Treatment Strategies in Women with Node-Negative Oestrogen ReceptorPositive Human Epidermal Receptor 2-Negative Breast Cancers
Department of General Surgery, Tan Tock Seng Hospital, Singapore
- *Corresponding Author:
- Ern Yu Tan
Department of General Surgery
Tan Tock Seng Hospital, Singapore
Tel: +65 6357 3755; E-mail: Ern_Yu_Tan@ttsh.com.sg
Received date: November 08, 2017; Accepted date: November 17, 2017; Published date: November 15, 2017
Citation: Chan PMY, Sin PY, Chen JJC, Goh MH, Lu SQH, et al. (2017) A Review of Treatment Strategies in Women with Node-Negative Oestrogen Receptor-Positive Human Epidermal Receptor 2-Negative Breast Cancers. J Mol Genet Med Vol.1 No.1:8
Abstract
Background: Women with node-negative ER-positive/HER2negative breast cancers respond well to hormonal therapy and generally have a good prognosis. The low frequency of events has led to re-evaluations of the need for chemotherapy in addition to hormonal therapy. In this study, we reviewed the long-term outcomes of women with ER-positive/HER2-negative tumours and evaluated factors associated with outcome, specifically that of hormonal therapy, alone or in combination with chemotherapy.
Methods: We identified 117 women with available longterm follow up data from our prospective database. Endpoints examined included disease recurrence, contralateral breast cancer and overall survival. These were correlated with standard clinicopathological parameters and treatments received.
Findings: Median overall survival was 128.23 months (ranging from 12.87 to 143.20 months). Recurrence developed in 14 women (12.0%) and contralateral breast cancer in another 7; events had developed after 5 years in 13 women. The majority of women (89 of 117) received hormonal therapy and 27 women also received chemotherapy. Chemotherapy produced a small nonsignificant effect on 10 year recurrence-free and event-free survival (P=0.295 and P=0.443, respectively). Lymphovascular invasion was the only factor with an independent association with 10 year recurrence-free and event-free survival (P=0.038 and P=0.009, respectively). Tumour size, grade and progesterone receptor status showed no association. Seventy-three women completed at least 5 years of hormonal treatment, which was found to significantly improve 10 year event-free survival (P=0.005).
Conclusion: Many women with node-negative ER-positive/ HER2 negative have a good prognosis on hormonal therapy alone.
Keywords
ER-positive; HER2-negative; Breast cancer; Hormonal therapy; Chemotherapy
Introduction
Chemotherapy has contributed greatly to improved treatment outcomes over the years. Chemotherapy eliminates systemic micrometastasis, thereby reducing the risk of dissemination and seeding of distant organs, which is responsible for much of the breast cancer specific mortality. Current modalities fail to detect micrometastasis and technologies to detect circulating tumour cells have not translated into clinical practice. Consequently, chemotherapy is being recommended based on the probability of micrometastasis and expected prognosis, with nodal status being one of the strongest indicators [1]. However, even when there is no nodal involvement, many women are deemed to be at high risk and are still being recommended chemotherapy. With existing guidelines recommending chemotherapy for tumours larger than 1 cm (T1c and larger), a significant proportion of women with node-negative disease are still being recommended chemotherapy.
Even with early detection and current treatment modalities, up to 30% of women with early breast cancer develop distant recurrence and the majority eventually die from disease dissemination. This seems to support the seemingly aggressive approach to adjuvant chemotherapy recommendations. However, it is acknowledged that many women are over-treated. Hormonal therapy is proven effective in reducing recurrence and mortality in women with Oestrogen receptor (ER)-positive/ Human Epidermal Growth Factor Receptor (HER)-2-negative tumours [2-4] and unlike with triple negative tumours (tumours negative for ER, progesterone receptors and HER2) and HER2- overexpressing tumours, the indication for chemotherapy is less strong in this group. Multigene assays have in fact demonstrated that many women with ER-positive/HER-2-negative tumours will have a good outcome on hormonal therapy alone and that chemotherapy confers little additional benefit. These multigene assays, such as Oncotype DX, Mammaprint and EndoPredict, appear to fulfil the inadequacies of traditional predictors and help limit the use of chemotherapy [5,6]. The use of these assays remains limited in our practice primarily because of its cost and the lack of reimbursement, but with the increasing emphasis on personalised treatments to reduce unnecessary treatment-related adverse side effects, we have now reviewed the treatment of women with ER-positive/HER2-negative tumours at our unit. In this present study, we reviewed the treatment strategies and clinical outcome in women diagnosed with node-negative ER-positive/HER2-negative invasive breast cancers, this being the group of women who would benefit from further evaluation with multigene assays. We specifically focused on women in whom long-term follow up data was available, given that recurrences occurring after 5 years are a particular problem in women with ER-positive/HER2-negative tumours.
Methods
Women were identified from the TTSH Prospective Breast Cancer Database (TTSH2017-00011) and ethnic’s approval was obtained from the National Healthcare Group Domain Specific Review Board (2013/00597). Women with histologically confirmed ER-positive/HER2-negative node-negative breast cancers staged as T1, T2 or T3 were included. Those who declined recommended adjuvant treatments were included, but those who did not undergo surgery were excluded. Women with T4 tumours were excluded, as well as women with complete pathological response in the nodes after neoadjuvant chemotherapy. Isolated tumour cells were considered nodenegative, but micrometastasis were considered N1 nodal involvement. Median follow up was 128.23 months (ranging from 12.87 to 143.20 months).
Tumor ER, PR and HER2 status were assessed with immunohistochemistry (IHC) using validated protocols at our laboratory, which has College of American Pathologists (CAP) accreditation. Formalin fixed paraffin embedded tumor sections were stained with anti-ER antibody (Neomarker MS750, Neomarkers Inc., Portsmouth, New Hampshire, United States of America) and anti-PR antibody (Dako M3569, Agilent Technologies, Santa Clara, United States of America), with Envision ChemMate (Agilent Technologies, Santa Clara, United States of America) used as the detection system for ER and the Ventana detection kit (Ventana Medical Systems Inc., Tucson, United States of America) for PR. The positive threshold was set at 10% or more tumor cells staining positive with at least moderate intensity. Tumor HER2 status was assessed first with IHC with anti-HER2 antibody (Neomarker MS-441, Neomarkers Inc., Portsmouth, New Hampshire, United States of America) and tumors were considered positive if at least 10% of tumor cells exhibited intense membranous staining and equivocal if at least 10% of tumor cells exhibited moderate membranous staining. In 2007, the anti-HER2 antibody and thresholds were changed. Anti-HER2 antibody (Neomarker RM-9103, Neomarkers Inc., Portsmouth, New Hampshire, United States of America) was used instead and tumors were considered positive if more than 30% of tumor cells exhibited uniform intense membrane staining; equivocal when at least 10% of tumor cells exhibited complete circumferential membrane staining that was non-uniform or weak in intensity, or if less than 30% of tumor cells exhibited intense complete membrane staining. Tumors not fulfilling these criteria were considered HER2-negative. Tumors with an equivocal IHC result were then sent to another institute for fluorescent in situ hybridization (FISH) where HER2/neu gene amplification was detected using Vysis PathVysion HER2 DNA Probe kit. Tumors were considered negative if the ratio of HER2 gene signals to chromosome 17 signals was less than 2.0.
All women were recommended hormonal therapy unless there were contraindications. Pre-menopausal women were offered tamoxifen while post-menopausal women were offered the choice between tamoxifen and aromatase inhibitors. Anastrozole and letrozole were used as first-line. At the time of the study, hormonal therapy was recommended for a total duration of 5 years. In the later years, the option of extended therapy was discussed with those who had completed 5 years of tamoxifen, but most patients declined. Chemotherapy was recommended to women with tumours larger than 1cm. In those with T1b tumours (0.5 cm to 1 cm), chemotherapy was discussed and factors such as tumor grade, lymphovascular invasion, and age at diagnosis were taken into consideration. Chemotherapy was not indicated in women with T1a tumours (smaller than 0.5 cm). Co-existing morbidities and performance status were also taken into consideration. Anthracycline-based regimens were preferred, with doxorubicin/cyclophosphamide followed by paclitaxel (AC/T) being the most commonly used. Surgery involved either a wide local excision (WLE) or a mastectomy; immediate breast reconstruction post-mastectomy was offered to suitable women. Sentinel lymph node biopsy was the standard at our unit and axillary clearance was done only in instances where the surgeon failed to identify the sentinel node. Surgical margins of at least 1mm were considered adequate. Whole breast radiation, often with a tumor bed boost, was standard in women treated with WLE, but chest wall radiation post-mastectomy was not recommended unless the tumor was larger than 5 cm.
Cox regression was used to identify independent factors associated with event-free and recurrence-free survival and was carried out using the Stata package release 11.0 (Stata Corporation, 4905 Lakeway Drive, College Station, Texas 77845, USA). A full model was first created to include all potentially important explanatory variables. At each step, the variable with the smallest contribution to the model was removed, until a final backward stepwise model was obtained. The effect of hormonal therapy, alone or in combination with chemotherapy, on survival was examined using Kaplan-Meier survival curves; these were calculated using GraphPad Prism, version 6 (GraphPad Software, San Diego, CA). Event-free survival was defined as the time interval from the date of definitive breast cancer surgery to the development of recurrence, whether locoregional or systemic, contralateral breast cancer, a new primary cancer or death in a previously non-metastatic patient; locoregional recurrence was defined as recurrent disease in the ipsilateral breast (post-wide local excision) or chest wall (postmastectomy) or in the ipsilateral axial nodes; contralateral cancer was defined as a metachronous cancer in the contralateral breast more than 6 months after the first diagnosis. Recurrence-free survival was defined as the time interval from definitive breast cancer surgery to the development of disease recurrence. For survival analyses, patients were censored after 10 years of follow up and also if they were lost to follow-up. A 2-tailed P value test was used for all analyses and a value of P<0.05 was considered statistically significant.
Results
A total of 117 women were included in this present study. Median age was 55 years (ranging from 32 to 84 years) and the women were predominantly Chinese. Details are included in Table 1. All women had node-negative cancers and the tumour was staged as T1 in 80 women, as T2 in 35 women and as T3 in 2 women. Isolated tumour cells were noted in the nodes of 5 women. Median tumour grade was 1. Median ER staining intensity was strong and the median proportion of cells staining positive was 90%; 109 tumours were negative for HER2 on IHC and another 3 were equivocal on IHC but negative on FISH assay.
| Parameter | Number of women (%) |
|---|---|
| Ethnicity Chinese Malay Indian Others |
91 (78) 10 (9) 11 (9) 5 (4) |
| Disease Stage I II |
80 (68) 37 (32) |
| Tumour Histology Ductal carcinoma (not otherwise specified) Lobular carcinoma Mixed ductal and lobular carcinoma Cribriform carcinoma Mucinous carcinoma Papillary carcinoma Tubular carcinoma |
90 (77) 6 (5) 2 (2) 5 (4) 12 (10) 1 (1) 1 (1) |
| Tumour grade 1 2 3 |
49 (42) 46 (39) 22 (19) |
| Lymphovascular invasion Present Absent |
 11 (9) 106 (91) |
| Surgery type Wide local excision Mastectomy |
57 (49) 60 (51) |
| Chemotherapy* AC/T AC FAC CMF |
27 (23) 10 (as a dose dense regimen in 3) 9 6 1 |
| Received hormonal therapy Completed hormonal therapy 5 years of tamoxifen 5 years of AI Switch to AI after 2 years of tamoxifen Extended therapy |
89 (76) 73 43 16 (14 anastrozole, 2 letrozole) 13 1 |
| Event Locoregional recurrence Distant recurrence Contralateral cancer New primary cancer |
21 (18) 7 7†6†2 |
Table 1: Details of clinicopathological parameters, treatment and outcome of the 117 women with ER-positive/HER2-negative node negative cancers,*chemotherapy regimens: AC/T: doxorubicin/cyclophosphamide followed by paclitaxel, FAC: fluorouracil/doxorubicin/cyclophosphamide, CMF: cyclophosphamide/metrotrexate/fluorouracil; details of regimen not available for 1 women. AI: Aromatase Inhibitors. †1 patient developed contralateral cancer at 101.17 months and distant recurrence at 111.23 months.
Table 1 Details of clinicopathological parameters, treatment and outcome of the 117 women with ER-positive/HER2-negative node negative cancers,*chemotherapy regimens: AC/T: doxorubicin/cyclophosphamide followed by paclitaxel, FAC: fluorouracil/ doxorubicin/cyclophosphamide, CMF: cyclophosphamide/metrotrexate/fluorouracil; details of regimen not available for 1 women. AI: Aromatase Inhibitors. †1 patient developed contralateral cancer at 101.17 months and distant recurrence at 111.23 months.
Recurrence developed in 14 women (12.0%) and occurred after 5 years in 9 women. Median time to locoregional recurrence was 97.86 months (ranging from 14.80 to 119.73 months) and to distant recurrence was 108.33 months (ranging from 18.60 to 115.30 months). Ipsilateral breast tumour recurrence occurred in 5 women, all of whom had adequate surgical margins after WLE and had completed whole breast radiation with a 10Gy tumour bed boost. Two women developed chest wall recurrence after mastectomy. None of these women were found with systemic disease. Distant recurrence developed in 7 women, with the lungs being most frequently involved. None had disease limited to the bones only.
We next examined the association between clinical outcome, standard clinicopathological factors and the treatments received. Lymphovascular invasion was the only factor that showed an independent association with 10 year recurrencefree and event-free survival (P=0.038 and P=0.009, respectively) (Tables 2 and 3). Tumour size, grade and progesterone receptor (PR) status all showed no association. Roughly equal numbers of women were treated with WLE and mastectomy, and 8 of the women with mastectomy opted for immediate breast reconstruction at the same setting. Six of 57 women who underwent WLE defaulted on adjuvant whole breast radiation, though one still received hormonal therapy. None of these 6 women developed recurrence or contralateral cancer. All women received 50 Gy in 25 fractions to the breast and 49 women also received a 10 Gy boost to the tumor bed. Locoregional recurrence developed in 5 women treated with breast conserving therapy compared to only 2 women treated with mastectomy, but the trend was not statistically significant (P=0.264) and the type of surgery did not show any independent association with 10 year recurrence survival (P=0.379) (Table 2).
| Parameter | Hazard Ratio | Standard Error | P value | 95% CI |
|---|---|---|---|---|
| Chemotherapy vs. Hormonal monotherapy | 0.519 | 0.480 | 0.478 | 0.085-3.178 |
| Completed at least 5 years of hormonal therapy | 1.356 | 1.115 | 0.709 | 0.272-6.786 |
| Mastectomy vs. breast conserving therapy | 0.531 | 0.382 | 0.379 | 0.129-2.175 |
| Age | 1.017 | 0.314 | 0.577 | 0.958-1.081 |
| Tumour T stage | 1.502 | 1.098 | 0.578 | 0.359-6.291 |
| Tumour grade | 1.000 | 0.823 | 1.000 | 1.999-5.021 |
| Lymphovascular invasion | 4.513 | 3.278 | 0.038 | 1.087-18.734 |
| Tumour PR status | 0.524 | 0.338 | 0.316 | 0.148-1.854 |
Table 2 Cox regression model stratified by 10-year recurrence-free survival (n=91). PR: progesterone receptor
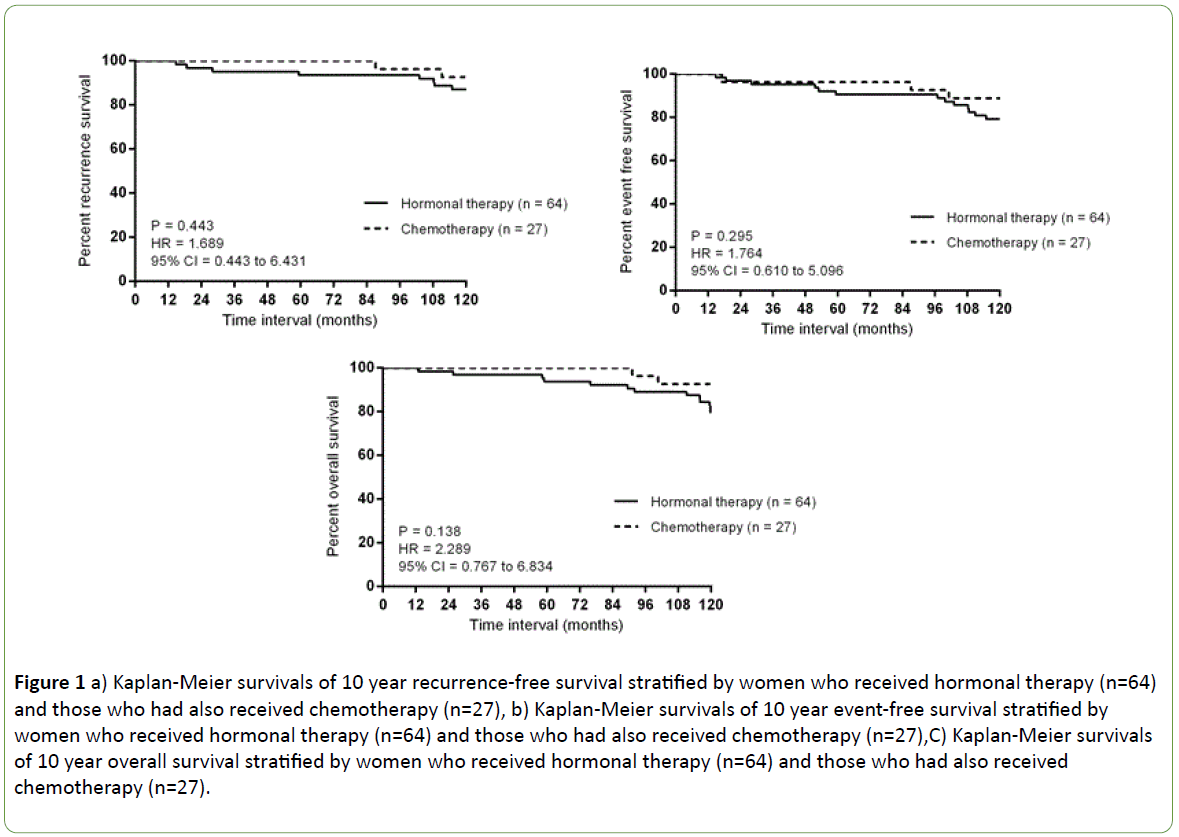
About a quarter of women (27 of 117, 23.7%) received chemotherapy, and more than half received an anthracyclinebased regimen. Only 3 women did not complete the recommended regimen; 2 due to intolerable side effects and 1 patient died from a non-breast cancer related cause before chemotherapy completion. Chemotherapy appeared to improve both 10 year recurrence and event free survival but the effect was small and not statistically significant (P=0.443 and P=0.295, respectively) (Figures 1a and 1b). Eighty-nine women (76%) agreed to and received hormonal therapy and 72 women completed 5 years of hormonal therapy while another woman received extended therapy, completing 2 years of letrozole after an initial 5 years of tamoxifen. Five years of hormonal therapy, regardless of whether it was tamoxifen or an aromatase inhibitor, produced a significant improvement on 10 year eventfree survival (P=0.005) (Table 3).
| Parameter | Hazard Ratio | Standard Error | P value | 95% CI |
|---|---|---|---|---|
| Chemotherapy vs. Hormonal monotherapy | 0.399 | 0.285 | 0.198 | 0.099-1.616 |
| Completed at least 5 years of hormonal therapy | 0.209 | 0.116 | 0.005 | 0.070-0.621 |
| Mastectomy vs. breast conserving therapy | 1.212 | 0.703 | 0.740 | 0.389-3.778 |
| Age | 1.129 | 0.721 | 0.849 | 0.324-3.941 |
| Tumour T stage | 0.537 | 0.330 | 0.311 | 0.161-1.791 |
| Tumour grade | 1.286 | 0.876 | 0.712 | 0.338-4.888 |
| Lymphovascular invasion | 5.368 | 3.454 | 0.009 | 1.521-18.943 |
| Tumour PR status | 1.179 | 0.659 | 0.769 | 0.394-3.524 |
Table 3 Cox regression model stratified by 10-year event-free survival (n=91). PR: progesterone receptor.
Contralateral cancer developed in 7 women after a median of 96.97 months (ranging from 17.07 to 110.87 months) and occurred after 5 years in 4. Two women were diagnosed with new primary cancers; one was diagnosed with malignant neuroendocrine tumour and another with hepatocellular carcinoma, neither had evidence of breast cancer recurrence. Fourteen women died during the study period, with median overall survival for the group being 128.23 months (ranging from 12.87 to 143.20 months). Overall survival appeared poorer among those who received only hormonal therapy, but again, this was not statistically significant (P=0.138) (Figure 1c).
Figure 1: a) Kaplan-Meier survivals of 10 year recurrence-free survival stratified by women who received hormonal therapy (n=64) and those who had also received chemotherapy (n=27), b) Kaplan-Meier survivals of 10 year event-free survival stratified by women who received hormonal therapy (n=64) and those who had also received chemotherapy (n=27),C) Kaplan-Meier survivals of 10 year overall survival stratified by women who received hormonal therapy (n=64) and those who had also received chemotherapy (n=27).
Discussion
In our present study, disease recurrence developed in 12% of women with ER-positive/HER2-negative tumours and another 6% of women developed contralateral breast cancer. This low frequency of events has been consistently observed and ERpositive/ HER2-negative tumours have the most favourable outcome among all the breast cancer subtypes [7,8]. Majority of the women in our study agreed to hormonal therapy, given the general perception that oral medications are less toxic. Tamoxifen was the preferred agent primarily because of the significant cost differences between tamoxifen and aromatase inhibitors. Of note was the observation that more than half the events occurred beyond 5 years, with the median times to recurrence and contralateral cancers being well beyond 60 months. Women completing 5 years of hormonal therapy gained a significant improvement in 10 year event-free survival, but the addition of chemotherapy produced only a small non-significant benefit beyond that of hormonal therapy.
The favourable prognosis frequently seen with these tumours have called into question the benefit of chemotherapy over and above that of hormonal therapy. Guidelines recommend chemotherapy for tumours larger than 1 cm (T1c) and call for chemotherapy to be considered in women with T1b tumours. Discussions are often hampered by difficulties in estimating the risk-benefit ratio. This, together with a general reluctance to receive chemotherapy, explain why only less than half the women with T1b and T1c tumours in our study received chemotherapy; only 1 of the 17 (6%) women with T1b tumours and 13 of the 34 (38%) women with T1c tumours received chemotherapy in addition to hormonal therapy. Majority of the women received anthracycline-based regimens, as nonanthracycline regimens were not yet widely used. Only 2 women suffered from intolerable side effects that required premature discontinuation, but many more women suffer adverse side effects related to the chemotherapy treatment, some of which, like taxane-induced peripheral neuropathy, may be permanent and irreversible.
The low recurrence risk among this group of women suggests that many are being over-treated with chemotherapy, but the difficulty comes with identifying those at risk. Clinical subtype appears to be of the greatest significance in node-negative disease. Tumours classified as ER-positive/HER2-negative, corresponding to the Luminal A molecular subtype, have been found with the lowest risk of recurrence, compared to triple negative and HER2-overexpressing tumours [9]. Reports are conflicting, but tumour size, tumour grade, lymphovascular invasion and age at diagnosis have variously been reported as predictors of recurrence [10-12]. Specifically, tumour PR status was found particularly relevant in ER-positive/HER2-negative tumours, but we did not observe a similar association [13]. In our study, lymphovascular invasion was the only independent predictor of outcome. Neither tumour size, grade nor age at diagnosis showed any correlation. However, it is unlikely that lymphovascular invasion alone has sufficient discriminatory power and in fact, more accurate risk stratification has been demonstrated with multigene assays [14-17]. Women with a low score are deemed to have a low risk of distant recurrence and are expected to do well on hormonal therapy alone, while those with a high score, which translates into a high risk of distant recurrence, are thought to benefit from a combination of chemotherapy and hormonal therapy [16,18]. Prospective data has demonstrated a favourable prognosis in women with low scores who received hormonal therapy alone, and the MINDACT study has further reported that chemotherapy conferred little additional survival benefit beyond that achieved with hormonal therapy in women with low genomic scores, even if they scored high on the clinical Adjuvant! Online model [15,19]. This once again suggests that tumor biology, as assessed by genomic parameters, is superior to traditional clinicopathological parameters.
Late recurrences is a particular problem in women with ERpositive/ HER2-negative cancers and the frequency of recurrences after 5 years is higher than that observed in triple negative and HER2-overexpressing tumours [20,21]. We made a similar observation in our study, where more than half the recurrences and contralateral breast cancers developed after 5 years, at a point where the women would have completed the recommended hormonal therapy regimen. We observed a greater reduction in contralateral cancers compared to recurrent events, although this could be because of the relatively small sample size. Regardless, the occurrence of these late events strengthens the rationale for extended therapy, although there remains doubt as to whether all women will benefit from a 10 year treatment [22-24]. Several clinicopathological parameters, including tumor size, grade, nodal involvement, are strong predictors of recurrence [25-27], but the predictive value of certain parameters such as tumor grade appears to be restricted to the first 5 years [25]. The EndoPredict assay was specifically evaluated with respect to the prediction of recurrences beyond 5 years and was a more accurate of 10 year distant recurrence compared to Oncotype DX [28]. This superior predictive power was found to be a result of its ER-signaling gene set [29]. In contrast, the ER module of Oncotype DX lost predictive power after 5 years [30]. The integration of nodal status and tumor size into the purely genomic EP score (EPclin score) further strengthens the prediction of late distant recurrence [16]. Women who score high on multigene assays are recommended chemotherapy in addition to hormonal therapy based on the assumption that combination therapy will reduce distant recurrence, although the assays do not strictly indicate the response to chemotherapy. It also remains unclear as to whether chemotherapy will actually reduce the frequency of late recurrences, particularly since it has been reported that chemotherapy is mainly effective in the first 5 years [31]. This will be clearer when the long-term results of the MINDACT study is reported.
Our study was limited by the low frequency of events occurring in women with ER-positive/HER2-negative tumours, which is inherent to tumours of this particular subtype. Furthermore, a long follow up is necessary since late events are more frequent compared to other subtypes and this can potentially make results more difficult to analyse since treatment regimens used in patients with sufficient follow-up data can be different from those currently used. Although looking at patients from earlier years would have increased our study numbers, we chose to include women from 2006 onwards as HER2 testing and trastuzumab treatment became routine only from then. Aromatase inhibitor use started during this period, although most women continued to receive tamoxifen because of the cost difference and this therefore did not allow us to analyse the efficacy of tamoxifen and aromatase inhibitors separately. This has changed in more recent years, and with the introduction of generic letrozole, aromatase inhibitors are now the preferred agent in post-menopausal women. Nevertheless, this present study affirms the good prognosis of women with node-negative ER-positive/HER2-negative cancers and provides support for the use of multigene assays to stratify recurrence risk and guide treatment recommendations. The high frequency of late events also highlights the need for long-term surveillance and calls for the consideration of extended hormonal therapy.
Funding Sources
Work was funded in part by the National Healthcare Group Small Innovative Grant (SIG/15025).
Competing Interests
All authors declare no conflict of interest.
References
- Rack B, Janni W, Gerber B, Strobl B, Schindlbeck C, et al. (2003) Patients with recurrent breast cancer: Does the primary axillary lymph node status predict more aggressive tumor progression? Breast Cancer Res Treat 82: 83-92.
- (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15 year survival: An overview of the randomised trials. Lancet 365: 1687-1717.
- Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, et al. (2008) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100 month analysis of the ATAC trial. Lancet Oncol 9: 45-53.
- Thurlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, et al. (2005) A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353: 2747-2757.
- Eiermann W, Rezai M, Kummel S, Kuhn T, Warm M, et al. (2013) The 21-gene recurrence score assay impacts adjuvant therapy recommendations for ER-positive, node-negative and node-positive early breast cancer resulting in a risk-adapted change in chemotherapy use. Ann Oncol 24: 618-624.
- Holt S, Bertelli G, Humphreys I, Valentine W, Durrani S, et al. (2013) A decision impact, decision conflict and economic assessment of routine oncotype DX testing of 146 women with node-negative or pNImi, ER-positive breast cancer in the U.K. Br J Cancer 108: 2250-2258.
- Hennigs A, Riedel F, Gondos A, Sinn P, Schirmacher P, et al. (2016) Prognosis of breast cancer molecular subtypes in routine clinical care: A large prospective cohort study. BMC Cancer 16: 734.
- Haque R, Ahmed SA, Inzhakova G, Shi J, Avila C, et al. (2012) Impact of breast cancer subtypes and treatment on survival: An analysis spanning two decades. Cancer Epidemiol Biomarkers Prev 21: 1848-55.
- Gamucci T, Vaccaro A, Ciancola F, Pizzuti L, Sperduti I, et al. (2013) Recurrence risk in small, node-negative, early breast cancer: A multicenter retrospective analysis. J Cancer Res Clin Oncol 139: 853-60.
- Fisher ER, Costantino J, Fisher B and Redmond C (1993) Pathologic findings from the National Surgical Adjuvant Breast Project (Protocol 4). Discriminants for 15 year survival. National Surgical Adjuvant Breast and Bowel Project Investigators. Cancer 71: 2141-2150.
- Fisher ER, Anderson S, Redmond C and Fisher B (1993) Pathologic findings from the National Surgical Adjuvant Breast Project protocol B-06. 10 year pathologic and clinical prognostic discriminants. Cancer 71: 2507-2514.
- Rosen PP, Groshen S, Saigo PE, Kinne DW and Hellman S (1989) Pathological prognostic factors in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma: A study of 644 patients with median follow-up of 18 years. J Clin Oncol 7: 1239-1251.
- Kurozumi S, Matsumoto H, Hayashi Y, Tozuka K, Inoue K, et al. (2017) Power of PgR expression as a prognostic factor for ER-positive/HER2-negative breast cancer patients at intermediate risk classified by the Ki67 labeling index. BMC Cancer 17: 354.
- Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, et al. (2010) Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: A TransATAC study. J Clin Oncol 28: 1829-1834.
- Cardoso F, van't Veer LJ, Bogaerts J, Slaets L, Viale G, et al. (2016) 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375: 717-729.
- Filipits M, Rudas M, Jakesz R, Dubsky P, Fitzal F, et al. (2011) A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res 17: 6012-1620.
- Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, et al. (2010) A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res 16: 5222-5232.
- Sparano JA, Paik S (2008) Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol 26: 721-728.
- Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, et al. (2015) Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 373: 2005-2014.
- Cossetti RJ, Tyldesley SK, Speers CH, Zheng Y, Gelmon KA (2015) Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. J Clin Oncol 33: 65-73.
- Ribelles N, Perez-Villa L, Jerez JM, Pajares B, Vicioso L, et al. (2013) Pattern of recurrence of early breast cancer is different according to intrinsic subtype and proliferation index. Breast Cancer Res 15: R98.
- Davies C, Pan H, Godwin J, Gray R, Arriagada R, et al. (2013) Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381:805-816.
- Jakesz R, Greil R, Gnant M, Schmid M, Kwasny W, et al. (2007) Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: Results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst 99: 1845-1853.
- Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, et al. (2005) Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 97: 1262-1271.
- Sestak I, Dowsett M, Zabaglo L, Lopez-Knowles E, Ferree S, et al. (2013) Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst 105: 1504-1511.
- Castano Z, Tracy K and McAllister SS (2011) The tumor macroenvironment and systemic regulation of breast cancer progression. Int J Dev Biol 55: 889-897.
- Mittempergher L, Saghatchian M, Wolf DM, Michiels S, Canisius S, et al. (2013) A gene signature for late distant metastasis in breast cancer identifies a potential mechanism of late recurrences. Mol Oncol 7: 987-999.
- Buus R, Sestak I, Kronenwett R, Denkert C, Dubsky P, et al. (2016) Comparison of EndoPredict and EPclin with oncotype DX recurrence score for prediction of risk of distant recurrence after endocrine therapy. J Natl Cancer Inst 108.
- Dubsky P, Brase JC, Jakesz R, Rudas M, Singer CF, et al. (2013) The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2-breast cancer patients. Br J Cancer 109: 2959-2964.
- Dowsett M, Sestak I, Buus R, Lopez-Knowles E, Mallon E, et al. (2015) Estrogen receptor expression in 21-gene recurrence score predicts increased late recurrence for estrogen-positive/HER2-negative breast cancer. Clin Cancer Res 21: 2763-2770.
- Early Breast Cancer Trialists' Collaborative G (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 365: 1687-717.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences