The Isomers of Clomiphene Citrate have Dissimilar Dispositions Once Ingested: Results of a Mouse ADME Study
Fontenot GK*, Wiehle RD, Hsu K and Podolski J
Repros Therapeutics, The Woodlands, Texas, USA
- *Corresponding Author:
- Gregory K Fontenot, PhD
Repros Therapeutics 2408 Timberloch Place B-7
The Woodlands, Texas 77380, USA
Tel: 281 719 3455
Fax: 281 719 3446
Email: gfontenot@reprosrx.com
Received date: January 17, 2017; Accepted date: January 18, 2017; Published date: January 25, 2017
Citation: Fontenot GK, Wiehle RD, Hsu K, et al. The Isomers of Clomiphene Citrate have Dissimilar Dispositions Once Ingested: Results of a Mouse ADME Study. Adv Tech Clin Microbiol. 2017, 1:1.
Copyright: © 2017 Fontenot GK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Clomiphene Citrate is composed of two structural isomers: cis- or Zuclomiphene citrate (Zu) and trans- or Enclomiphene citrate (En). Clomiphene, as well as isolate isomers, has recently been shown to have effects on Ebola virus infections. It has been shown that the enclomiphene molecule is an estrogen antagonist and that zuclomiphene is inactive. We demonstrate here that the two isomers have different fates once ingested and the tissues that absorb each are distinct from each other to lead to different biologic effects. Methods and findings: We studied these molecules by employing 14C-labelled versions of each in C-57 black mice. Mice were given the same oral dose, but sacrificed at different time periods. Each isomer could be followed separately. Enclomiphene was rapidly lost such that the majority was found in low amounts after 24 h. Zuclomiphene was distributed to more organs and remained associated with discrete tissues for longer periods of time. Remarkable exceptions were the pigmented organs of the eye, which retained both compounds. Notable was the specific absorption in individual tissues and the lack of clearance in certain cases. The ratio of zuclomiphene to enclomiphene (Zu/En) demonstrated the promiscuous nature of the zuclomiphene and the specific absorption. The tissue/plasma ratios demonstrated those tissues that were accrued or failed to clear compounds. Important differences were found in the lack of clearance of isomers in the eye, gall blabber/bile, brain, lung, fat, adrenals, kidneys and reproductive tissues. Conclusion: The adverse effects of Clomiphene citrate in the eye and male reproductive organs may be rationalized by lack of clearance of higher levels of zuclomiphene as well as effects of Ebola virus infection. An additional element of this study was to determine levels that would not be expected to represent a significant radiation exposure risk to human male volunteers in future ADME studies.
Keywords
Clomiphene; Enclomiphene; Zuclomiphene; Blood-brain barrier; Metabolism; Uveal tract.
Introduction
Clomiphene citrate has been approved for use in women to induce ovulation [1-3] and has been used off-label in men to raise serum testosterone [4-9]. The latter use was rationalized early [10] by the recognition that a bolus dose of Clomiphene citrate could be used as a diagnostic tool to determine the functionality of the hypothalamic-pituitary axis by the stimulation of release of Luteinizing Hormone (LH) in a subject with low gonadotropins. Clomiphenes and its isolated isomes, have also been shown to have an effect on progression of disease after Ebola virus infection in cell culture and mouse model systems [11, 12].
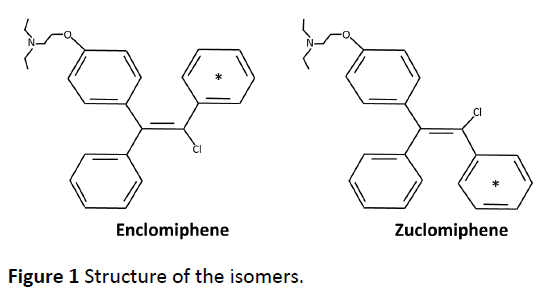
Clomiphene citrate is considered to be a SERM, i.e., a compound possessing estrogen agonist or antagonist properties depending on the tissue. Clomiphene citrate was described as a mixture of two isoforms, enclomiphene (trans-isomer) and zuclomiphene (cis-isomer), with estrogen agonist or antagonist properties [13-16]. The structure of the isomers described in the initial papers is shown in Figure 1. Clomiphene citrate is available commercially as a mixture of the two isomers. Clomiphene citrate is approximately 62% enclomiphene and 38% zuclomiphene.
Enclomiphene citrate has anti-estrogenic properties [17] and appears to block the negative feedback inhibitory effects of estradiol on the hypothalamic-pituitary axis resulting in increased levels in both LH and follicle stimulating hormone (FSH) which stimulate endogenous intragonadal testosterone production and spermatogenesis in men [8, 18].
Clomiphene citrate presented no adverse sequelae due to zuclomiphene which is, at worst, an inactive congener. That view was not blunted despite the known much longer half-life of zuclomiphene [19, 20] and the absence of estrogen antagonism [17, 21], the key feature of the activity of the mixture. Early ADME studies of Clomiphene citrate describe loss through the feces and urine after a single dose [13, 22]. Previous descriptions of The ADME profiles of Clomiphene citrate are thus inadequate since the longer-lived zuclomiphene would be in excess over the more active enclomiphene.
We have determined that one of the two isomers of Clomiphene citrate, Zuclomiphene citrate, a component of a drug believed to be safe, has deleterious effects on the male reproductive organs of mice [23]. There are known effects of clomiphene on the eyes [24-26] as has been recognized in the product label [27]. These effects might be a result of an accumulation of the zuclomiphene isomer over time.
Materials and Methods
The 14C-labelling of Enclomiphene and Zuclomiphene was done at Ricerca Concord, OH. The labeled compounds as citrate salts were done after synthesis. The positon of the 14C-label was distributed among the carbons of one of the rings (Figure 1). The in-life dosing to C-57 black mice was performed at Covance Laboratories Inc. Madison, WI under the direction of Randall Press. All procedures performed in animals were under the guidance of the “Guide for the Care and Use of Laboratory Animals”. Tissue distribution of 14CEnclomiphene related radioactivity was assessed following a single oral administration of 14CEnclomiphene citrate to male and female pigmented (C57 black) mice (Group 1). Tissue distribution of 14CZuclomiphene related radioactivity was also investigated following a single oral administration of 14CZuclomiphene citrate to male pigmented (C57 Black) mice (Group 2). Dose formulations were prepared as solutions on the day of dose administration by combining appropriate amounts of radiolabeled and nonradiolabeled Enclomiphene citrate or radiolabeled and non-radiolabeled Zuclomiphene citrate in 0.5% methylcellulose and 0.2% Tween 80 in reverse osmosis water at a nominal concentration of 2.0 mg/mL and a target radioactivity dose of 100 μCi/20 mg/kg. In Group 1, two animals/sex/time points were sacrificed at 0.25, 1, 4, 24, 72, 168, 240, 336, 504 and 840 h. For Group 2, two animals/time points were sacrificed at 1, 4, 24, 72, 168, 240, 336, 504, 840 and 1008 h post dose. Blood was collected and plasma was harvested at specified time points and carcasses were prepared for QWBA. Sections were collected from one carcass per time point and exposed to phosphor imaging screens. The images were processed for determination of the radioactivity concentrations in selected tissues. Blood and plasma were analyzed for concentrations of radioactivity using liquid scintillation counting (LSC). The radioactivity concentrations were expressed as ng equivalents 14CEnclomiphene/g or ng equivalents 14CZuclomiphene/g of sample, as applicable. The pharmacokinetic analysis of 14CEnclomiphene citratederived and 14CZuclomiphene citratederived radioactivity were conducted for blood, plasma and tissues and radiation dosimetry parameters were calculated. There was no attempt to separate and thus determine the identity of the 14C-derived products, i.e., to determine the metabolites of each drug. A description of the kinds of metabolites derived from each of the drugs in women has already been shown [28, 29]. We have also determined metabolites of each in liver hepatocytes of four species and characterized the metabolites detected in dogs after both acute and chronic dosing using non-labelled materials (internally generated data not shown).
Results
Test article and dose formulation analysis
Radiopurity and stability
The HPLC analyses performed by Covance showed the radiopurities of 14C-Enclomiphene and 14C-Zuclomiphene to be 98.2 and 96.6%, respectively, prior to dose preparation. The mean radiopurity values from HPLC analysis for 14CEnclomiphene of predose and postdose aliquots were 98.0 and 97.9%, respectively. The mean radiopurity values from HPLC analysis for 14C-Zuclomiphene of pre-dose and post-dose aliquots were 97.2 and 97.3%, respectively. These values confirmed stability of the test articles under conditions of the study.
Concentrations of radioactivity in blood and plasma
Group 1, 14C-Enclomiphene
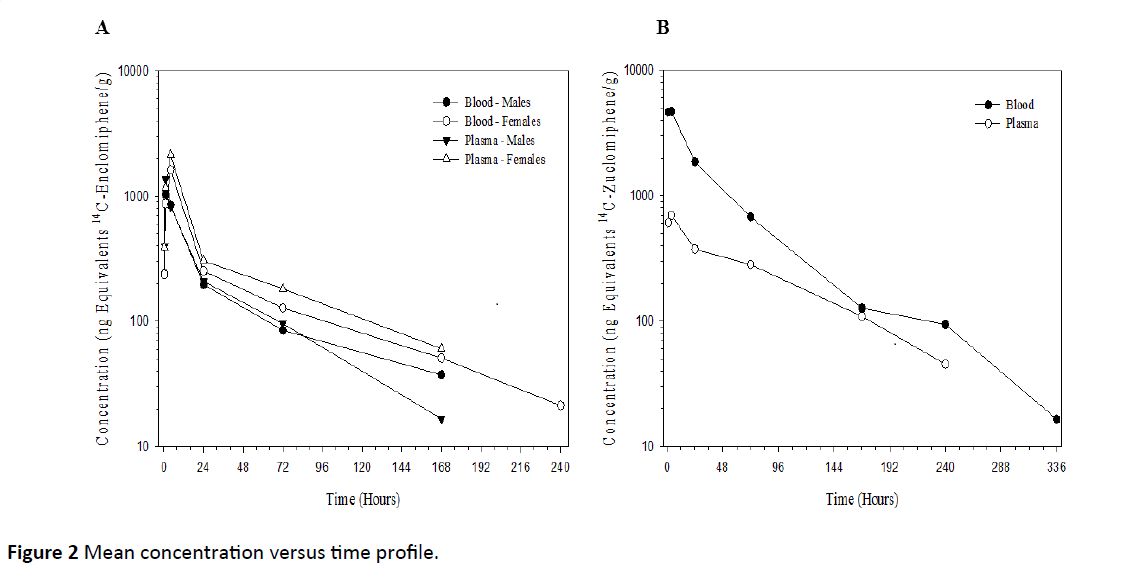
After a single 20 mg/kg oral dose of 14C-Enclomiphene to male and female mice, the maximum average plasma concentrations of radioactivity were 1370 and 2140 ng equivalents 14C-Enclomiphene/g, respectively, observed at 1 and 4 h post-dose, respectively, concentrations declined to BLQ, by 240 h post-dose. The mean concentration versus time profile is presented graphically in Figure 2A. All reported concentrations are in ng equivalents of free base/g of matrix.
Group 2, 14C-Zuclomiphene
After a single 20 mg/kg oral dose of 14C-Zuclomiphene to male mice, the maximum average plasma concentration of radioactivity was 697 ng equivalents 14C-Zuclomiphene/g, observed at 4 h post-dose, concentrations declined to BLQ by 336 h post-dose. The mean concentration versus time profile is presented graphically in Figure 2B.
Tissue distribution of radioactivity by QWBA
Group 1, 14C-Enclomiphene
The concentrations of radioactivity in tissues are presented in Table 1 (for males) and Table 2 (for females). All reported concentrations are in ng equivalents of free base/g of matrix.
| Tissue | 4 h | Tissue | 24 h |
|---|---|---|---|
| Bile | 728000 | Eye uveal tract | 6680 |
| Gall bladder | 583000 | Liver | 4180 |
| Liver | 29800 | Gall bladder | 3960 |
| Pancreas | 14200 | Bile | 2420 |
| Kidney cortex | 12700 | Harderian gland | 2210 |
| Kidney medulla | 12700 | Eye(s) | 1560 |
| Kidneyls) | 12700 | Pancreas | 1380 |
| Harderian gland | 11600 | Intra-orbital lacrimal gland | 1280 |
| Eye uveal tract | 10300 | Kidney cortex | 1220 |
| Lung(s) | 9840 | Preputial gl | 1170 |
| Urine | 9710 | Kidneyls) | 1130 |
| Small intestine | 9340 | Kidney medulla | 1040 |
| Pituitary gland | 6160 | Cecum | 977 |
| Urinary bladder | 6010 | Pituitary gland | 970 |
| Esophagus | 5690 | Prostate gland | 961 |
| Exorbital lacrimal gland | 5550 | Small intestine | 689 |
| Intra-orbital lacrimal gland | 5020 | Exorbital lacrimal gland | 676 |
| Salivary(s) | 4260 | Large intestine | 635 |
| Spleen | 4180 | Salivary(s) | 469 |
| Thyroid | 4120 | Epididymis | 456 |
| Bone marrow | 3850 | Thyroid | 444 |
| Lymph node(s) | 3760 | Testisles) | 442 |
| Diaphragm | 3010 | Diaphragm | 440 |
| Adrenals | 2880 | Thymus | 436 |
| Cecum | 2830 | Lymph node(s) | 431 |
| Fat (brown) | 2810 | Lung(s) | 384 |
| Stomach | 2770 | Esophagus | 383 |
| Thymus | 2760 | Spleen | 349 |
| Eye Is) | 2450 | Urinary bladder | 302 |
| Large intestine | 2360 | Fat (brown) | 258 |
| Myocardium | 1840 | Skin (pigmented) | 233 |
| Preputial gland | 1660 | Bone marrow | 222 |
| Prostate gland | 1390 | Adrenals | 221 |
| Skin (pigmented) | 1260 | Stomach | 197 |
| Fat (abdominal) | 1210 | Fat (abdominal) | BLQ |
| Muscle | 919 | Muscle | BLQ |
| Brain cerebrum | 855 | Brain cerebrum | BLQ |
| Seminal vesicle(s) | 823 | Seminal vesicle(s) | BLQ |
| Blood | 815 | Blood | BLQ |
| Brain cerebellum | 786 | Brain cerebellum | BLQ |
| Spinal cord | 716 | Spinal cord | BLQ |
| Brain medulla | 678 | Brain medulla | BLQ |
| Bone | 672 | Bone | BLQ |
| Testisles) | 669 | Myocardium | BLQ |
| Brain olfactory lobe | 595 | Brain olfactory lobe | BLQ |
| Nasal turbinates | 417 | Nasal turbinates | BLQ |
| Epididymis | 382 | Urine | BLQ |
| Eye lens | BLQ | Eye lens | BLQ |
Table 1: ng equivalents [14C]-enclomiphene/g in Group 1 males.
| Tissue | 4 h | Tissue | 24 h |
|---|---|---|---|
| Bile | 383000 | Gall bladder | 19400 |
| Gall bladder | 256000 | Bile | 16400 |
| Liver | 28500 | Liver | 7650 |
| Kidney medulla | 12100 | Cecum | 4310 |
| Pancreas | 11700 | Eye uveal tract | 3720 |
| Harderian gland | 11400 | Small intestine | 2890 |
| Small intestine | 10500 | Kidney cortex | 1970 |
| Lung(si_ | 10400 | Kidney(s) | 1940 |
| Cecum | 9340 | Kidney medulla | 1870 |
| Kidney(s) | 8830 | Pancreas | 1510 |
| Eye uveal tract | 7350 | Harderian gland | 1470 |
| Kidney cortex | 5800 | Large intestine | 1170 |
| Large intestine | 5200 | Intra-orbital lacrimal gland | 1140 |
| Adrenals | 4310 | Stomach | 1090 |
| Exorbital lacrimal gland | 4130 | Adrenals | 997 |
| Pituitary gland | 3810 | Thyroid | 916 |
| Esophagus | 3340 | Diaphragm | 901 |
| Spleen | 3310 | Esophagus | 778 |
| Salivary(s) | 3240 | Eye(s) | 773 |
| Lymph node(s) | 3160 | Exorbital lacrimal gland | 730 |
| 0vary(ies) | 3000 | lung(s) | 713 |
| Thyroid | 2920 | Ovary(ies) | 628 |
| Bone marrow | 2880 | Spleen | 577 |
| Fat (brown) | 2860 | Pituitary gland | 542 |
| Intra-orbital lacrimal gland | 2720 | Fat (brown) | 458 |
| Diaphragm | 2710 | Uterus | 458 |
| Urine | 2700 | Salivary(s) | 448 |
| Stomach | 2610 | Urinary bladder | 423 |
| Thymus | 1990 | Urine | 423 |
| Fat (abdominal) | 1780 | Lymph node(s) | 377 |
| Eye(s) | 1520 | Thymus | 304 |
| Uterus | 1480 | Blood | 236 |
| Myocardium | 1440 | Myocardium | 198 |
| Urinary bladder | 1290 | Fat (abdominal) | 197 |
| Blood | 1060 | Bone marrow | BLQ |
| Skin (pigmented) | 999 | Skin (pigmented) | BLQ |
| Muscle | 687 | Muscle | BLQ |
| Brain medulla | 594 | Brain medulla | BLQ |
| Spinal cord | 558 | Spinal cord | BLQ |
| Brain cerebrum | 549 | Brain cerebrum | BLQ |
| Brain cerebellum | 454 | Brain cerebellum | BLQ |
| Brain olfactory lobe | 427 | Brain olfactory lobe | BLQ |
| Nasal turbinates | 397 | Nasal turbinates | BLQ |
| Bone | 292 | Bone | BLQ |
| Eye lens | BLQ | Eye lens | BLQ |
Table 2 ng equivalents [14C]-enclomiphene/g in Group 1 females.
The single 20 mg/kg oral dose of 14C-Enclomiphene to male and female pigmented mice was well absorbed and widely distributed to tissues by 1 h post-dose. Peak tissue concentrations were reached at 1 or 4 h post-dose and the concentrations of radioactivity in tissues declined over time in both genders. For both genders, the highest concentrations of drug-derived radioactivity were in the contents of the gastrointestinal (GI) tract and bile. The radioactivity concentrations in the contents of the GI tract were based on qualitative visual assessment of the autoradiographic data. In male and female mice, bile had a maximum concentration of 728000 and 383000 ng equivalents 14C-Enclomiphene/g, respectively, observed at 4 h post-dose. Hepato-biliary excretion was an important route of elimination.
| Tissue | 4 h | Tissue | 24 h |
|---|---|---|---|
| Bile | 105000 | Harderian gland | 43900 |
| Gall bladder | 90200 | Bile | 39800 |
| lung(s) | 72400 | Gall bladder | 33600 |
| Kidneycortex | 53500 | lung(s) | 33600 |
| Kidneyls) | 43700 | Eye uveal tract | 30000 |
| Pituitary gland | 34200 | Kidneycortex | 29400 |
| Kidney medulla | 32400 | Kidney(s) | 24600 |
| Spleen | 31600 | Liver | 18200 |
| Harderian gland | 29300 | Kidney medulla | 17800 |
| Liver | 29100 | Exorbital lacrimal gland | 15000 |
| Adrenals | 26100 | Adrenals | 14800 |
| Pancreas | 24900 | Intra-orbital lacrimal gland | 14000 |
| Small intestine | 21900 | Spleen | 13500 |
| Cecum | 21000 | Pancreas | 12700 |
| Salivary(s) | 20300 | Salivary(s) | 12700 |
| Exorbital lacrimal gland | 20000 | Pituitary gland | 12600 |
| Fat (brown) | 19800 | Thymus | 11700 |
| Bone marrow | 19500 | Fat (brown) | 11400 |
| Intra-orbital lacrimal gland | 18700 | Lymph node(s) | 10600 |
| Eye uveal tract | 18400 | Epididymis | 10500 |
| Thyroid | 17400 | Small intestine | 10000 |
| Diaphragm | 15200 | Bone marrow | 9930 |
| Stomach | 14900 | Urinary bladder | 9630 |
| Myocardium | 14700 | Cecum | 9260 |
| Fat (abdominal) | 14000 | Diaphragm | 8190 |
| Thymus | 12500 | Thyroid | 7410 |
| Lymph node(s) | 11900 | Brain cerebrum | 7110 |
| Esophagus | 10700 | Testisles) | 6810 |
| Urinary bladder | 9140 | Myocardium | 6790 |
| Large intestine | 7820 | Large intestine | 6070 |
| Preputial gland | 7630 | Eye(s> | 5760 |
| Brain cerebrum | 7070 | Fat (abdominal) | 5530 |
| Muscle | 5860 | Prostate gland | 4760 |
| Brain cerebellum | 5310 | Spinal cord | 4460 |
| Spinal cord | 5000 | Stomach | 4170 |
| Seminal vesicle(s) | 4630 | Nasal turbinates | 4020 |
| Brain medulla | 4620 | Brain cerebellum | 3920 |
| Prostate gland | 4460 | Brain medulla | 3800 |
| Brain olfactory lobe | 4390 | Esophagus | 3770 |
| Epididymis | 4390 | Seminal vesicle(s) | 3490 |
| Nasal turbinates | 4290 | Brain olfactory lobe | 3300 |
| Blood | 4250 | Skin (pigmented) | 3220 |
| Bone | 3060 | Preputial gland | 3140 |
| Eye Is) | 2840 | Muscle | 2910 |
| Testis(es) | 2830 | Urine | 2530 |
| Skin (pigmented) | 2680 | Blood | 1670 |
| Urine | 2350 | Bone | 1440 |
| Eye lens | BLQ | Eye lens | 302 |
Table 3: ng equivalents [14C]-zuclomiphene in Group 2 males.
The highest peak tissue radioactivity concentrations in males were in gall bladder, liver, kidney cortex, kidney and pancreas with values of 583000, 46800, 16800, 15600 and 14200 ng equivalents 14C-Enclomiphene/g, respectively. All other analyzed tissues had peak concentration values less than 13000 ng equivalents 14C-Enclomiphene/g. For females, the highest peak tissue radioactivity concentrations were gall bladder, liver, kidney medulla, pancreas and harderian gland with values of 256000, 51700, 12100, 11700 and 11400 ng equivalents 14CEnclomiphene/ g, respectively. All other analyzed tissues had peak concentration values less than 11000 ng equivalents 14CEnclomiphene/ g. The lowest peak concentrations were detected in nasal turbinates (448) of males and bone (292) of females.
Group 2, 14C-Zuclomiphene
The concentrations of radioactivity in tissues are presented in Table 3 (males only). All reported concentrations are in ng equivalents of free base/g of matrix.
14C-Zuclomiphene was well absorbed and widely distributed to tissues by 1 h post-dose. Peak tissue concentrations were reached at 1 or 4 h post-dose for most tissues, with some tissues having peak concentrations at 24 and 72 h post-dose. The highest concentrations of drug-derived radioactivity were in the contents of the GI tract and bile. The radioactivity concentrations in the contents of the GI tract were based on qualitative visual assessment of the autoradiographic data. Bile had a maximum concentration of 105000 ng equivalents 14CZuclomiphene/ g observed at 4 hours post-dose. Hepato-biliary excretion was an important route of elimination.
Dosimetry
Group 1, 14C-Enclomiphene
Dosimetry calculations from pigmented male mice data predict that uveal tract, eye, liver, pancreas and renal cortex will be exposed to the highest doses of radiation in humans following a single oral dose of 14C-Enclomiphene. In man, these matrices are estimated to be exposed to 215, 53.1, 11.4, 8.45 and 6.02 mRad or mrem, respectively (2.15, 0.531, 0.114, 0.0845 and 0.0602 mGy, respectively).
Dosimetry calculations from pigmented female mice data predict that uveal tract, eye, liver, small intestine and pancreas will be exposed to the highest doses of radiation in humans following a single oral dose of 14C-Enclomiphene. In women, these matrices are estimated to be exposed to 186, 49.7, 16.7, 9.93 and 8.01 mRad or mrem, respectively (1.86, 0.497, 0.167, 0.0993 and 0.0801 mGy, respectively).
Group 2, 14C-Zuclomiphene
Dosimetry calculations from pigmented male mice data predict that uveal tract, epididymis, eye, testes and renal cortex will be exposed to the highest doses of radiation in humans following a single oral dose of 14C-Zuclomiphene. In man, these matrices are estimated to be exposed to 365, 185, 91.8, 88.9 and 62.0 mRad or mrem, respectively (3.65, 1.85, 0.918, 0.889 and 0.620 mGy, respectively).
Discussion
There were no apparent gender related differences in the tissue concentration data for male and female mice. Tissue to plasma concentration ratios were greater than one for most tissues through 24 h post-dose and were all above one where measurable through 168 h post-dose. Radioactivity concentrations in central nervous system tissues protected by the blood:brain barrier (cerebellum, cerebrum, medulla, olfactory lobe and spinal cord) were low and dropped to nonmeasurable levels by 24 h post-dose. Low levels of 14CEnclomiphene- derived radioactivity distributed to the reproductive tissues of the male and female mice, but cleared by 72 h post-dose. Elimination was nearly complete for most tissues by 72 h post-dose. By the final sampling time of 840 h post-dose, no tissues, other than the eye and uveal tract of the eye, contained measurable concentrations of radioactivity.
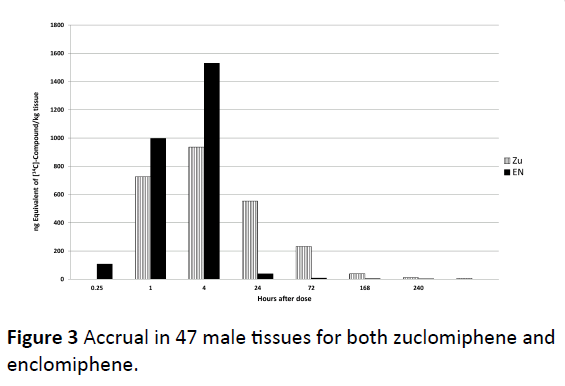
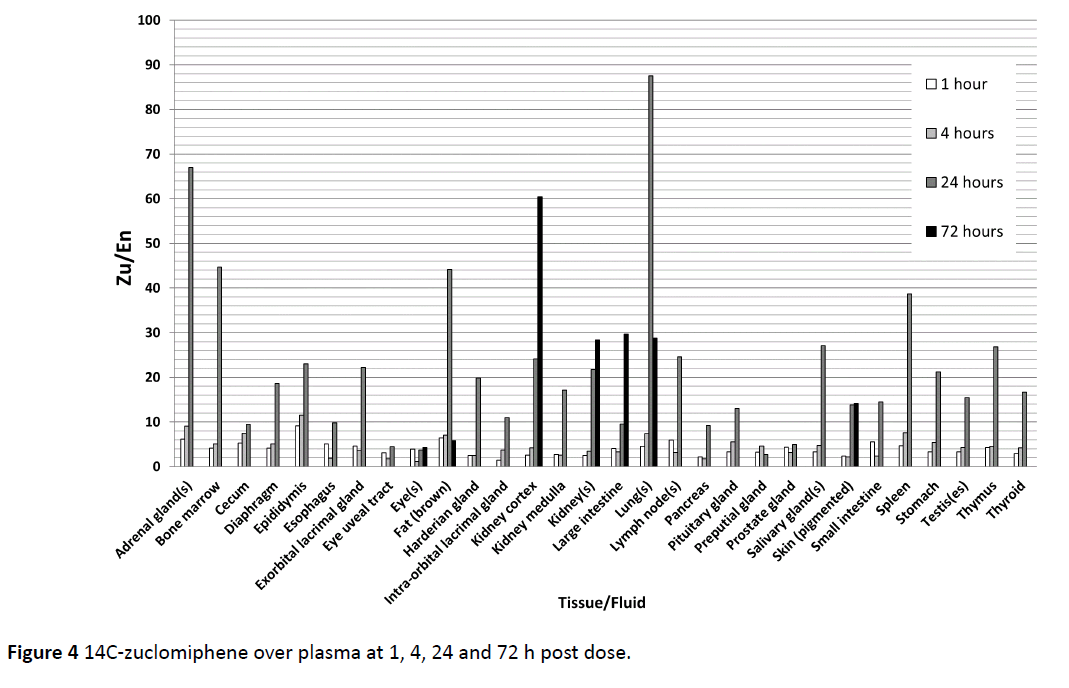
The accrual in 47 male tissues individually assessed are summed and given for both zuclomiphene and enclomiphene in Figure 3. We chose to use “accrual” to indicate amounts found in tissue in distinction to “accumulation” which might indicate an active process of tissue uptake or lack of clearance over that found in biological tissues. Peak tissue concentrations were reached at 1 or 4 h post-dose for most tissues, with some tissues having peak concentrations at 24 and 72 h post dose. There were major differences in the uptake and disposition of zuclomiphene and enclomiphene. Enclomiphene has been shown to have an approximate half-life of 7 h compared to the 14 day half-life of zuclomiphene. Every tissue examined demonstrated high lack of clearance of 14C-zuclomiphene over plasma at 1, 4, 24 and 72 h post dose (Figure 4). These differences were an effect of the differing half-lives of the two isomers. We propose there is not a differential accumulation of the two isomers, rather a difference of half-life and of each of the isomers resulting in a differing elimination of each isomer in a specific tissue.
The highest concentrations of radioactivity in eye uveal tract (10300 and 7350 ng equivalents 14C-Enclomiphene/g in male and female pigmented mice, respectively) were observed at 4 h post-dose. Radioactivity concentrations in eye uveal tract declined, but remained measurable through 840 h post-dose (1410 and 1150 ng equivalents 14C-Enclomiphene/g in male and female pigmented mice, respectively). The radioactivity concentrations in eye uveal tract at 840 h post-dose in male and female mice represented approximately 7- and 6-fold decreases in radioactivity concentrations, respectively, from the observed peak concentrations. The highest concentrations in pigmented skin were 1260 ng equivalents 14C-Enclomiphene/g at 4 h postdose in males and 999 ng equivalents 14C-Enclomiphene/g at 4 h post-dose in females. The radioactivity concentrations in pigmented skin declined over time and dropped to BLQ by 72 and 24 h post-dose in male and female mice, respectively. These data suggested that 14C-Enclomiphene-related radioactivity was selectively associated with melanin-containing tissues of the eye.
For pigmented male mice, the 14C-Enclomiphene-derived radioactivity declined with half-life (T1/2) values ranging from 2.47 h (myocardium) to 1012 h (eye), with most matrices showing T1/2 values less than 20 h. The area under the concentration-time curve from 0 to infinity (AUC0-∞) ranged from 11064 ng equivalents 14C-Enclomipheneh/g in spinal cord to 7567172 ng equivalents 14C-Enclomipheneh/g in gall bladder. In pigmented female mice, the 14C-Enclomiphene-derived radioactivity declined with half-life values ranging from 5.17 hours (lung) to 707 h (eye), with most matrices showing T1/2 values less than 20 h. The AUC0-∞ ranged from 5115 ng equivalents 14C-Enclomipheneh/g in the olfactory lobe to 3973695 ng equivalents 14C-Enclomipheneh/g in the gall bladder.
The highest peak tissue radioactivity concentrations in male mice were in gall bladder, lung, kidney cortex, liver and harderian gland with values of 90200, 72400, 53500, 45900 and 43900 ng equivalents 14C-Zuclomiphene/g, respectively. All other analyzed tissues had peak concentration values less than 43800 ng equivalents 14C-Zuclomiphene/g. The lowest peak concentrations were detected in plasma (644) and lens of eye (302). Elimination was nearly complete for most tissues by 504 h post-dose. By the final sampling time of 1008 hours post-dose, no tissues, other than the eye and uveal tract of the eye, contained measurable concentrations of radioactivity. Unlike 14C-Enclomiphene, tissue to plasma concentration ratios following single oral dosing with 14C-Zuclomiphene were greater than one for all tissues with measurable concentrations of radioactivity over the time course examined, except the lens of the eye. Radioactivity concentrations in central nervous system tissues protected by the blood:brain barrier (cerebellum, cerebrum, medulla, olfactory lobe and spinal cord) were low and dropped to non-measurable levels by 168 h post-dose. Unlike 14C-Enclomiphene, 14C-Zuclomiphene-derived radioactivity concentrations remained measurable in the noncircumventricular CNS tissues through 72 h post-dose. Low levels of 14C-Zuclomiphene-derived radioactivity distributed to the testis, but cleared by 240 h post-dose.
The highest concentration of radioactivity in eye uveal tract (30000 ng equivalents 14C-Zuclomiphene/g) was observed at 24 h post-dose. Radioactivity concentrations in eye uveal tract declined, but remained measurable through 1008 hours postdose (1020 ng equivalents 14C-Zuclomiphene/g). The radioactivity concentrations in eye uveal tract at 1008 h postdose represented approximately a 29-fold decrease in radioactivity concentration from the observed peak concentration. The highest concentration in pigmented skin was 3220 ng equivalents 14C-Zuclomiphene/g at 24 h post-dose. The radioactivity concentration in pigmented skin declined over time and dropped to BLQ by 168 h post-dose.
The 14C-Zuclomiphene-derived radioactivity declined with half-life (T1/2) values ranging from 18.7 h (urinary bladder) to 411 h (uveal tract of the eye), with most matrices showing T1/2 values less than 40 h. The AUC0-∞ ranged from 60644 ng equivalents 14C-Zuclomiphene h/g in plasma to 6204197 ng equivalents 14C-Zuclomipheneh/g in uveal tract of the eye.
Conclusion
In conclusion, 14C-Enclomiphene and 14C-Zuclomiphenerelated radioactivity were widely distributed in tissues and organs of mice and were selectively associated with melanincontaining tissues. For 14C-Enclomiphene, there were no apparent gender related differences in the tissue concentration data for male and female mice. In general, 14C-Zuclomiphenerelated radioactivity had a longer biological half-life in most tissues as compared to 14C-Enclomiphene. Unlike 14CEnclomiphene, 14C-Zuclomiphene-related radioactivity was preferentially distributed to the cellular components of blood and tissue to plasma concentration ratios were greater than one for all tissues with measurable concentrations of radioactivity over the time course examined, except the lens of the eye. Based on the pharmacokinetic and dosimetry data, administration of a single 100 μCi (3.7 MBq) oral dose of 14CEnclomiphene would not be expected to represent a significant radiation exposure risk to human male or female volunteers. Administration of an oral dose of 14C-Zuclomiphene at the same level would not be expected to represent a significant radiation exposure risk to human male volunteers.
Acknowledgement
Repros would like to acknowledge and thank Randy Press and Erin Ballard at Covance for their work in performing this study.
Authorship Contributions
Participated in research design: Fontenot, Wiehle, Podolski.
Conducted experiments: Covance Laboratories Inc. Madison, WI (Under direction of Randall Press).
Contributed new reagents: Hsu
Performed data analysis: Fontenot, Wiehle
Wrote or Contributed to writing of the manuscript: Fontenot, Wiehle, Podolski.
References
- Hughes E, Collins J, Vanderkerckhove P (1996) Clomiphene citrate for ovulation induction in women with oligo-amenorhea. Cochrane Database Syst Rev 22: CD000056.
- Jugheim ES, Odibo AO (2010) Fertility treatment in women with polycystic ovary syndrome: A decision analysis of different oral ovulation induction agents. Fertil Steril 94: 2659-2664.
- Ghobadi C, Amer S, Lashen H, Lennard MS, Ledger WL, et al. (2009) Evaluation of the relationship between plasma concentrations of en- and zuclomiphene and induction of ovulation in anovulatory women being treated with clomiphene citrate. Fertil Steril 91: 1135-1140.
- Reyes FI, Faiman C (1974) Long-term therapy with low-dose cis-clomiphene in male infertility: Effects on semen, serum FSH, LH, testosterone and estradiol and carbohydrate tolerance. Int J Fertil 19: 49-55.
- Ioannidou-Kadis S, Wright PJ, Neely RD, Quinton R (2006) Complete reversal of adult-onset isolated hypogonadal hypogonadism with clomiphene citrate. Fertil Steril 86: 1513.
- Kaminetsky J, Hemani ML (2009) Clomiphene and ENC for treatment of hypogonadal deficiency. Expert Opin Investig Drugs 18: 1947-55.
- Moradi M, Moradi A, Alemi M, Ahmadnia Abdi H, Ahmadi A, et al. (2010) Safety and efficacy of clomiphene citrate and L-carnitine in idiopathic male infertility. Urol J 7: 188-193.
- Katz DJ, Nabuisi O, Tal R, Mulhall JP (2011) Outcomes of clomiphene citrate treatment in young hypogonadal men. BJU Int 110: 573-578.
- Chua ME, Escusa KG, Luna S, Tapia LC, Difitas B, et al. (2013) Revisiting oestrogen antagonists (clomiphene and tamoxifen) as medical empiric therapy for idiopathic male infertility: A meta-analysis. Androl 1: 749-757.
- Guay AT, Bansal S, Hodge MB (1991). Possible hypothalamic impotence. Male counterpart to hypothalamic amenorrhea? Urol 38: 317-322.
- Johansen LM, Brannan JB, Delos SE, Shoemaker CK, Stossel A, et al. (2013) FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci Transl Med 19: 1-28.
- Nelson EA, Barnes AB, Wiehle RD, Fontenot GK, Hoenen T, et al. (2016) Clomiphene and its isomers block Ebola virus particle entry and infection with similar potency: Potential therapeutic implications. Viruses 8: 206.
- Schreiber E, Johnson JJ, Plotz EJ, Weiner M (1966) Studies with 14C labeled clomiphene citrate. Clin Res 14: 287.
- Charles D, Klein T, Luhn SF, Loraine JA (1969) Clinical and endocrinological studies with the isomeric components of clomiphene citrate. J Obstet Gynaec Brit Cwlth 76: 1100-1110.
- Blohm TR, Stevens VL, Kariya T, Alig, HN (1970) Effects of clomiphene cis and trans-isomers on sterol metabolism in the rat. Biochem Pharmacol 19: 2231-2241.
- Clark J, Guthrie SC (1981) Agonistic and antagonistic effects of clomiphene citrate and its isomers. Biol Reprol 25: 667-672.
- Fitzpatrick SL, Berrodin TJ, Jenkins SF, Sindori DM, Deecher DC, et al. (1999) Effect of estrogen agonists and antagonist on induction of progesterone receptor in a rat hypothalamic cell line. Endocrinol 140: 3928-3937.
- Hussein A, Ozgok Y, Ross L, Niederberger C (2005) Clomiphene administration for cases of non-obstructive azoospermia: A multicenter study. J Androl 26: 787-791.
- Mikkelson TJ, Kroboth PD, Cameron WJ, Dittert LW, Chungi V, et al. (1986) Single-dose pharmacokinetics of clomiphene citrate in normal volunteers. Fertil Steril 46: 392-396.
- Szutu M, Morgan DJ, McLeish M, Phillipou G, Blackman GL, et al. (1989) Pharmacokinetics of intravenous clomiphene isomers. Br J Clin Pharmacol 27: 639-640.
- Jimenez MA, Magee DE, Bryant HU, Turner RT (1997) Clomiphene prevents cancellous bone loss from tibia of ovariectomized rats. Endocrinol 38: 1794-1800.
- Schulz KD, Hölzel F, Bettendorf G (1971) The uptake and distribution of 14C-clomiphene citrate in different organs of new-born female guinea pigs. Acta Endocrinologia 68: 605-613.
- Fontenot GK, Wiehle RW, Podolski J (2016) Differential effects of isomers of clomiphene citrate on reproductive tissues in male mice. BJU Int 117: 344–350.
- Roch LM, Gordon DL, Barr AB, Paulson CA (1976) Visual changes associated with clomiphene citrate therapy. Arch Ophthalmol 77: 14-17.
- Lee M, Fried WI, Sharifi R (1983) Ocular adverse events of human chorionic gonadotrophin. Fertil Steril 40: 266-268.
- Bishai R, Arbour L, Lyons C, Koren G (1999) Intrauterine exposure to clomiphene and neonatal persistent hyperplasic primary vitreous. Teratology 60: 143-145.
- https://www.products.sanofi.us/clomid/clomid.pdf
- Ganchev B, Heinkele G, Kerb R, Schwab M, Mürdter TE (2011) Quantification of clomiphene citrate metabolite isomers in human plasma by rapid-resolution liquid chromatography-electrospray ionization-tandem mass spectroscopy. Anal Bioanal Chem 400: 3429-3441.
- Mürdter T, Kerb R, Turpeinen M, Schroth W, Ganchev B, et al. (2012) Genetic polymorphism of cytochrome P450 2D6 determines oestrogen receptor activity of the major infertility drug clomiphene via its active metabolites. Hum Mol Genet 21: 1145-1154.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences