ISSN : 0976-8505
Der Chemica Sinica
The Effect of pH and Surfactant on the Dissolution Profile of Poorly Water Soluble Drug and it’s in vivo Implications
Urooj Haroon1*, M. Hashim Zuberi2, Sameera Razi1
1Department of Chemistry, Federal Urdu University for Arts, Science and Technology, Karachi
2Department of Environmental Studies, Sindh Madressatul Islam University, Karachi
Abstract
Use of surfactants has widely increased in molecular biology, in formulating novel and in improving existing pharmaceutical preparations. This is because to some degree, surfactants perform the functions of cellular membranes, emulsification, solubilization, and of transport of substances that are otherwise insoluble in living tissues. In present study, the combined effect of pH and surfactant on the dissolution profile of poorly water soluble acidic drug; Telmisartan in physiological pH environment using dissolution bath and UV spectroscopy has been reported. For the study, nonionic (polysorbate S=80, Tween T=80, polyoxy ethylene (20) cetyl ether) and cationic surfactant (CTAB) was used. Literature survey reveals that bioavailability of pharmaceuticals is highly influenced by changes in pH environment within gastrointestinal tract. Hence for this purpose, three pH media, 1, 4 and 7.4, simulating human body compartment were selected. It is well recognized that poor aqueous solubility can inhibit the efficiency of drugs while certain drugs can also exhibit drastic side effects like gastrointestinal mucosal toxicity due to their poor solubility. Therefore in order to increase the efficacy and reduce the side effects of these drugs, increasing aqueous solubility can thus be a valuable aid. Results of our study showed that cationic surfactant CTAB was found to be most effective for dissolution of Telmisartan in all pH mediums. While availability of Telmisartan significantly increased in presence of polyoxy ethylene (20) cetyl ether in pH 4. The increase in dissolution rates of Telmisartan in presence of CTAB is probably due to the creation of electrostatic interactions between acidic drugs and cationic surfactant. Improvement in dissolution rate of drug in presence of non-ionic surfactants is possibly due to the decrease in surface tension of drug brought by surfactant combined with their low critical micelle concentration (CMC) and low toxicity making them effective for drug solubilization.
Keywords
Bromhexine hydrochloride, Buccal drug delivery system Carbopol, Pectin, Sodium alginate, HPMC, Mannitol, MCC, Magnesium stearate, Talc, Moisture absorption, Surface pH, Swelling index, In vitro drug release studies, Release kinetics and Stability studies.
Introduction
Telmisartan is class of Angiotensin II Receptor Antagonists drug which is essential for treatment of hypertension. It is a benzimidazole derivative antagonist of sub type of angiotensin II receptors, having imidiazole moiety condensed with bi phenyl ring. It is chemically described as 4-[(1, 4-dimethyl-2-propyl [2, 6-1H –benzimidazole]-1-yl]-[1, 1-biphenyl]-2-carboxylic acid]. It is a white to slightly yellowish solid, practically poorly soluble in water in the pH range of 3 to 9, sparingly soluble in strong acid (but insoluble in hydrochloric acid), and soluble in strong base. Its molecular formula is C33H30N4O2 with molecular weight of 514.63 [1-4].
There are many techniques which can be used in enhancing aqueous solubility. To enhance the aqueous solubility of the drug, techniques generally employed includes micronization, chemical modification, pH adjustment, solid dispersion, complexation, co-solvency, micellar solubilization, hydrotropy etc (Figure 1).
The cell membranes of almost all living organisms are based on a lipid bilayer. Surfactants are amphiphilic in nature having separate hydrophobic and hydrophilic parts with the ability to self-associate in aqueous dispersion due to the hydrophobic effect above the critical aggregation concentration. All these properties are similar to lipid based cellular membranes e.g. phospholipids. Therefore surfactants possess variety of applications and biological functions one of which is studied in the present work which is to improve the aqueous solubility of poorly water soluble Telmisartan for their sufficient absorption from the gastrointestinal tract following oral administration. We have chosen surfactants over other dissolution media for in vitro dissolution testing of water-insoluble drugs because of its mechanistic similarities to in vivo dissolution [5,6]. In our study, we report the combined effect of pH and surfactant on the dissolution profile of Telmisartan drug in physiological pH simulating human body environment using dissolution bath and UV spectroscopy. We studied the effects of non-ionic (polysorbate S=80, Tween T=80 and polyoxy ethylene (20) cetyl ether) and cationic surfactant (CTAB) on the dissolution profile of Telmisartan with the aim to improve its bio-availability.
Experimental
Materials
Telmisartan: Telmisartan reference standard was a gift form pharm evo pharmaceutical company. Telmisartan (Micardis, 20 mg) tablets were obtained from Getz Pharma (pvt) Ltd Karachi.
Reagents: All the reagents used were of analytical. De-ionized water portion used was double distilled, de-ionized and filtered.
Glass wares: All the glass wares were of Pyrex type and were washed with chromic acid followed by a thorough washing with water and at last rinsed with de-ionized water which was freshly prepared in the laboratory.
Equipments
Electrical balance (Mettler Toledo # AB54), pH meter (Mettler Toledo, MP 220 with temperature compensation capability), BMS UV/Visible spectrophotometer with 1 cm rectangular quartz cells, Deionizer, Water distillation unit were used.
Dissolution test apparatus
The dissolution equipment was manufactured to the B.P 2006 standards, which included the dissolution motor and variable speed controller with a stainless steel basket assembly. The top of the basket was modified and replaced by a conical head in order to eliminate air entrapment during dissolution, which is not inconsistent with the present apparatus description. The dissolution vessel was flat bottom glass vessel with an internal diameter of 100 mm and with a capacity of 1 litre dissolution fluid. The variable speed motor was modified to reduce unwanted vibrations by the incorporation of 1000 230 mF capacitor in the speed control circuit and was maintained with in ± 0.5%of the required speed. The rotation speed of the basket assembly was fixed at 100 ± 0.5 rpm throughout the experiment; dissolution assembly was immersed in a water bath at 37°C ± 0.1°C. The absorbance of the drug drawn from the dissolution medium was scanned at a range 190-400 nm.
Methods
De-ionized water
Deionized water was prepared by passing double distilled water through the deionizer distillation assembly while monitoring the conductance of the effluent.
Preparation of buffers
Preparation of 0.1 N hydrochloric acid: 9.1 ml hydrochloric acid of analytical grade (36%; 11 N) was taken in a litre volumetric flask and the volume was made up to the mark with de-ionized water.
B Preparation of Buffer of pH 4: 14.9 g of potassium chloride was dissolved in 1000 ml sufficient de-ionized water and adjust the pH with 0.1 N HCl.
C Preparation of Buffer of pH 7.4: Dissolve about 0.6 g of Potassium dihydrogen phosphate, 6.4 g of diSodium hydrogen phosphate and 5.85 g of Sodium chloride in sufficient de-ionized water. Adjust the pH with 0.1 N HCl.
Assay methods of Telmisartan
There are number of assay methods of Telmisartan reported in the literature [7-12]. However, UV spectroscopic method which is easy and rapid was employed for analysis. Telmisartan shows strong absorbance at 207 nm [13-15]. Measurement of absorbance has been employed for the assay, which followed Beer Lambert’s law in the concentration range of 1 ppm to 25 ppm in pH 1, 4, and 7.4.
Preparation of solutions
A Primary solution of Telmisartan (100 ppm): Telmisartan (0.01 g) were weighed exactly and introduced with the help of funnel in 100 mL volumetric flask, dissolved in 0.1 N HCl, buffer of pH 4 and 7.4 the volume was made with the same solvent up to the mark.
B Stock solution of Telmisartan: 25 ppm of primary solution of Telmisartan was transferred in 100 mL of volumetric flask and the volume was made up to the mark with same solvent. The concentration of stock solution was 25 ppm.
C Working standard solution: Different dilutions ranging from 1, 2, 4, 8, 10, 16, 20, 25 were prepared (for buffers of pH 4, 7.4, and 0.1 N HCl) by diluting stock solution in different 25 mL volumetric flasks diluted to the mark with the required buffer.
Calibration curve of Telmisartan
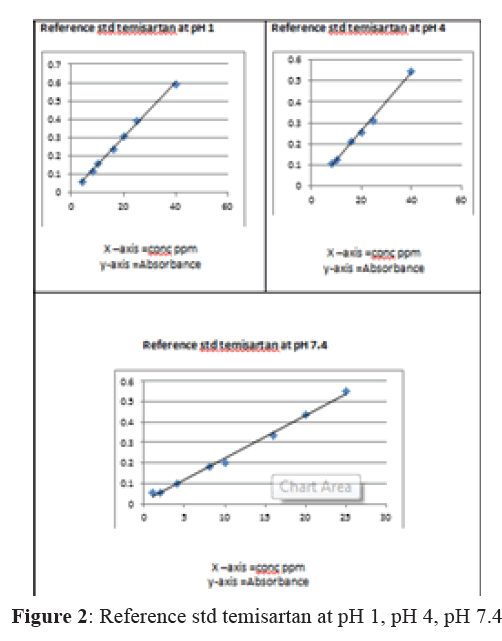
For construction of calibration curve, working standard solutions of each concentration ranging from 1 ppm to 25 ppm were prepared. A popup scan was taken in the region of 200-400 nm for these solutions against the reagent blank. Maxima appeared at 207 nm. Graph was plotted for absorbance against concentration and straight line was obtained which obeyed the Beer Lambert’s law. Epsilon values were calculated at each pH from these observations.
In vitro availability of Telmisartan in pH 1, buffer pH 4 and 7.4 at 37°C.
The in vitro availability of Telmisartan dosage was studied in simulated gastric juice (0.1 N HCl) and in buffer of pH 4 and 7.4 on a modified B.P 2002 dissolution apparatus described earlier. 10 mg of drug was added in 1 liter (in simulated gastric juice and in pH 4 and pH 7.4. dissolution medium already maintained at specified temperature (37°C) at the start of the experiment. Aliquots were withdrawn (5 ml for simulated gastric juice and buffer pH 4 and 5 ml for pH 7.4) periodically at fifteen minutes time intervals for 120 minutes. The volume of the dissolution fluid was maintained by adding an equivalent amount of dissolution fluid withdrawn, which had previously been maintained at the temperature in the same bath. The aliquots drawn were properly diluted and assayed for drug contents spectrophotometrically (Figure 2).
Results and Discussion
It is well recognized that bioavailability of pharmaceuticals is highly influenced by the changes in pH within the gastrointestinal tract. After oral administration of drug, its degree of solubility is probably affected as it passes through the intestine. In the stomach the pH is around 1 to 2, blood pH is 7.4 and in the duodenum the pH is between 5-7.5. Hence in our study, dissolution was performed in three pH mediums, 1, 4 and 7.4, simulating human body compartment.
Literature reports that poor aqueous solubility can severely limit the efficacy of drugs while certain drugs can also show side effects like gastrointestinal mucosal toxicity due to their poor solubility. The ability to increase aqueous solubility can thus be a valuable aid in increasing efficiency and reducing side effects for certain drugs. Consequently the present study was performed with the objective to improve the solubility and bioavailability of poorly water soluble acidic drugs.
The availability of Telmisartn in pH 1 after 2 hours without surfactant was less than 50%, which increased to nearly 100% when CTAB was added to dissolution medium. The availability of Telmisartan in media containing poly sorbate(S=80), Tween (T=80) and polyoxyethylene 20 cetyl were nearly 88, 75 and 79%. In pH 4, Telmisartan was only 30% available while the availability increased to nearly 100 % in presence of CTAB and polyoxyethylene while in presence of surfactants S=80 and T=80 the availability remained unchanged. In pH 7.4 availability of Telmisartan was 49% which increase to 81% with CTAB, 76% with T=80 while with surfactants S=80 and polyoxy ethylene, there was only a small increase in availability i.e. 51% and 53% respectively.
Results indicate that degree of dissolution of Telmisartan and hence its availability depends upon the class of surfactant used and pH of the medium. The cationic surfactant CTAB was found to be mostly effective for the dissolution of the drug among the surfactant tested. Improvement in the availability of Telmisartan in presence of surfactants was more prominent at pH 1 in presence of all the surfactants studied. The pH of the stomach is nearly 1 which decreases the dissolution of acidic drugs in stomach environment. Results of our study suggest that using surfactant can be very helpful to overcome this problem. Surfactant reduces the aggregation of the drugs particles and therefore increases the region (area) of particles available and activates the drug to dissolution. Improvement in the dissolution rate of drugs is possibly due to the decrease in surface tension of the drugs brought by the surfactants combined with their low critical micelle concentration (CMC) and low toxicity making them effective for drug solubilization [16]. The significant increase in dissolution rates of Telmisartan in presence of CTAB is probably due to the possibility of electrostatic interactions between acidic drug and cationic surfactant.
Conclusion
Conclusively our work describes the effect of surfactant and pH on the dissolution profile of poorly water soluble Telmesartan. In presence of CTAB the availability of Telmisartan was maximum at pH1 followed by pH4 and pH7.4. Similarly in the presence of non-ionic surfactant the availability of the drug was also increased and was more prominent in presence of polyoxy ethylene 20 cetyl ether). Results suggest that careful selection of surfactant could be beneficial for the enhancement of solubility of less aqueous soluble drugs. In our study, cationic surfactant (CTAB) was found to be most effective in solubilizing the selected acidic drug. Improving the aqueous solubility of poorly soluble drug has been a consistent challenge for the pharmaceutical scientists. Results of our study could serve the pharmaceutical companies in their quest to improve formulation design of poorly water soluble drugs.
References
- Kumar AS, Ghosh S, Mehta GN, Soundararajan R, Sarma PSR et al. (2009) New and Improved Synthesis of Telmisartan: An Antihypertensive Drug, Synthetic Communications 39: 4149-4157.
- Muthu AK, sankala R,. prasad CS, satheesh D, Manavalan R (2011) Simultaneous Estimation of Telmisartan and Amlodipine by uv Spectrophotometric Method using Multi Component Mode of Analysis. IRJP 2: 175-180.
- Galzerano D, Capogrosso C, Di Michele S, Galzerano A, Paparello P et al. (2010) New standards in hypertension and cardiovascular risk management: focus on telmisartan. Vasc Health Risk Manag 6: 113-33.
- Sawant RL, Raskar MA, Ahmed R, Pawar S (2012) Validated spectrophotometric methods for simultaneous estimation of telmisartan and indapamide in pharmaceutical dosage form. Der Pharma Chemica 4: 633-638.
- Rahman SMH, Telny TC, Ravi TK, Kuppusamy S (2009) Role of Surfactant and pH in Dissolution of Curcumin, Indian J Pharm Sci 71: 139-142.
- Sjokvist E, Nystrom C, Alden M, Caram LN (1992) Physicochemical aspects of drug release. XIV. The effects of some ionic and non-ionic surfactants on properties of a sparingly soluble drug in solid dispersions. Int J Pharm 79: 123-33.
- Pandey A, Sawarkar H, Singh M, Kashyap P, Ghosh, P (2011) UV Spectrophotometric Method for estimation of Telmisartan in Bulk and Tablet Dosage Form. Int J Chemtech Res 3: 657–660.
- Thomas AB, Jagdale SN, Dighe SB, Nanda RK (2010) Simultaneous Spectrophotometric Estimation of Amlodipine Besylate and Telmisartan in Tablet Dosage Form. IJPRIF 2: 1334-1341.
- Pradhan KK, Mishra US, Sahoo A, Sahu KC, Mishra D (2011) Method development and validation of telmisartan in bulk and pharmaceutical dosage forms by UV spectrophotometric method, Int. J. Res. Pharm. Sci 2: 526-530.
- Nandipati S, Reddy VK, Reddy TR (2012) Development and Validation of RP-HPLC Method for Estimation of Telmisartan in Bulk and Tablet Dosage Form. Int. Res. J. Pharm. App Sci 2: 39-43.
- Jat RK, Sharma S, Chippa RC, Singh Rambir, Alam Imranzzz (2012), Quantitative estimation of telmisartan in bulk drug and tablets by uv spectroscopy. IJDRT 2: 268-272.
- Ilango K, Shiji KPS (2012) Spectrophotometric Methods for Simultaneous Determination of Telmisartan and Hydrochlorothiazide in Tablet Dosage Form. RJPBCS 3: 1438-1445.
- Shen J, Jiao Z, Shi XJ Zhong MK, Li ZD (2005) HPLC determination of telmisartan in human plasma and its application to a pharmacokinetic study. Pharmazie 60: 418-20.
- Rekha G, Sunil K, Paras S (2011) Spectrophotometric Simultaneous Determination of Hydrochlorothiazide and Telmisartan in Combined Dosage Form. 1: 46-49.
- Yogesh, Shrivastava A (2009) Isocratic RP-HPLC-UV Method Development And Validation for the Simultaneous Estmation of Ramipril And Telmisartan in Tablet Dosage Form. Asian J Pharm Clin Res 2: 104-111.
- Realdon N, Dal Zorro M, Franceschinis E, Punchina A (2012) Effect of the surfactant on the availability of piroxicam as a poorly hydrosoluble drug from suppositories. Pharmazie 67: 37-45.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences