by day. Various attempts have been done in combating this disease worldwide and among these cures, vitamin D is more prominent [6]. Various factors that may possibly affect the occurrence and development of TB has been reported, one of them is Vitamin D Deficiency (VDD) [7,8]. Researchers have found that vitamin D is essential for activating our immune defense system and that with insufficient intake of the vitamin D, the killer cells of the immune system for example T cells and other cells will not be able to respond to and combat serious infections in the body as it has been found that T cells first search for vitamin D for their activation and if they cannot find enough of it the condition will inhibit the process of activation [9]. This manuscript provides the essential aspects of vitamin D, which are crucial for inhibiting growth of M. tuberculosis and affects its survival inside the host cell.
Vitamin D deficiency related to disease development
There are various evidences that prove linkage between vitamin D deficiency & autoimmune diseases and to facilitate progression of existing autoimmune disease such as multiple sclerosis (MS), rheumatoid arthritis (RA), diabetes mellitus (DM), inflammatory bowel disease and systemic lupus erythematosus (SLE) [10] .Also with decreased in utero exposure to vitamin D and islet cell autoimmunity. Lower in utero exposure evaluated by a lower intake of vitamin D by women in pregnancy causing abnormality in foetus [11]. Studies showed that group progressed to a definitive disease, patients or diseased person has been found with significantly lower level of vitamin D than healthy or a disease free person. In other diseases also, similar correlation have been observed between disease activity and severity and low levels of vitamin D [12]. The possible reason for vitamin D deficiency might be lack in Vitamin D Acquisition mechanism, Vitamin D Deficiency induced by infection or it could be due to Vitamin D Deficiency induced by drug [13].
Vitamin D synthesis mechanism
As shown in Figure 1, in human the main source of vitamin D is sunlight. By the action of sunlight the 7-DHC present in skin gets converted into vitamin D (cholecalciferol; a sterol essential for deposition of calcium in bones with the help of sunlight) through photolytic reaction. Vitamin D may also be consumed through dietetic supplements. In liver, the vitamin D is first hydroxylated to form 25-OH vitamin D3 (25D) [14]. Though it is inactive but is a major serum of vitamin D. In kidney, this inactive serum converted into active 1,25 dehydroxy vitamin D (1,25D) via enzyme calcidiol by 1-α- hydroxylase (CYP27B1). Further this active 1, 25 D metabolized to the inactive 1,24,25 vitamin D by 24-hydroxylase (CYP24). 1,25 D acts on the intestine and stimulates calcium reabsorption, and upon bone, it promotes osteoblast differentiation and matrix calcification. 1,25D reaches to the tissues and binds to the vitamin D receptor (VDR) [15] that includes forming a heterodimer with the retinoid X receptor (RXR). It further interacts with co-activators and co-repressors that translocate the 1, 25D-VDR-RXR heterodimer to the nucleus where it binds to vitamin D responsive elements (VDRE) in the promoter region and prompts expression of vitamin D [16].
Figure 1: Mechanism of vitamin D and its effect on immune system On UV-B exposure, by the action of DHCR7 photolytic conversion of 7-DHC takes place in the skin and produce vitamin D. Vitamin D can also be consumed as supplements in diet. In the liver, vitamin D is converted into 25D by CYP2R1. Vitamin D status in the blood is primarily determined by the 25D bound to DBP, while some of them 25D are bound to other serum proteins, such as albumin, or can also be present as ‘free’ 25D. On glycosylation DBP generates a MAF (DBP-MAF) that activates macrophages and inhibits infection to grow further. Remaining free 25D can enter into immune cells and then get activated via the enzyme CYP27B1. 1,25D can act in both intracrine fashion within the same cell if the VDR is expressed or in a paracrine fashion on neighbouring cells expressing VDR. The enzyme CYP24A1 degrades 1,25-dihydroxyvitamin D3 to vitamin D3 24-hydroxylase which weakens both intracrine and paracrine responses. Interaction between 1,25D and VDR promotes transcription of genes associated with innate and adaptive immunity. Thus, sufficient bioavailability of vitamin D may be an essential factor in producing correct immune responses towards pathogens such as Mycobacterium tuberculosis and in order to provide immunity against TB.
Vitamin D and the immune system
Immune system provide protection from disease causing pathogen, inhibit their growth inside the body and boost up the body to produce specific immune response against the invading species in order to promote self- tolerance. Vitamin D is an important regulator of immune system that inhibits growth of various pathogens. By each passing years studies on consequence of vitamin D deficiency on immune system have been more classified and it has been found that vitamin D deficient person is more prone to infection [17]. Vitamin D acts as a modulator for human immune system by various cellular and molecular mechanisms it carried out [18]. It performs various important roles in addition to maintaining calcium homeostatis and promoting bone health [19].
Influence of Vitamin D to innate immunity of the host
Vitamin D modulates immune responses via both innate as well as adaptive immunity. Vitamin D3 is a steroid hormone that plays a critical role in modulating and functioning of various immunogenic responses. Studies proved that M. tuberculosis shows reduced proliferation in macrophages that were treated with 1,25D and this effect was enhanced by addition of the cytokine, usually IFN-γ which stimulates the activity of macrophage CYP27B1 [20]. Interactions between intracellular receptor protein and vitamin D receptor (VDR) produce cellular responses to 1, 25-D3 which acts as a ligand dependent transcription factor. During this VDR-mediated genomic signalling, the vitamin D3–VDR complex gets activated and heterodimerizes with the retinoid X receptor and formed complex [21]. This heterodimer complex then binds to the vitamin D response element (VDRE) consensus sequence, which is located in the upstream of the 1, 25-D3- activated genes and then directly regulates the transcription of antimicrobial peptide cathelicidin [22]. Many factors contribute to the vitamin D3-induced antimicrobial effects against mycobacteria such as transcriptional activation of the antimicrobial peptide cathelicidin mediated through the binding of the vitamin D3–VDR complex to VDRE sites on the cathelicidin promoter and also non genomic regulatory mechanisms such as PI3K signalling pathway. It has been found that M. tuberculosis inhibits PI3K trafficking pathway by its cell wall contents such as Lipoarabinomannan (LAM) which is a large molecule of glycosylated phosphatidylinositol composed of domains that include a glycosylphosphatidyl anchor which attaches the molecule to the cell wall, a D-mannan core and a terminal D-arabinan as a carbohydrate skeleton. The LAM present in the wall of M. tuberculosis contains product that blocks the delivery of Cathepsins and Proton pump from Trans Golgi Network (TGN) to phagosome and thereby the recruitment of PI3K complex which is required for phagosome maturation. LAM also blocks the fusion of endosome with phagosome and prevents killing of bacteria. It has been observed that treatment with 1, 25-D3 persuades the formation of a VDR–phosphatidylinositol 3-kinase (PI3K) complex and its activation in monocytes. Phagosome maturation followed by phagolysosomal fusion through PI3K pathway kills bacteria and helps to inhibit M. tuberculosis survival in host tissue and induce antimicrobial activity [23] as shown in Figure 2.
Action of Vitamin D by Autophagy, ROS, NO & Antimycobacterial peptide
Autophagy is the destruction of damaged or redundant cellular components also non-functional proteins occurring in vacuoles within the cell and has also been found to link with immune processes and TB disease. The basic principle that autophagy works on is associated with developed mycobactericidal activity i.e. the ability to eliminate intracellular virulent M. tuberculosis by the host cell through the maturation of mycobacterial phagosomes and its further fusion with lysosome to form phagolysosomes. Also, recent progresses have revealed mechanisms of regulation of autophagic process in innate immune responses towards mycobacteria [24]. Autophagy is a defence mechanism inhibiting M. tuberculosis survival in infected macrophages. It has been showed that 1, 25 D promotes autophagy in monocytes, which inhibits autophagosome formation that suppress antibacterial activity [25]. Also the antimicrobial peptide shows great response towards killing of M. tuberculosis and its survival in human body. The only member of the cathelicidin family that has been identified in human body is HCAP-e18/LL-37. As proved earlier, the binding of the vitamin D3–VDR complex to VDRE sites present on the cathelicidin promoter results in activation of cathelicidin which triggers and initiates antimicrobial activity against intracellular infection caused by M. tuberculosis in body and in addition cathelicidins also serves multiple roles as moderators of inflammatory responses, chemoattractants, binding and defusing bacterial cell wall (LPS), and promoting reformation of epithelial cells over a wound for healing. Innate antibacterial mechanisms also include the generation of reactive oxygen species (ROS), and monocytes treated with 1,25D showed increased ROS generation against M. tuberculosis consequently decreases its survivability in host body. Another ROS, nitric oxide (NO) has an important key role in the eradication of bacteria from human body [26]. Studies also showed linkage between vitamin D prominence and its NO-mediated killing of mycobacteria and absence of the enzyme that synthesizes NO has showed poor response to infection irrespective of vitamin D prominence [27]. Similar vitamin D-mediated NO mechanism in humans is less clear, but NO production stimulated by 1,25D has been shown to be sufficient to suppress M. tuberculosis proliferation in host tissues [28].
Vitamin D and adaptive immunity
Although innate immunity has been proved to be more responsive in killing M. tuberculosis but sometimes host body also requires adaptive immunity to eradicate pathogens completely. Immune response generated by adaptive immunity may influence by innate induced factors to facilitate progression of immune responses for existing disease and it also gets modulated by the action vitamin D which utilizes the ability of T-cell cytokines to produce antibacterial responses, [29]. Vitamin D inhibits the T and B cell proliferation, differentiation and immunoglobulin secretion [30]. TH cells, with 1, 25(OH) 2D exerting effects on T-cell proliferation and cytokine production. Studies also confirmed that, Vitamin D has potential to inhibit T cell proliferation. Antigen activates the naïve TH cells which differentiate into further TH1 & TH2 subgroups with distinct cytokines supports both cell mediated as well as humoral immunity. Vitamin D has found to inhibit TH1 (IFNϒ, TNF) as they can alter the monocyte differentiation into DCs that helps the bacteria to escape from killing whereas promotes TH2 formation (anti-inflammatory cytokines) as it promotes the expression of antigen binding to MHC class I molecules. This shift in the phenotype from TH1 to TH2 is responsible for suppressing TB infection and other autoimmune diseases [31]. Due to this, there is decrease in production of inflammatory cytokines (IL-17, IL-21) which otherwise causes tissue damage and increase in production of anti-inflammatory cytokines such as IL-10. It also inhibits production of monocytes of inflammatory cytokines such as IL-1, IL-6, IL-8, IL-12 and TNFα. It also inhibits DC differentiation and maturation as mature DC produce immune response while presenting antigen to T-cell whereas immature DC produce tolerance which is important in context of autoimmunity and abolition of self-tolerance. Due to physiological cell death and tissue turnover number of self-antigens increases which are then presented by immature DCs with decreased expression of MHC class II molecules & IL-12 to maintain self-tolerance. It has been found that treatment with 1,25D shows increase in S this tolerance of immature DC hence increases the production of chemokines and to promote suppressor T-cell function that decreases bacterial survivability. This shows the versatility of vitamin D in regulation of DC function and differentiation [29,32].
It has been observed that the antibacterial activity of vitamin D cannot completely determined by pathogen–TLR interaction but may also involve number of cytokines associated with adaptive immunity such as T lymphocyte (Tcell) cytokine, IFN-γ are the inducer of monocyte CYP27B1 activity which enhances intracrine antibacterial responses to 25D [33]. Other cytokine such as IL-1 also shows similar effect. In contrast to this another cytokine, IL-4, which shows suppressive response, promotes the catabolic vitamin D enzyme CYP24A1 and reduces the induction of LL37 by vitamin D [34]. Many T-cell cytokines are themselves able to apply powerful antibacterial effects for example; IL-1β promotes phagosome maturation and autophagy, while IL-4 inhibits starvation and IFN-γ-induced autophagy [35]. Studies have proved that 1,25D shows dual nature. Depending on the contained expression of VDR 1,25D can act in a paracrine fashion on neighbouring cells, or in an intracrine fashion on the same cell [36]. In this manuscript authors exploits various ways of vitamin D to boost immunity and treatment of TB as shown in Figure 2.
Figure 2: Schematic diagram showing vitamin D synthesis and its effects on the survival of M. tuberculosis. Vitamin D can be acquired directly from exposure of sunlight and can also be consumed as daily diet supplement. Vitamin D is a steroid hormone can act as an immunomodulator for human immune system by various cellular and molecular mechanisms it carried out. Vitamin D modulates immune responses via both innate as well as adaptive immunity. Vitamin D uses various innate immunity factors such as the antimicrobial peptide i.e. cathelicidins which effects the survivability of M. tuberculosis in host body, Autophagy- a self killing mechanism , nitrogen oxide (NO) and reactive oxygen species(ROS) has also been proved to be effective against infection caused by M. tuberculosis. Host body also requires adaptive immunity to eradicate pathogens completely. Adaptive immune response may influence by innate induced factors to assist development of immune responses from host immunity for existing disease and utilizes the ability of T-cell cytokines, interleukins, TNF, chemokines, T helper and T regulatory cells to produce antibacterial responses for killing of this bacterium.
Conclusion and Future Perspective
Vitamin D deficiency is associated with various health conditions ranging from soft bones and skeletal deformities to cancer and person suffering from VDD is more susceptible to TB. Recent studies have clearly shown that vitamin D is a diverse regulator of both innate and adaptive immune responses. The current link between vitamin D and TB is mainly based on bacterial killing through combined innate and adaptive immune responses but there are many other aspects of this disease where vitamin D is least effective. Although no current data have been reported for this aspect but this is likely to be an important feature for future studies. With the discovery of antimicrobial peptide gene regulation by the vitamin D pathway a different concern in regarding its impact on the immune system has arisen. Significant progress has been observed in vitamin D3-mediated innate immunity and autophagy which upon activation contributes to antimycobacterial responses through phagosomal maturation. The current article showed that linkage between VDD and most of the prominent diseases such as AIDS, Diabetes, complications in pregnancy and many more but the concern is that this list is increasing continuously. Also relation of vitamin D with respiratory disorders has arisen as a new area of interest. Epidemiologic studies showed that innate immune responses by vitamin D may not only restricted to bacterial infections but also to other infections such as cold n flu, influenza etc. The use of vitamin D as a preventive drug for influenza has shown great effect in preventing of illness and reduction of asthma like disease. Although the mechanism is yet not clear but has broad implications for influenza research. Clinical relevance showed that vitamin D3-induced antituberculosis therapy produces effects that act as supplementation on TB treatment and essential for future therapeutic modalities. The therapeutic use of vitamin D to boost immunity is an exciting possibility from future perspective.
Acknowledgement
The authors acknowledge economic sustain from the project GAP0145 of the Department of Science and Technology (SERB). We also acknowledge the support from scientific and administrative staff of CSIR-IGIB.
Conflict of Interest
None
References
- Meena LS, Rajni (2010) Survival mechanisms of pathogenic Mycobacterium tuberculosis H37Rv. FEBS J 277: 2416-2427.
- Jo EK (2010) Innate immunity to mycobacteria: vitamin D and autophagy. Cellular Microbiology 12: 1026-1035.
- World Health Organization, Global Tuberculosis Report 2014 (WHO, 2014).
- World Health Organization, Global Tuberculosis Report 2017 (WHO, 2017).
- Rajni, Meena LS (2010) Guanosine triphosphatases as novel therapeutic targets in tuberculosis. Int J Infect Dis 14: 682-687.
- DeLuca HF. (2004) Overview of general physiologic features and functions of vitamin D. Am j Clin Nutr 80: 1689S–1696S.
- Kumari P, Meena LS (2014) Factors affecting susceptibility to Mycobacterium tuberculosis: A close view of immunological defence mechanism.SAppl Biochem Biotechnol 174: 2663-2673.
- Huang SJ, Wang XH, liu ZD, Cao WL, Han Y, et al. (2017) Vitamin D deficiency and the risk of tuberculosis: a meta-analysis. Drug Des Devel Ther 11: 91–102.
- Essen MRV, Kongsbak M, Schjerling P, Olgaard K, Odum N, et al. (2010) Vitamin D controls T cell antigen receptor signalling and activation of human T cells. Nat Immunol 11: 344–349.
- Adorini L (2005) Intervention in autoimmunity: the potential of vitamin D receptor agonists. Cell Immunol 233: 115-124.
- Fronczak CM, Baron E, Chase HP, Ross C, Brady HL, et al. (2003) In utero dietary exposures and risk of islet autoimmunity in children. Diabetes Care 26: 3237-3242.
- Patel S, Farragher T, Berry J, Bunn D, Silman A, et al. (2007) Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis Rheum 56: 2143–2149.
- Coussens AK., Martineau AR, Wilkinson RJ (2014) Anti-Inflammatory and Antimicrobial Actions of Vitamin D in Combating TB/HIV. Scientifica (Cairo).
- Bikle DD, Siiteri PK, Ryzen E, Haddad JG (1985) Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab 61: 969–975.
- MacDonald PN, Baudino TA, Tokumaru H, Dowd DR, Zhang C (2001) Vitamin D receptor and nuclear receptor coactivators: crucial interactions in vitamin D-mediated transcription. Steroids 66: 171–176 .
- Dusso A, Brown A, Slatopolsky E (1994) Extrarenal production of calcitriol. Semin Nephrol 2: 144–155.
- Zehnder D, Bland R, Williams MC, Mcninch RW, Howie AJ, et al. (2001) External Expression of 25-Hydroxyvitamin D3-1a-Hydroxylase.J ClinEndocrinol Metab 86: 888-894.
- Chun RF, Adams JS, Hewison M (2011) Immunomodulation by vitamin D: implications for TB. Expert Rev Clin Pharmacol 4: 583–591.
- Aranow C (2011) Vitamin D and the Immune System. J Investig Med 59: 881–886.
- Adams JS, Gacad MA (1985) Characterization of 1 α-hydroxylation of vitamin D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. J Exp Med 161: 755–765.
- Pike JW, Meyer MB, Watanuki M, Kim S, Zella LA, et al. (2007) Perspectives on mechanisms of gene regulation by 1,25-dihydroxyvitamin D3 and its receptor. J Steroid Biochem Mol Biol 103: 389–395.
- Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, et al. (2001) Extrarenal expression of 25-hydroxyvitamin D (3)-1 α-hydroxylase. J Clin Endocrinol Metab 86: 888–894.
- Ullrich HJ, Beatty WL, Russell DG (1999) Direct delivery of procathepsin D to phagosomes: implications for phagosome biogenesis and parasitism by Mycobacterium. Eur J Cell Biol 78: 739-748.
- Vergne I, Chua J, Deretic V (2003) Mycobacterium tuberculosis Phagosome Maturation Arrest: Selective Targeting of PI3P-Dependent Membrane Trafficking. Traffic 4: 600–606.
- Jo EK (2010) Innate immunity to mycobacteria: vitamin D and autophagy. Cell Microbiol 12: 1026-1035.
- Sly LM, Nauseef WM, Reiner NE (2001) 1α, 25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J Biol Chem 276: 35482-35493.
- Waters WR, Palmer MV, Nonnecke BJ, Whipple DL, Horst RL (2004) Mycobacterium bovis infection of vitamin D-deficient NOS2-/- mice. Microb Pathog 36:11-17.
- Rockett KA, Brookes R, Udalova I, Vidal V, Hill AV, et al. (1998) 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect Immun 66: 5314– 5321.
- Hewison M (2011) Vitamin D and Innate and Adaptive Immunity. Vitam Horm 86: 23-62.
- Bhalla AK, Amento EP, Serog B, Glimcher LH (1984) 1, 25-Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol 133: 1748–1754.
- Salgame P (2005) Host innate and Th1 responses and the bacterial factors that control Mycobacterium tuberculosis infection. Curr Opin Immunol 17: 374–380.
- Szeles L, Keresztes G, Torocsik D , Balajthy Z , Krenacs L, et al. (2009) 1,25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J Immunol 182: 2074-2083.
- Koeffler HP, Reichel H, Bishop JE, Norman AW (1985) γ-interferon stimulates production of 1,25-dihydroxyvitamin D3 by normal human macrophages. Biochem Biophys Res Commun 127: 596-603.
- Edfeldt K, Liu PT, Chun R, Fabri M, Schenk M, et al. (2010).T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci U S A 107: 22593-22598.
- Harris J, Haro SAD, Master SS, Keane J, Roberts EA, et al. (2007) T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity 27: 505–517.
- Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C (2010) Vitamin D: modulator of the immune system.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences