Update on Diagnosis and Management of Severe Sepsis and Septic Shock
Arun Janakiraman1* and R Phillip Dellinger2
1UPMC Presbyterian-Shadyside, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
2Cooper University Health Care, Cooper Medical School of Rowan University, Camden, NJ, USA
- *Corresponding Author:
- Arun Janakiraman
UPMC Presbyterian-Shadyside
University of Pittsburgh School of Medicine
Pittsburgh, PA, USA
Tel: 4126924882
E-mail: janakiramana@upmc.edu
Received Date: November 29, 2017; Accepted Date: December 15, 2017; Published Date: December 28, 2017
Citation: Janakiraman A, Dellinger RP (2017) Update on Diagnosis and Management of Severe Sepsis and Septic Shock. J Clin Med Ther. 2:28.
Abstract
Since 1992 consensus recommendations have defined sepsis as a known or suspected infection plus systemic manifestations of infection, and originally included only the sepsis inflammatory response syndrome (SIRS). A decade later the definition was broadened to include other systemic manifestations of sepsis. The newer definition encouraged identification of patients with moist severe systemic manifestations of infection. The 3rd Sepsis Definitions Consensus Conference in 2015 redefined sepsis to simplify the terminology and definitions based on analyses conducted using several large databases. The last fifteen years have been a remarkable time in the evolution of management principles for severe sepsis and septic shock. It is generally agreed that early identification, early antibiotics and early fluid resuscitations are paramount to decreasing mortality of severe sepsis and septic shock.
Keywords
Infection; Sepsis; Antibiotics; Septic shock
History
Throughout early history, there have been numerous infections that have led to significant changes in how we view healthcare, from the bubonic plague to the Spanish flu of the early 20th century. Unlike these notorious infections, “sepsis” cannot be defined by one period or by one epidemic. The concept of sepsis is more than 2000 years old and a complete mastery of the subject has yet to be achieved. The word “sepsis” is derived from the word σηψις, which is Greek for “decomposition of animal or vegetable organic matter”, with the word first appearing in Homer’s poems [1]. Initial theories of infection from antiquity remained unchanged and unchallenged until advancements by Lister, Koch, Semmelweis, and Pasteur. Then in the 20th century molecular theory allowed for greater understanding of the disease process [1]. The first reference was noted to be in 1858 with the inclusion of the word “sepsis” in the Oxford English Dictionary; however, referenced in previous specialized works. It was not until Louis Pasteur’s work on germ theory that the connection was made between the presence of bacteria and putrefaction [2]. Pasteur’s work was the foundation for the discoveries in the field of sepsis research.
Definitions
Since 1992 consensus recommendations have defined sepsis as a known or suspected infection plus systemic manifestations of infection, and originally included only the sepsis inflammatory response syndrome (SIRS) [1].
• Tachycardia (heart rate > 90 beats per min)
• Tachypnea (respirations > 20 breaths per min)
• Fever ((> 38.3°C [100.9 °F]) or hypothermia (core temperature < 36°C [96.8 °F])
• High or low white blood cell count (>12,000 × 109 /L or < 4,000 109 /L), or a normal count with more than 10% immature cells) [3].
The definition was broadened in 2002 to include other systemic manifestations of sepsis such as changes in blood glucose levels and organ dysfunction. This definition of sepsis encourages the early identification of patients with more severe systemic manifestations of infection that require more aggressive monitoring and treatment measures. Severe sepsis has been defined as sepsis plus either acute organ dysfunction or tissue hypoperfusion, with tissue hypoperfusion defined as:
• Hypotension (systolic blood pressure <90 mm Hg, or a drop in systolic blood pressure of >40 mm Hg)
• Elevated lactate
• Low urine output
• Altered mental status
The 3rd Sepsis Definitions Consensus Conference in 2015 redefined sepsis to simplify the terminology and definitions based on analyses conducted using several large databases [4]. This document recommended abandoning the term severe sepsis and using “sepsis” to describe infection induced organ dysfunction or tissue hypoperfusion. The document also recommended de-emphasizing the SIRS criteria. What was previously called sepsis (infection plus systemic manifestations of infection) would not be called infection with no differentiation from infection without systemic manifestation of infection. Organ dysfunction was defined as an increase in the sequential organ failure assessment (SOFA) score of 2 points or more. Septic shock was defined as vasopressor requirement to maintain a mean arterial pressure (MAP) of 65 mmHg or greatr and serum lactate greater than 2 mmol/L (>18 mg?dL) in the absence of hypovolemia after intravascular volume repletion.
Despite attempts to better define sepsis and septic shock, early recognition remains a challenge as there is no specific test to define infection induced organ dysfunction other than clinical criteria. Tissue hypoperfusion can occur in the absence of hypotension and could be present for hours before organ dysfunction manifests. Patients presenting to the ER with infection induced organ dysfunction may have had onset of this dysfunction an hour ago or a day ago. Since the 2001 definitions are currently used for both ICD-10 codes and Centers for Medicare and Medicaid Services (CMS) quality metrics, any major shift to the new definitions is likely to be slow and will require much planning and coordination.
Clinical Presentation and Course
In severe sepsis, organ dysfunction is caused by a systemic pro-coagulant and pro-inflammatory response to blood-borne toxins and involves acute lung or kidney injury, coagulopathy (thrombocytopenia or increased international normalized ratio [INR]), liver dysfunction and cardiovascular dysfunction. It is known that many conditions can cause delirium in critical illness, but sepsis associated encephalopathy (SAE) is the most severe [5]. When patients with SAE undergo imaging there is generally no focal lesions present and electroencephalography (EEG) are consistent with nonfocal encephalopathy [6].
Septic shock is present when a patient had persistent hypotension requiring vasopressors following adequate intravascular volume repletion.
Epidemiology and Burden of Disease
In spite of the advances that have been made with the recognition and treatment of sepsis, it remains a great burden in health care. Of the 750 million hospitalizations between 1979 through 2000, there were 10,319,418 cases of sepsis [7]. This represents 1.4% of all admissions during this course of time. Even though patients admitted with sepsis is a mere fraction of total hospital admissions, the mortality rate continues to be above those of other diseases. In-hospital mortality rate fell from 27.8% during the period from 1979 through 1984 to 17.9% during the period from 1995 to 2000; yet the total number of deaths continued to increase [7]. Of the individuals diagnosed with sepsis, mortality was noted to be highest in black men [7].
When sepsis is present, an unregulated systemic response that may progress to multiple organ failures. Survivors of sepsis may have persistently compromised organ function, which may result in symptoms such as dyspnea, fatigue, depression, and impaired functional status [8]. The term cognitive impairment refers to clinically significant abnormalities in one or more brain functions. Many critically ill patients have significant chronic neurocognitive impairments at 2 months, 6 months, 9 months, 1 year, 2 years, and up to 6 years. The impairments improve during the first 6-12 months post-hospital discharge. A study concluded that 70% of sepsis survivors had neurocognitive impairments at one year [8].
Burden of Sepsis and Performance Improvement
In 2008, an estimated $14.6 billion was spent on hospitalizations for severe sepsis, and from 1997 through 2008, the inflation-adjusted aggregate costs for treating patients hospitalized for this condition increased on average annually by 11.9% [9]. The economic burden is enormous: it is the most expensive condition treated in US hospitals, costing an estimated $20.3 billion in 2011, of which $12.7 billion came from Medicare [10]. Age-adjusted rates for severe sepsis hospitalization and mortality increased annually by 8.2% (p<. 001) and 5.6% (p<.001), respectively, whereas case fatality rate decreased by 1.4% (p<.001) [11]. The financial burden caused by sepsis continues to be enormous.
Physician and nursing education along with the implementation of hospital screening tools have allowed for the early treatment of sepsis and severe sepsis. Nursing orientation includes recognition of sepsis as well as annual recertification. At the physician level, annual training through lectures and simulation sessions are needed. At various institutions, “sepsis champions” have been developed to be an advocate who can assist in the identification and early treatment of sepsis. These champions act as the “eyes and ears” for each unit. Success is achieved with the use of screening tools that are available to the nursing staff as well as incorporation of practice alerts that are accessible to all providers. According to results of the Multiple Urgent Sepsis Therapies (MUST) protocol, there were improvements in outcomes in the intervention group that received algorithmbased treatment when compared to the standard therapy. In the MUST study, patients who met criteria for severe sepsis underwent a pathway of treatment that involved alerting a sepsis team, which included the ICU attending and resident, an emergency room attending and resident, and nursing bed supervisor. In addition to alerting the key providers, this approach involved empiric antibiotics, fluid resuscitation, insulin therapy, as well as ventilation if applicable. Patients in the treatment arm were noted to have received more fluids (4.0 L vs. 2.5 L), antibiotics earlier (90 min vs. 120 min), and more vasopressors (80% vs. 45%) [12]. A key advancement in the management of sepsis has been linking treatment to early diagnosis through screening processes and early identification. With the advent of the electronic medical record (EMR), simple algorithms can be written to alert the provider of abnormalities in either vital sign of laboratory findings which may be an early warning sign of sepsis. For example, Mayo Clinic has developed a Multidisciplinary Epidemiology and Translational Research in Intensive Care (METRIC) Data Mart to include syndrome surveillance, decision support, reporting, and modeling of critical illness [13].
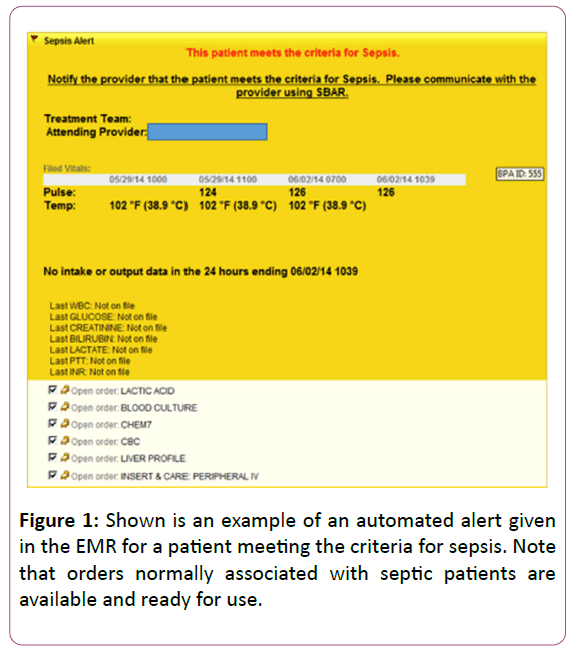
Sepsis alarms notify the provider that the patient, based on numerical values screened from vital signs and laboratory findings may warrant diagnostic or therapeutic intervention (Figure 1).
Management
Early identification and early antibiotics
Routine screening of the potentially infected seriously ill patient for severe sepsis may lead to early identification of sepsis and allow implementation of early therapy to include early administration of antibiotics, early and adequate fluid resuscitation [14]. Early identification has been documented to improve outcomes in septic patients [14]. The use of appropriate (organism is sensitive) antibiotics as an early intervention has been shown to have a positive effect on outcome (Table 1).
| Time to Antibiotics (Hr) | ORa | 95% CI | p | Probability of Mortality (%)b | 95% CI |
|---|---|---|---|---|---|
| 0-1c | 1.00 | 24.6 | 23.2-26.0 | ||
| 1-2 | 1.07 | 0.97-1.18 | 0.165 | 25.9 | 24.5-27.2 |
| 2-3 | 1.14 | 1.02-1.26 | 0.021 | 27.0 | 25.3-28.7 |
| 3-4 | 1.19 | 1.04-1.35 | 0.009 | 27.9 | 25.6-30.1 |
| 4-5 | 1.24 | 1.06-1.45 | 0.006 | 28.8 | 25.9-31.7 |
| 5-6 | 1.47 | 1.22-1.76 | < 0.001 | 32.3 | 28.5-36.2 |
| > 6 | 1.52 | 1.36-1.76 | < 0.001 | 33.1 | 30.9-35.3 |
OR = odds ratio
A) Hospital mortality odds referent group is 0-1 h for the time to antibiotics and is adjusted by the sepsis severity score (SSS), ICU admission source (ED, ward, vs. ICU), and geographic region (Europe, United States, and South America)
B) Probability of hospital mortality is estimated using the generalized estimating equation population averaged logistic regression model and is based on the subject having the following characteristics: from the United States, admission source is the ED, and the SSS is 52 (median of all observations)
C) Antibiotics administered in the first hour are the referent group and thus the odds ratio by definition is 1.00 while the 95% CI and the p value are not generated by the regression model.
Table 1: Probability of Mortality.
As the time to appropriate antibiotics increases so does the probability of mortality, with rates greater than 30% when administration of appropriate antibiotics is delayed to 5 h or more [15].
The ProCESS and ARISE trials showed the ability to lower mortality of septic shock to 18% across all treatment arms of these studies pointing to the importance of early identification, early antibiotics and early fluid resuscitation (Table 2) [16,17].
| Process | Arise | |
|---|---|---|
| Enrollment | < 2 h from detection shock | 2.8 h (median) from presentation to ED |
| Antibiotics | 75% received prior to enrollment | 70 min (median) from presentation to ED |
| Fluids | > 2 Liters prior to enrollment | 2515 (mean) prior to enrollment |
| Initial mean ScvO2 | 71 ± 13% | 72.7 ± 10.5% |
Table 2: Comparison of the ProCESS and ARISE trials.
Fluid resuscitation
Severely septic patients at the time of presentation are typically significantly volume depleted, and although fluid resuscitation is a mainstay in the treatment of sepsis the recommendations of which type of fluid and how much to use are debated. Current recommendation in general for septic patients presenting with hypotension or lactate ≥ 4 mmol/L is a minimum of 30 mL/kg crystalloid over the first three hours of therapy. Crystalloids have been traditionally used as the initial resuscitation fluid in patients with septic shock. A study published in 2004, compared cystalloids with albumin as a general resuscitation fluid in ICU patients and included a large cohort of patients. There was no difference in outcome between the two fluids, however, subset analysis showed better outcome with albumin in patients with severe sepsis and septic shock. A subsequent meta-analysis also favored albumin. Publications comparing hetastarch with crystalloids reveal increased mortality, increased renal replacement therapy, or no difference in outcomes when hetastarch is used and therefore hetastarch is not recommended. The Surviving Sepsis Campaign recommends that when large amounts of fluids are required to maintain MAP, that albumin be added to the fluid resuscitation regimen.
Choices of cystalloids include normal saline, (an unbalanced, i.e., high chloride, unbuffered crystalloid) or crystalloids that are balanced, (chloride similar to plasma) and buffered. The presence of an organic anion and correspondingly lower chloride content that more closely resembles the composition of plasma are supportive of a more “balanced” crystalloid [18]. The most commonly used crystalloid, normal saline is far from “normal” with a pH much less than 7.0 and a supraphysiologic chloride content of 154 mmol/L [18]. Normal saline predisposes patients to hyperchloremic metabolic acidosis, with potential for adverse effects [18]. A before and after study of critically ill patients showed that balanced versus unbalanced fluid solution was associated with a lower incidence of acute kidney injury (8.4% vs. 14%) and renal replacement therapy (6.3% vs. 10%) but no difference in hospital mortality [19]. Regardless of the crystalloid, the current recommendation as outlined by the Surviving Sepsis Campaign is that patients receive 30 cc/kg as part of the initial resuscitation. The debate over “balanced” versus “unbalanced” solutions remains. Since there have not been any randomized trials to compare balanced and unbalanced solutions, no definitive answer is currently available [20].
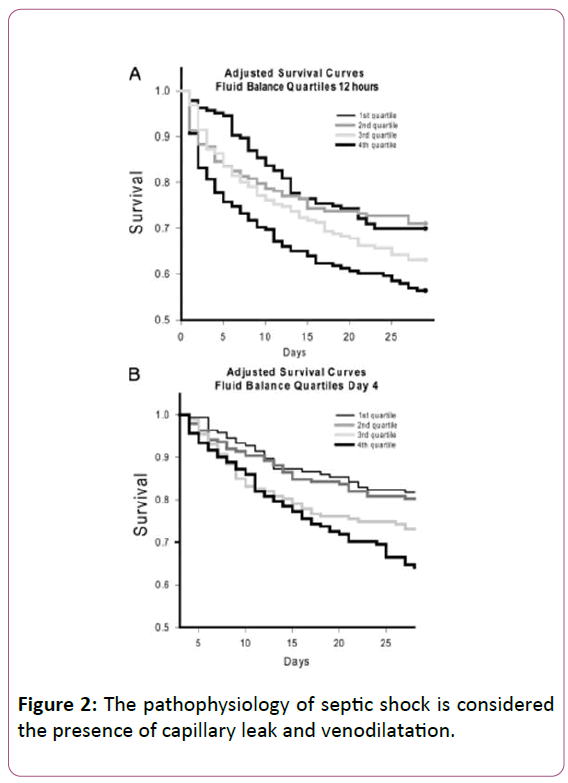
When the pathophysiology of septic shock is considered the presence of capillary leak and venodilatation (Figure 2) [21] leads to decreased blood return (preload) to the right ventricle and subsequently to the left ventricle.
Therefore, the nature of the pathophysiology of septic shock lends to the need for fluid resuscitation to reestablish intravascular volume, stroke volume and cardiac output which decreased in the early unresuscitated phase of septic shock. The original Surviving Sepsis Campaign performance improvement program (2005) recommended a 20 mL/kg actual body weight crystalloid fluid bolus in patient with sepsis induced hypo perfusion (hypotension or high lactate, defined as lactate >4 mEq/L). This remained the case until the publication of the 2012 guidelines which recommended 30 mL/kg crystalloid or albumin equivalent. The current CMS measures recommend that same amount, i.e. 30 mL/kg actual body weight crystalloid (note that albumin equivalent is not an alternative in the CMS measure). Considerable discussion and even controversy has ensued around this recommendation related to (a) the use of actual body weight and what that would mean with fluid resuscitation of morbidly obese patients and (b) fluid resuscitation of patients at risk for volume overload to include end-stage renal disease (ESRD) and preexisting congestive heart failure and (c) literature that links high input/output ratios to poor outcome.
Fluid resuscitation of the obese and the morbidly obese patient
As body weight increases past ideal body weight there in increase in vascular space. However, the proportion of increase in intravascular space relateive to increase in body weight in obesity does not maintain the same relationship as is present in someone with ideal body weight. Sophisticated formulas have been created to allow one to better calculate what the intravascular space would be in obesity and morbid obesity. However, for the purposes of a sepsis performance improvement program the use of such a sophisticated formula is counterproductive.
“How does the fluid resuscitation of patients with ESRD and cardiomyopathy (ischemic or otherwise) who develop septic shock differ from those without these pre-existing conditions?”
For example, let’s assume the worst case scenarios of an anuric ESRD patient on dialysis. While the question of fluid requirements is easy to answer, the treatment itself is a little more challenging. The amount of fluids required for successful resuscitation and replacement of intravascular volume is no different for this patient from other patients. Because the capillary leak and venous capacitance increase would be the same with the same amount of sepsis pathophysiology, this patient has the same intravascular fluid replacement needs as someone without ESRD. However, because the kidneys are non-functioning, the result of over-resuscitation with fluids must be considered, as described below.
“What about cardiomyopathy?” Again, the fluid loss from the intravascular space and the venous capacitance changes would be the same as in a patient without cardiomyopathy. Theoretically, the patient requires the same amount of fluid therapy to reach the baseline status (which is elevated filling pressures which produce benefit in allowing compensation for the cardiomyopathy with higher end-diastolic volume increasing contractile force due to the Starling principle). This is tolerated to some degree because the lymphatic system increases its drainage capability as a compensatory mechanism. As with ESRD patients, over-resuscitation is more of a problem due to underlying cardiomyopathy. Furthermore, because further decrease in myocardial contractility occurs in the majority of patients with septic shock, this group of patients will have more cardiac dysfunction than the group of patients without cardiomyopathy at baseline and will be more likely to need or benefit from dobutamine added to norepinephrine.
In both scenarios, because the same degree of septic shock requires the same amount of fluid resuscitation but with increased risk of hypoxemia with over-resuscitation, a logical approach is to use smaller boluses of fluid, repeating again and again, while observing for any deterioration in oxygenation until fluid replacement is judged to be adequate. So an approach of 250 mL bolus, stop and assess for any clinical significant change in oxygenation, if none another 250 mL bolus, and on and on until you get the 30 mL/kg target or see some clinically significant change in oxygenation. Also, remember that some patients, even with ESRD or cardiomyopathy may need more than 30 mL/kg crystalloid and assessments of intravascular volume state such as changes in CVP with fluid administration, ultrasound of the inferior vena cava (IVC), echocardiogram and response of measured flow to passive leg raise or fluid bolus may be useful.
There is clearly an association between high input/output (I/O) and worse outcome in patients with septic shock has been reported. The problem with linking this association to cause and effect is that patients with greater severity of septic shock will by definition have greater capillary leak and greater venodilation and require greater amounts of fluid resuscitation. Therefore, it is hard to separate out the downside of fluids with the need for high amounts of fluid resuscitation to maintain intravascular volume and hemodynamic stability in the most severe patients. It is clear however, that there is a price to be paid for aggressive early fluid resuscitation even if it is a lifesaver and that is the fluid accumulating in the “third spaces”. This interstitial fluid if potentially problematic in lung, brain, and kidney and subacutely could lead to an impediment to organ recovery, i.e. improvement in mental status, improvement in oxygenation and weaning from ventilator and kidney issues.
Balance of vasopressors and fluids to maintain MAP. One can maintain MAP by either increasing left ventricular preload and stroke volume with more aggressive fluid resuscitation versus use of higher doses of vasopressors to achieve MAP with arteriolar and vasoconstriction. One intervention increases MAP by increasing flow and the other intervention increases perfusion pressure with vasoconstriction. It is likely that for every patient there is an ideal mix of fluid resuscitation and vasopressors to achieve MAP.
Unfortunately, in 2016 we do not know what that mixture is for any individual patient. One can postulate that as fluids are given more aggressively to maintain MAP that CVP and the associated renal vein pressure will raise and that renal perfusion pressure (MAP – CVP) will decrease. As one resuscitates a patient with acute kidney injury due to sepsis it is important to keep renal perfusion pressure in mind and the potential for increasing MAP should be considered in the presence of higher CVP.
Vasopressor therapy
A study by LeDoux et al., compared the effects of MAP targets of 65 mmHg, 75 mmHg and 85 mmHg in patients with septic shock. There was no difference in systemic oxygen metabolism, skin microcirculatory blood flow, urine output, or splanchnic perfusion as the MAP increased (Table 3) [22].
| 65 mmHg | 75 mmHg | 85 mmHg | F/LT | |
|---|---|---|---|---|
| Urinary Output (mL) | 49 ± 18 | 56 ± 21 | 43 ± 13 | .60/.71 |
| Capillary blood flow (mL/min/100 g) | 6.0 ± 1.6 | 5.8 ± 1.1 | 5.3 ± 0.9 | .59/.55 |
| Red Cell Velocity (au) | 0.42 ± 0.06 | 0.44 ± 0.16 | 0.42 ± 0.06 | .74/.97 |
| PiCO2 (mmHg) | 41 ± 2 | 47 ± 2 | 46 ± 2 | .11/.12 |
| Pa-PiCO2 (mmHg) | 13 ± 3 | 17 ± 3 | 16 ± 3 | .27/.40 |
Table 3: Mean Arterial Pressure (Adapted from page 2731, from LeDoux, Astiz ME, Carpati CM, Rackow ED. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med 2000; 28: 2729-2732).
Another study looked at variables associated with better outcome in septic shock and identified a MAP of 65 mmHg area under the curve as the strongest predictor of good outcome. When septic shock patients continue to be hypotensive despite adequate intravascular volume repletion the hypotension must come from two potential sources, vasodilation and depressed cardiac contractility. Therefore, the optimum vasopressor would have both inotropic and vasopressor activity. Three drugs satisfy this requirement, norepinephrine, dopamine, and epinephrine. The SOAP trial published in 2010 revealed a strong trend toward better outcome with norepinephrine and increased arrhythmias with dopamine, and therefore dopamine has fallen into disfavor as a choice for vasopressor therapy.
The SSC designates norepinephrine as the first choice vasopressor. Epinephrine can be added to and potentially substituted for norepinephrine (NE), when NE alone fails to achieve MAP target.
Vasopressin up to 0.03 units/min is an alternate to epinephrine in this circumstance, or can be routinely combined with NE as part of a vasopressor regimen. Finally, dopamine and phenylephrine are discouraged as empiric therapy but have niche uses. Septic shock patients with sinus bradycardia and high output septic shock respectively.
Source control
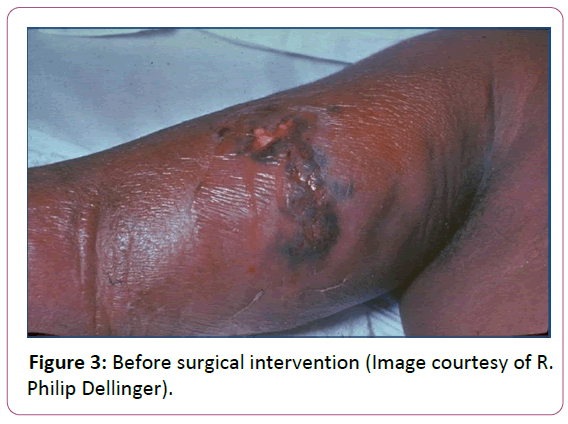
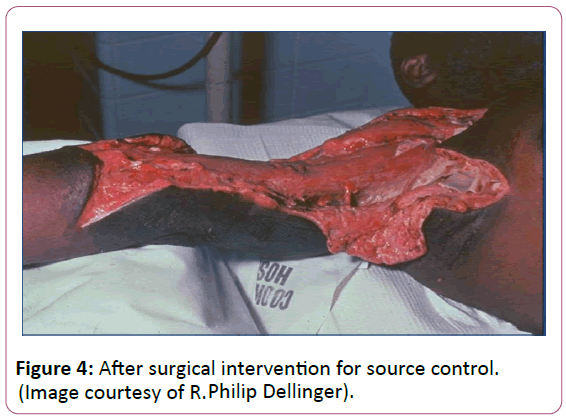
Source control is paramount in sepsis management when antibiotics alone will not clear bacteria burden. A specific anatomical diagnosis of infection requiring consideration for emergent source control should be sought and diagnosed or excluded as rapidly as possible [23]. In many cases this involves surgical debridement of a wound or percutaneous drainage of an abscess (Figures 3 and 4).
The role of steroids in the treatment of septic shock remains controversial and steroids are not indicated if adequate fluid resuscitation and vasopressor therapy are able to restore hemodynamic stability. If the patient remains hemodynamically unstable then hydrocortisone 50 mg every 6 h intravenously (IV) or 50 mg IV followed by 200 mg as a continuous infusion over 24 h for up to seven days is recommended with taper when the shock resolves.
The role of lactate measurement in the management of sepsis continues to evolve. It has been well established that as mitochondrial oxidative phosphorylation fails and energy metabolism becomes dependent on anaerobic glycolysis, the production of cellular lactate increases sharply, resulting in eventual diffusion into the blood during prolonged cell ischemia [21]. Current studies confirm the strong and independent prognostic value of hyperlactatemia in the setting of sepsis-related circulatory dysfunction [21]. Therapy that decreases lactate indicates improvement in tissue perfusion. A single elevated lactate confers a higher mortality risk, and patients who decrease admission hyperlactatemia over time do better than those manifesting incremental increase. Some sepsis patients are noted to have normal lactate levels, but are in shock.
The 2004 and 2008 Surviving Sepsis Campaign (SSC) recommends targeting a CVP of 8-12 mmHg and ScvO2 of 70% in septic shock. The 2012 SSC guidelines maintained CVP and ScvO2 target recommendations for measurement, but deemphasized specific targets by requiring measurement only. Following publication of the ProCESS and ARISE trials which demonstrated no difference in various resuscitation approaches in septic shock including “Early Goal Directed Therapy” (EGDT) that required CVP and ScvO2 targets, the measurement of CVP and ScvO2 could no longer be given preferential treatments of resuscitation. The PROMISE trail conferred similar findings. The National Quality Forum (NQF) had initially presented the SSC 2012 guidelines revised bundles. Following the ProCESS and ARISE trials changes were incorporated into these measures that no longer mandated measurement of CVP and ScvO2. The Centers for Medicare and Medicaid Services subsequently adopted similar measures. Mandated collection began in late 2015 [24].
CMS measures
Within 3 h
A. Measure lactate level.
B. Obtain blood cultures prior to antibiotics.
C. Administer broad spectrum antibiotics.
D. Administer 30 mL/kg crystalloid for hypotension or lactate = 4 mmol/L.
Within 6 h
E. Apply vasopressore (for hypotension that does not respond to initial fluid resuscitation to maintain mean arterial pressure = 65).
F. In the event of persistent hypotension after initial fluid administration (MAP <65 mmHg) or if initial lacate was = 4 mmol/L, re-assess volume status and tissue perfusion and document findings.
*To meet the requirements, a focused exam by licensed independent practicioner (LIP) to include vital signs, cardiopulomary, capillary refill, pulse and skin findings, or any 2 other items below are required.
• Measure CVP.
• Measure ScvO2.
• Bedside cardiovascular ultrasound.
• Dynamic assessment of fluid responsiveness with passive leg raise or fluid challenge.
G. Re-measure lactate if initial lactate is elevated.
Conclusion
The last fifteen years have been a remarkable time in the evolution of management principles for severe sepsis and septic shock. It is generally agreed that early identification, early antibiotics and early fluid resuscitations are paramount to decreasing mortality of severe sepsis and septic shock. It has been disappointing that some perspective literature showing interventions made a difference in severe sepsis and septic shock, but was later found not to be the case. Research continues in the area of innovative therapy which to date has been very lacking. Controversy remains as to fluid resuscitation targets and the best to fine tune resuscitation as it relates to tissue perfusion. The CMS measures are, in the opinion of these authors, a significant step forward although clearly in the alpha/beta testing mode as to reassessment tools. In 2016, the resuscitation of the septic shock patient remains both a science and a bedside art. As new research is published the SSC guidelines will continue to adopt new knowledge in new recommendations advancing our understanding and management approach of severe sepsis and septic shock forward. The next revision of the SSC guidelines is scheduled for 2018.
References
- Botero JSH, Pérez MCF (2012) The history of sepsis from ancient Egypt to the XIX century. INTECH.
- Mello P, Gusmao-Flores D, Dellinger RP (2016) Sepsis 28. Surgical Intensive Care Medicine 373.
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, et al. (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 101: 1644-1655.
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, et al. (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315: 801-810.
- Sonneville R, Verdonk F, Rauturier C, Klein IF, Wolff M, et al. (2013) Understanding brain dysfunction in sepsis. Ann Intensive Care 3: 15.
- Angus DC, van der Poll T (2013) Severe sepsis and septic shock. N Engl J Med 369: 840-851.
- Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348: 1546-1554.
- Streck EL, Comim CM, Barichello T, Quevedo J (2008) The septic brain. Neurochem Res 33: 2171-2177.
- Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A (2011) Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief pp. 1-8.
- Dellinger RP (2015) The Surviving Sepsis Campaign: Where have we been and where are we going? Cleve Clin J Med 82: 237-244.
- Dombrovskiy VY, Martin AA, Sunderram J, Paz HL (2007) Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med 35: 1244-1250.
- Shapiro NI, Howell MD, Talmor D, Lahey D, Ngo L, et al. (2006) Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med 34: 1025-1032.
- Herasevich V, Pickering BW, Dong Y, Peters SG, Gajic O, et al. (2010) Informatics Infrastructure for Syndrome Surveillance, Decision Support, Reporting, and Modeling of Critical Illness. Mayo Clin Proc 85: 247-254.
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, et al. (2013) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39: 165-228.
- Domingo-Salvany A, Lamarca R, Ferrer M, Garcia-Aymerich J, Alonso J, et al. (2002) Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 166: 680-685.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, Barnato AE, et al. (2014) A randomized trial of protocol-based care for early septic shock. N Engl J Med 370: 1683-1693.
- ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, Delaney A, Bailey M, et al. (2014) Goal-directed resuscitation for patients with early septic shock. N Engl J Med 371: 1496-1506.
- Rochwerg B, Alhazzani W, Sindi A, Heels-Ansdell D, Thabane L, et al. (2014) Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med 161: 347-355.
- Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, et al. (2012) Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 308: 1566-1572.
- Dellinger RP (2014) Crystalloids for fluid resuscitation in sepsis: where is the balance? Ann Intern Med 161: 372-373.
- Jones AE, Puskarich MA (2011) Sepsis-induced tissue hypoperfusion. Crit Care Nurs Clin North Am 23: 115-125.
- LeDoux D, Astiz ME, Carpati CM, Rackow EC (2000) Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med 28: 2729-2732.
- Schorr CA, Zanotti S, Dellinger RP (2014) Severe sepsis and septic shock: management and performance improvement. Virulence 5: 190-199.
- https://www.qualityforum.org/QPS/0500
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences