ISSN : 2574-0431
Synthesis and Catalysis: Open Access
Synthesis, Characterization and in vitro Biological Activities of Pyrazolylpalladium(II) Complexes towards Selected Strains
Munyaneza A1*, Kumar G1* and Morobe IC2
1Chemistry Department, University of Botswana, Private Bag 00704 Gaborone, Botswana
2Biological Sciences Department, University of Botswana, Private Bag 00704 Gaborone, Botswana
- *Corresponding Author:
- Apollinaire Munyaneza & Kumar G
Chemistry Department
University of Botswana
Private Bag 00704 Gaborone, Botswana
Tel: +2673552491
E-mail: amunyaneza@gmail.com kumarg@mopipi.ub.bw
Received Date: February 02, 2018; Accepted Date: February 28, 2018; Published Date: March 03, 2018
Citation: Munyaneza A, Kumar G, Morobe IC (2018) Synthesis, Characterization and in vitro Biological Activities of Pyrazolylpalladium(II) Complexes towards Selected Strains. Synth Catal. 3:2. doi: 10.4172/2574-0431.100020
Abstract
Four palladium(II) complexes vz. C1, [PdCl2(L1)] (L1 = 3-ferrocenyl-1H-pyrazole), C2, [PdCl2(L2)] (L2 = 3-ferrocenylpyrazolyl-1-methylenepyridine), C3, [PdCl2(L3)] (L3 = 3,5-dimethylpyrazole) and C4, [PdCl2(L4)] (L4 = 3-phenyl-1H-pyrazole) have been successfully synthesized. The ligands as well as the complexes were characterized by 1H NMR, IR, UV-vis, thermogravimetric analysis (TGA), cyclic voltammetry, magnetic measurements and mass spectrommetry. The antibacterial and antifungal activities of the ligands and the respective complexes were carried out using the Agar disk diffusion method on two gram positive bacteria (Staphylococcus aureus, Bacillus subtilis), two gram negative bacteria (Escherichia coli, Pseudomonas aeruginosa) and one fungal, Candida albicans.
All the compounds displayed biological activity to some extent. However, L3 was more active than most of other compounds towards all the microorganisms screened. The activity was found to generally improve after coordination to palladium metal especially for ligands which do not contain a ferrocenyl moiety.
Keywords
Pyrazole; Palladium; Characterization; Antimicrobial activity
Introduction
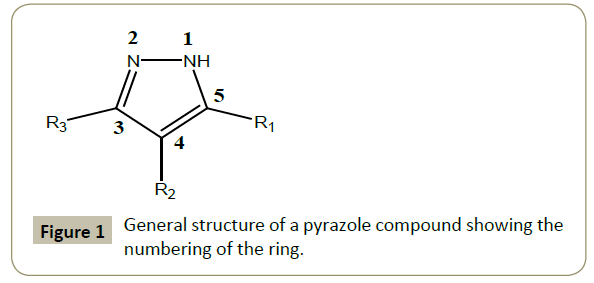
Pyrazole is a five membered ring molecule with three carbons and two contiguous nitrogen atoms [1,2]. It exhibits aromatic character due to the presence of delocalized π-electrons. Furthermore, pyrazole is a better π-donor and a weaker π-acceptor than most six membered ring heterocycles [3]. During coordination to metal ions, pyrazole may act as a monodentate or bidentate ligand depending on whether the N(1)-H atom is deprotonated or not (Figure 1).
Pyrazole and its derivatives have been extensively reported in the literature [4-8]. The first account on pyrazole synthesis was given by Knorr in 1884 and the reported pyrazole compound had antipyretic properties [9]. Althoughpyrazole compounds can be relatively easy to synthesize, they are rarely found in natural products. This might be due to difficulty of living organisms to form N-N bond. Less than 20 pyrazole containing natural products are so far known [10]. The classical synthetic routes to pyrazoles can proceed by (i) the condensation of hydrazines with 1, 3-diketone compounds in a suitable solvent (ii) 1,3-dipolar cycloaddition of diazo compounds with alkynes and (iii) reaction of α,β-unsaturated aldehydes and ketones with hydrazines [11,3]. Modern approaches to pyrazole synthesis make use of greener techniques such as the ultrasonic and microwave assisted reactions [12-14].
However, the interest in the pyrazoles synthesis does not emanate from a mere scientific curiosity. These compounds have been found to have important medicinal applications. In 1954, Kosuge and Okeda synthesized 3-n-nonylpyrazole which was reported to have good antimicrobial activity [15]. Many other activities such as anti-bacterial [16] anti-cancer [17] anti-inflammatory [18] are also known for pyrazole compounds. The structure of pyrazoles can be fine-tuned to enhance a specific biological activity. For example, Dora Currion et al. [19] reported that the presence of methoxy electron donating groups in the structure of 4,5-dihydro-1H-pyrazoles improves the ability of these compounds as nNOS and iNOS inhibitors. Furthermore, the presence of a ferrocenyl moiety in pyrazole derivatives has been demonstrated to enhance the in vitro cytotoxic activity against some cancer cells [20]. With regard to the antimicrobial activities, pyrazoles have been undoubtedly confirmed to be active against a broad spectrum of gram-positive and gram-negative strains [16]. These activities can be modified by the presence of a given substituent on the pyrazole ring or by coordination to a transition metal ion. For example, Saha et al. [21,22] observed that for the same pyrazole ligand, Co(III) gives much better activity than Cu(II) towards the same microorganisms. This difference in activity was rationalized by the overtone’s concept of cell permeability as well as by the Tweed’s chelation therapy [23,24]. According to the overtone’s principle, the liposoluble material will enter the cell easily hence good activity while Tweed’s theory favors Co(III) as it is a cationic species compared to Cu(II).
In particular, palladium(II) complexes with various ligands have been screened for biological activities [25-27]. In most cases, Pd(II) complexes have higher activity than the corresponding free ligands. The chelating effect as well as the high nuclearity of the complex have proved to have a positive effect on the antimicrobial activity to some extent as compared to monodentate ligands and mononuclear complexes [28]. Furthermore Pd(II) systems are more active than analogue metal complexes such as Ni(II) and Pt(II) [29,30].
Generally, when analyzing the biological activities of metal complexes, factors such as chelate effect, nature of the ligand, total charge of the complex, nature of the counter ion (if any) and the nuclearity of the complex should be considered [31]. All these parameters seek to explain the interaction between the active agent and the microbe. Therefore the activity will be high in the case where the agent is able to easily penetrate the cell membrane and stay longer in contact with the microbe.
It is in this context of studying the effect of ligands and metal complexes on common microorganisms that herein we report on the synthesis and characterization of four pyrazolylpalladium(II) complexes (C1, C2, C3 and C4) as well as the antibacterial activities of all ligands (L1-L4) and complexes against selected strains.
Experimental Instrumentation
Infrared spectra were recorded on a Perkin Elmer FT-IR 2000 spectrometer in 4000-400 cm-1 range (University of Botswana, Indian Institute of Science). NMR spectra were recorded on a BRUKER AVANCE 300 MHz instrument (University of Botswana). MS spectra were recorded on AGILENT GC/MS 5975C instrument (University of Botswana). Magnetic susceptibility was measured at the University of Botswana using Johnson Matthey Alfa magnetic susceptibility balance. Thermogravimetric analysis (TGA) was performed using a Mettler Toledo TGA/SDTA 851e instrument (Indian Institute of Science, Bangalore) between 20- 1000°C at a heating rate of 10°C/min under nitrogen atmosphere. Solid state UV-vis spectra were recorded on a Perkin Elmer UV/ Vis/NIR Spectrometer Lambda 750 (Indian Institute of Science, Bangalore). Cyclic voltammograms were recorded on a CH Instrument (Electrochemical Analyzer CH I 645, Indian Institute of Science, Bangalore). Melting points were determined using STUART SMP3 version 5.0 apparatus (University of Botswana). The antibacterial activities were measured in the Biological Sciences Department (University of Botswana).
Chemicals
The chemicals were purchased from Sigma-Aldrich and used as received. Solvents were dried and distilled using conventional methods. Toluene was dried using sodium wires and distilled in the presence of benzophenone. 3, 5-dimethyl-1H-pyrazole (L3) and 3-phenyl-1H-pyrazole (L4) were purchased from Sigma- Aldrich and were used without any further purification.
Experimental
Synthesis of ligands
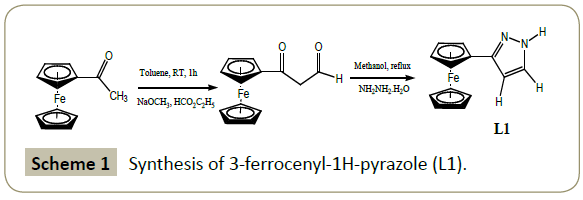
Synthesis of 3-ferrocenyl-1H-pyrazole (L1)
3-ferrocenyl-1H-pyrazole was synthesized with slight modification of published procedure [32] as follows:
To 0.237 g (4.38 mmol) of dry sodium methoxideslurried in 30 mL of toluene was added with stirring a solution of 1g (4.38 mmol) of acetylferrocene and excess of ethyl formate (3.5 mL, 43.8 mmol). The reaction was allowed to proceed for forty minutes to ensure the completion of the diketone intermediate formation. The precipitate was filtered and transferred quantitatively to a round bottomed flask. The diketone intermediate was then dissolved in methanol and reacted with aqueous solution of hydrazine dihydrochloride (0.460 g, 4.38 mmol). The product was extracted with dichloromethane and the solvent dried on a rotary evaporator. The resulting residue was refluxed in methanol with hydrazine monohydrate (0.100 mL, 2.194 mmol) for one hour. The hot yellow solution was poured in water cooled ice and a yellow precipitate immediately formed. The precipitate was allowed to settle overnight and filtered by gravity. The product was washed with water and allowed to dry in air.
Yield: 53%.
1H NMR (CDCl3,δ ppm): 7.54 (s, 1H, pz); 6.39 (s, 1H, pz); 4.63 (s, 2H, η5-C5H4); 4.27 (s, 2H, η5-C5H4); 4.05 (s, 5H, η5-C5H5). IR (neat, cm-1): 3250 (w, N-H); 3092 (w, =C-H); 1662 (s, C=N); 1598(vs, C=C).
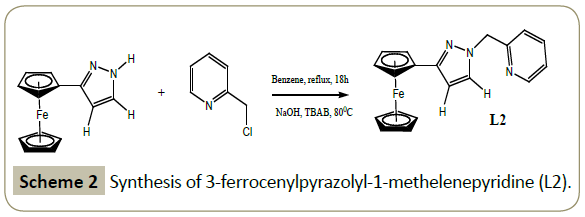
Synthesis of 3-Ferrocenylpyrazolyl-1-methylenepyridine (L2): The synthesis of L2 was adapted from the reported literature procedure [33] as follows: To a solution of 3-ferrocenylpyrazole (0.504 g, 2 mmol) and 2-picolyl hydrochloride (0.326 g, 2 mmol) in benzene (30 mL) was added 12 mL of 40% aqueous NaOH solution and 10 drops of 40% solution of tribulylammonium bromide (TBAB). The reaction mixture was refluxed for eighteen hours. After cooling to room temperature, the organic phase was separated from the water layer using a separating funnel followed by drying over MgSO4.The solvent was pumped to afford a red orange solid.
Yield: 45%.
1H NMR (CDCl3,δ ppm): 8.53 (d, 1H, J = 4.5 Hz, py); 7.58 (t, 1H, J = 7.8 Hz, py); 7.41(s, 1H, pz); 7.16 (t, 1H, J = 7.2 Hz, py); 6.91(d, 1H, J = 7.8 Hz, py); 6.32 (s, 1H, pz); 5.42 (s, 2H, CH2); 4.66 (s, 2H, η5- C5H4); 4.22 (s, 2H, η5-C5H4); 4.02 (s, 5H, η5-C5H5). IR (neat, cm-1): 3095(w, =C-H); 1664 (s, C=N); 1556(vs, C=C) Schemes 1 and 2.
Synthesis of palladium complexes
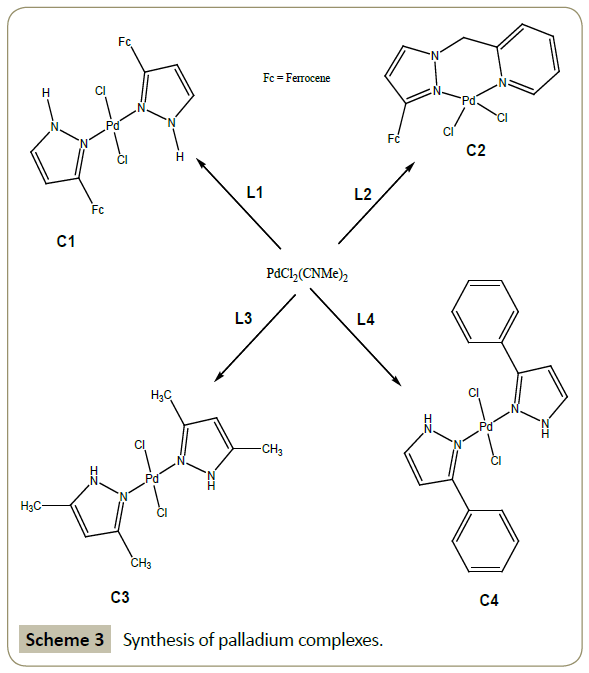
Synthesis of [{bis(3-ferrocenyl-1H-pyrazole)}palladium(II) chloride] (C1): To 0.200 g of L1 (0.793 mmol) dissolved in 20 mL of dichloromethane in a 50 mL round bottomed flask, was added 0.102 g (0.396 mmol) of palladium dichloride acetonitrile. The reaction mixture was stirred at room temperature overnight and the solvent removed to afford the desired product.
Yield: 75 %; M. p.: 230-232°C.
1H NMR (CDCl3,δ ppm): 11.48 (s, 1H, pz); 7.93 (s, 1H, pz); 7.21 (s, 1H, pz); 6.23 (s, 1H, pz); 4.55 (s, 2H, η5-C5H4); 4.34 (s, 2H, η5-C5H4); 4.13 (s, 5H, η5-C5H5). IR (neat, cm-1): 3202 (w, N-H); 3098 (w, =C-H); 1605(s, C=N); 1443(s, C=C). TOF MS (EI): m/z 681.90 [M+].
Synthesis of [(3-ferrocenylpyrazolyl-methylenepyridine) palladium(II) chloride] (C2): To 0.200 g of L2 (0.582 mmol) dissolved in 20 mL of dichloromethane in a 50 mL round bottomed flask, was added 0.150 g (0.582 mmol) of palladium dichloride acetonitrile. The reaction mixture was stirred at room temperature overnight and the solvent removed to afford the desired product.
Yield: 70%.
1H NMR (CDCl3,δ ppm): 8.54 (d, 1H, J = 4.2 Hz, py); 7.60 (t, 1H, J = 7.8 Hz, py); 7.42(s, 1H, pz); 7.16 (t, 1H, J = 6.6 Hz, py); 6.91(d, 1H, J = 7.8 Hz, py); 6.33 (s, 1H, pz); 5.43 (s, 2H, CH2); 4.70 (s, 2H, η5-C5H4); 4.23 (s, 2H, η5-C5H4); 4.06 (s, 5H, η5-C5H5). IR (neat, cm-1): 3095(w, =C-H); 1661(s, C=N); 1403(vs, C=C).
Synthesis of [{bis(3, 5- dimethylpyrazole)} palladium(II) chloride] (C3): To 0.045 g (0.46 mmol) of 3,5-dimethylpyrazole (L3) in 10 mL dichloromethane was added 0.060 g (0.23 mmol) of palladium dichloride acetonitrile. The reaction mixture was vigorously stirred over night at room temperature. The reaction was stopped and the solvent allowed to evaporate slowly to afford the title compound.
Yield: 85%
1H NMR (CDCl3,δ ppm): 1.89 (s, 3H); 2.64 (s, 3H); 5.67 (s, 1H); 11.81 (s, 1H).
IR (KBr, cm-1): 3205(s, N-H); 1583(s, C=N); 1413(s, C=C).
Synthesis of [{bis(3-phenyl-1H-pyrazole)} palladium(II) chloride] (C4): Complex C4 was synthesized in a similar way to complex C3 starting from 0.066 g (0.46 mmol) of 3-phenyl-1H-pyrazole (L4) and 0.060 g (0.23 mmol) of palladium dichloride acetonitrile.
Yield: 92 %; M. p.: 213-215°C
1H NMR (CDCl3,δ ppm): 6.41 (s, 1H, pz); 7.53-7.42 (m, 5H, Ph); 7.98 (s, 1H, pz); 11.83 (s, 1H, pz).
IR (KBr, cm-1): 3284 (s, N-H); 1553(s, C=N); 1473(s, C=C).
GC-MS: 463.4 [M-2H]; 145.0 [PhPz, free ligand] Scheme 3.
Biological activities: The pyrazolyl palladium metal complexes (C1-C4) and their respective ligands (L1-L4) were tested for antibacterial and antifungal activity using Agar disc diffusion method [34]. The microorganisms were grown overnight at 37°C in 20 mL of Müller-Hinton broth (Oxoid). The cultures were adjusted with sterile saline solution to obtain turbidity comparable to that of McFarland no. 5 standard (1.0 x 108) CFU/ ml. 90 mm Petri dishes (Merck, South Africa) containing 12 mL of sterilized Müller-Hinton agar (Oxoid) were inoculated with these microbial suspensions. Sterile Whatman No.1 (6 mm) discs papers were individually placed on the surface of the seeded agar plates and 10 μl of the complexes was applied to the filter paper disk. The plates were incubated at 37 °C for 24 h and the diameter of the resulting zones of inhibition (mm) of growth was measured. All tests were performed in triplicates. Kanamycin (30 μg) was used as a control (standard antibiotic) [35].
Results and Discussion
Spectroscopic analysis
The spectroscopic analysis of the ligands as well as complexes has confirmed the successful synthesis of compounds in this report. 3-ferrocenyl-1H-pyrazole (L1) and 3-ferrocenylpyrazolyl-methylenepyridine (L2) displayed characteristic 1H NMR chemical shifts for the pyrazole backbone proton as well as the ferrocenyl group. The proton backbone from pyrazole appeared at 6.49 ppm and 6.32 ppm while the singlet for the five protons on the unsubstitutedcyclopentadienyl ring of ferrocenyl moiety appeared at 4.05, 4.02 for L1 and L2 respectively. This is in agreement with previous literature reports on these compounds [32,33].
In L2, the methylene protons at 5.42 ppm together with two doublets (8.53, 6.91 ppm) and two triplets (7.58, 7.16 ppm) confirmed the presence of the pyridyl ring in the molecule. The mass spectrometry results also confirmed the molecular mass for L1 and L2 which were 252.03 (M+) and 342.07 (M+) respectively. 1H NMR of the complexes displayed the expected chemical shifts and positions for key protons in the structures. In complex C1, the pyrazole backbone proton was observed at 6.23 ppm and the N1-H proton at 11.48 ppm. The rest of the protons appeared also at expected positions with a downfield shift compared to the free ligand. The synthesis of complex C1 was also confirmed by mass spectrometry which showed a peak at 681.90 corresponding to the molecular ion (supplementary information Figure S1). In complex C2, the backbone proton is seen at 6.33 ppm. While the methyl group in 3,5-dimethylpyrazole (L3) are in the same chemical environment and hence expected to have one singlet, the situation is not the same after coordination to palladium metal in complex C3. The CH3 protons at position 3 in complex C3 appear at 1.89 ppm as a singlet and the protons of the methyl group at position 5 show a singlet at 2.64 ppm. The backbone proton is seen at 5.67 ppm and the proton at position 1 appears at 11.81 ppm (supplementary information Figure S2).
The 1H NMR of complex C4 showed the pyrazolebackbone proton at 6.41 ppm and the one at position 1 appeared 11.83 ppm. A multiplet for the phenyl protons is observed at 7.53-7.42 ppm and the proton on position 4 at 7.98 ppm (supplementary information Figure S3).
The IR spectral data for ligands and complexes showed the presence of important vibrations for key groups such as N-H between 3200-3000 cm-1, C=N around 1600 cm-1 and C=C in 1500- 1400 cm-1 region (supplementary information Figures S4-S15).
UV-vis studies
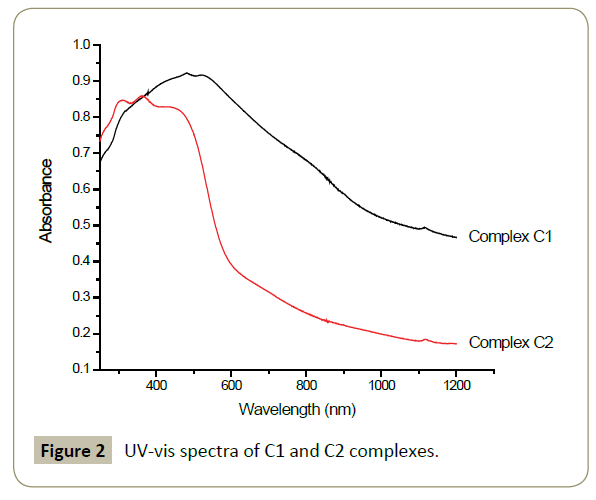
The electronic spectra of the ligands as well as the complexes were recorded on Lambda instrument in the UV-vis/NIR regions (100- 1300 nm). For 3-ferrocenyl-1-pyrazole ligand, a band observed at 354 nm is assigned to the π-π* transition of the cyclopentadienyl ring while the broad band at 463 nm is attributed to the metal to ligand charge transfer (MLCT) from iron (II) to cyclopentadienyl ring characteristic of square-planar compounds [36].
In complex C1, the π-π* transition band shows a bathochromic shift and possibly overlaps with metal to ligand charge transfer (MLCT) to produce a broad band at around 500 nm . Complex C2, displays the intraligand transfer bands at 308 nm and MLCT band at 363 nm for iron(II) to cyclopentadienyl ligand. Another MLCT band is observed as a broad peak at 445 nm accounting for the transfer band from pyrazole ring to palladium(II) (Figure 2) [37].
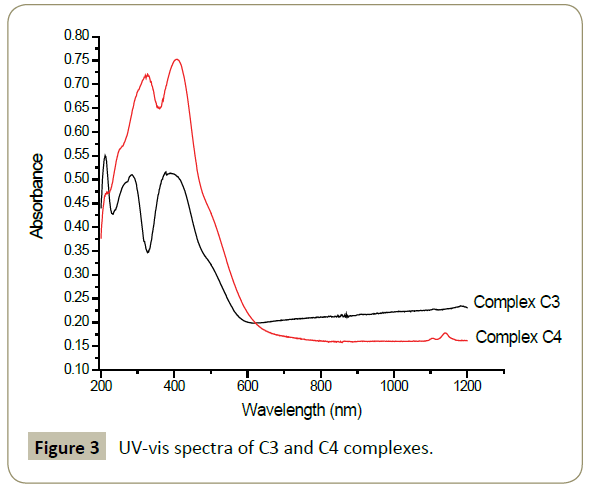
The complexes C3 and C4 do not contain the ferrocenylmoiety, hence the bands from ferrocene are absent. Locally excited intraligand transitions are observed at 283 nm in complex C3 and 326 nm in complex C4. The MLCT band which might be overlapping with d-d transitions are observed at 386 nm in complex C3 and 405 nm in complex C4. These results are in correlation with previous reports on similar compounds (Figure 3) [38].
Cyclic voltammetry
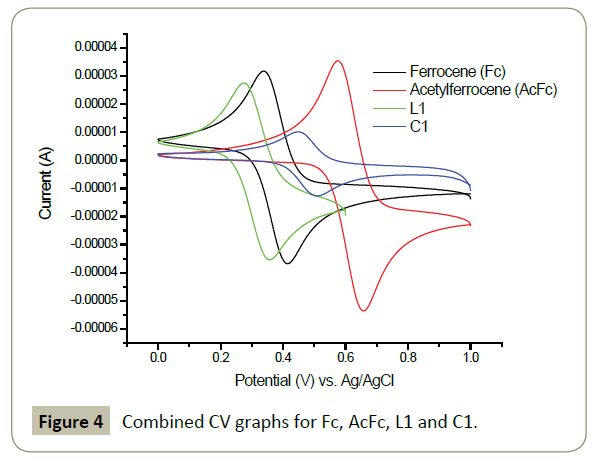
In order to understand the electrochemical behaviour around the iron centre, the cyclic voltammetry studies were perform on pure ferrocene, acetyl ferrocene, 3-ferrocenyl-1H-pyrazole (L1) and complex C1. Acetonitrile was used as a solvent and tetrabutylammonium perchlorate (Bu4NClO4) as an electrolyte. The working electrode was the carbon glass, platinum wire, counter electrode, and Ag/AgCl couple as a reference electrode with a scan rate of 0.1 Vs-1.
In general, all the compounds showed one redox couple corresponding to Fe2+/Fe3+. The oxidation potential (Epa) for ferrocene was observed at 0.414 V while the reduction potential appeared at 0.337 V giving ΔEp of 77 mV. This falls in the range of a one electron reversible system of 70-100 mV range [39]. The other ferrocenyl containing compounds showed ΔEp values in this range except complex C1 which was slightly lower (60 mV). The electronic properties of substituents on ferrocene can be followed by the values of Epa. In principle, electron withdrawing groups make the iron centre electron poor thus difficult to oxidize. This is seen by increased value of Epa compared to the one of pure ferrocene. The electron donating groups act contrarily to the electron withdrawing groups. The increased electron density around the iron centre makes it prone to loosing electron hence easy oxidation which is observed by a low value of Epa compared to pure ferrocene [39]. Accordingly, the acetyl group in acetyl ferrocene displayed electron withdrawing behaviour while pyrazole ring in 3-ferrocenylpyrazole acts as electron donating. The coordination to palladium centre makes the oxidation of iron difficult as compared to the initial ligand (L1) and this is obvious as the palladium centre is cationic and surrounded by Cl atoms which are also electron withdrawing. The redox properties of the palladium centre are not observed and this is in agreement with previously reported studies [40]. Table 1 shows detailed electrochemical values for each analysed compound (Figure 4).
| Compound | Epa(mV) | Epc(mV) | E1/2 (mV) | ΔE(mV) |
|---|---|---|---|---|
| Ferrocene | 414 | 337 | 376 | 77 |
| Acetylferrocene | 659 | 574 | 617 | 85 |
| L1 | 358 | 277 | 318 | 81 |
| C1 | 507 | 447 | 477 | 60 |
Table 1: CV data for selected ferrocenyl compounds.
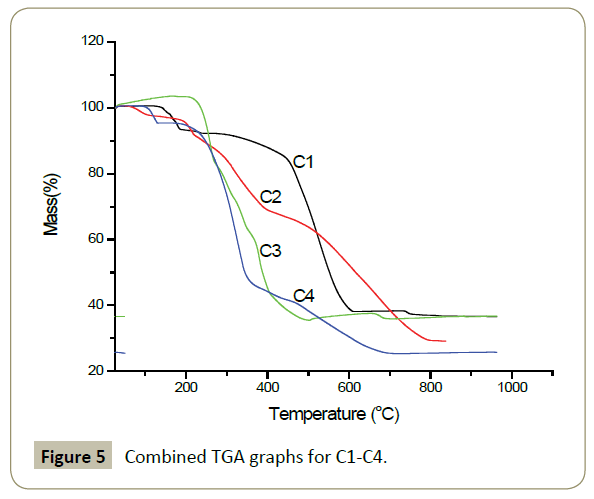
Thermogravimetric analysis: The thermal stability of complexes C1-C4 were studied by thermogravimetric analysis using Metler Toledo 851e instrument under nitrogen atmosphere at a heating rate of 10°C/min. In terms of thermal stability, complex C3 was the most stable (217°C), followed by complex C1 (137°C) and complex C4 (102°C) while complex C2 is the least stable and starts to decompose at 78°C.
The first decomposition step is the same for complexes C1, C2, C4 and corresponds to the loss of a chlorine atom while the final stage involves the formation of either palladium metal or a mixture of palladium metal a fraction of iron(II) chloride or palladium(II) chloride. In contrast to other compounds, complex C3 showed a continuous decomposition from 217°C until the final residue formed which could be a mixture of palladium metal and palladium (II) chloride. The proper analysis of the residue could help establish with no ambiguity the exact composition of the residue. The presence of palladium metal residue is in agreement with literature reports on the thermal decomposition studies of palladium (II) complexes (Table 2 and Figure 5) [40,41].
| Complex | Stability (°C) | Temp. Range (°C) | Group removed | Mass loss (%) | |
|---|---|---|---|---|---|
| Found | Calculated | ||||
| C1 | 137 | 137-189 800- |
Cl Residue Pd+FeCl2 |
6.69 36.75 |
5.21 34.22 |
| C2 | 78 | 78-185 827- |
Cl Residue Pd+Fe |
3.60 29.20 |
5.86 31.16 |
| C3 | 217 | 217-500 | Residue Pd+1/7PdCl2 | 35.93 | 35.64 |
| C4 | 102 | 102-129 129-720 |
Cl Residue Pd |
5.00 25.01 |
7.62 22.84 |
Table 2: TGA data for complexes C1-C4.
Magnetic measurements
The magnetic susceptibility for complexes C1, C2 and C4 were measured. The values for these compounds which are less than the value of the magnetic moment for one unpaired electron confirm the diamagnetic nature of the d8Pd(II) centres and they are in agreement with other reported Pd(II) compounds [42]. However, the reported values for the magnetic susceptibility are higher than zero especially for complexes C1 and C2 that contain ferrocenyl group implying some paramagnetism in the molecule. This might be due to the distortion from a pure square planar geometry which was also observed in previous reports on dinuclear complexes [43]. Table 3 contains detail of the magnetic measurement experiments.
| Complex | Temp K | χg x 10-6cgs | χM x 10-6cgs | χM x 10-6cgs | μeffB M |
|---|---|---|---|---|---|
| C1 | 301 | 0.731 | 498.24 | 605.84 | 1.21 |
| C2 | 299 | 0.1271 | 66.15 | 195.39 | 0.683 |
| C4 | 291 | 0.3323 | 154.75 | 115.11 | 0.056 |
Table 3: Magnetic moments results for complexes C1, C2 and C4.
Biological activities: The ligands (L1-L4) and complexes (C1- C4) have been screened for their antibacterial activities. The compound L3 showed remarkable antibacterial activities at the concentration of 30 μg/disc with respect to the standard antibiotic Kanamycin and across all the Gram positive and Gram negative bacteria. Complexes C1, C2 and C3 also showed a remarkable activity against all microorganisms while C4 showed a moderate activity against B. subtilis, S. aureus and C. albicans respectively. Complex C4 was not active against E. coli and P. aeruginosa. From the antibacterial activities of ligands, it emerges that the presence of a pyridyl group improves the activity (L2 generally more active than L1) while the phenyl group affects negatively the activity (L3 more active than L4). Similarly, the ferrocenyl moiety seems to have a negative impact on the antibacterial activities as the ligands which do not contain ferrocene are generally more active than those that incorporate the ferrocenyl group. This behaviour of ferrocenyl group towards the antibacterial activity was also observed from previous reports and it was stipulated that the bulkiness of the ferrocenyl group might hinder the penetration of compound in the cell hence reduced activity [21].
After coordination to palladium, the activities of ligands containing the ferrocenyl group are significantly improved (C1 and C2 compared to L1 and L2). The same observation was drawn from earlier reports where the coordination of a ligand to a metal cation improved the antibacterial activity of the free ligand. This behaviour was rationalized by the fact that coordination to the metal centre increases the lipophilicity of the compound hence a better penetration of the antibacterial molecule in the cell membrane [43]. Details on the zone of inhibition for each studied compound can be accessed in the supplementary information Table S1.
Conclusions
Palladium(II) complexes of pyrazolyl based ligands (C1-C4) have been successfully synthesized and characterized by spectroscopy, thermal analysis, magnetic measurements as well as cyclic voltammetry. Antibacterial and antifungal activities for all the ligands and complexes have been measured using Agar Disk Diffusion Method against gram positive bacteria (S. aureus, B. subtilis), gram negative bacteria (E. coli, P. aeruginosa) and one fungal, C. albicans. The results of the antibacterial activities reveal that the presence of bulky groups such as ferrocene and phenyl reduces the activity while coordination to palladium metal generally enhances the antibacterial activity.
Acknowledgements
The authors are grateful for the financial support from the University of Botswana, Indian Institute of Science and The World Academy of Sciences (TWAS). Mr. I. S. Jarali (Indian Institute of Science) for TGA data collection. Tamilarasan Subramani and Sourav Laha (Indian Institute of Science) for assistance on solid state UV-vis data collection. Rudra Narayan Samajdar (Indian Institute of Science) for assistance on CV data collection.
References
- Ehrlich HW (1960) the crystal and molecular structure of pyrazole. Acta Crystallogr13: 946-952.
- Mighell AD and Reiman CW (1967) Structure of pyrazole. J Phys Chem 71: 2375-2376.
- Kumar KA, Jayaroopa P (2013) Pyrazoles: Synthetic strategies and their pharmaceutical applications-an overview. Inter J PharmaTech Res5: 1473-1486.
- Mo O, Yanez M, Llamas-Saiz AL, Foces-Foces C, Elguero J (1995) Ab initio study of the effect of N- substituents on properties of pyrazoles. Tetrahedron 51: 7045-7062.
- Qiao J, Liu B, Liao Z, Li Y, Ma L, et al. (2014) One-pot to fused pyrazoles by a double cyclization of o-alkynylaldehydes with ketones and hydrazine under metal-free condition. Tetrahedron 70: 3782-3787.
- Zanetta N, Amaral SS, dos Santos JM, da Silva AMPW, Schneider JMFM, et al. (2013) One-pot to fused pyrazoles by a double cyclization of o-alkynylaldehydes with ketones and hydrazine under metal-free condition. Tetrahedron Lett54: 4076-4079.
- Tang M, Zhang FM (2013) efficient one-pot synthesis of substituted pyrazoles. Tetrahedron69: 1427-1433.
- Sairem S, Anna VR, Wang P, Das B, Kollipara MR (2012) η 5 and η 6 - cyclic π-perimeter hydrocarbon platinum group metal complexes of 3-(2-pyridyl)pyrazole derived ligands with a pendant nitrile group: Syntheses, spectral and structural studies. J Chem Sci 124: 411-419.
- Knorr L (1884) Einwirkung von Acetessigester auf Hydrazinchinizinderivate. Chem Ber 17: 546.
- Kumar V, Kaur K, Gupta GK, Sharma AK (2013) Pyrazole containing natural products: Synthetic preview and biological significance. Eur J Med Chem69: 735-753.
- Kucukguzel SG, Senkardes S (2015) Recent advances in bioactive pyrazoles. Eur J Med Chem97: 786-815.
- Shabalala NG, Pagadala R, Jonnalagadda SB (2015) Ultrasonic-accelerated rapid protocol for the improved synthesis of pyrazoles. Ultrason. Sonochem27: 423-429.
- Nemati F, Nikkhah SH, Elhampour A (2015) An environmental friendly approach for the catalyst-free synthesis of highly substituted pyrazoles promoted by ultrasonic radiation. Chin Chem Lett26: 1397-1399.
- Ganapathi M, Jayaseelan D, Guhanathan S (2015) Microwave assisted efficient synthesis of diphenyl substituted pyrazoles using PEG-600 as solvent - A green approach. Ecotoxicol Environ Saf121: 87-92.
- Kosuge T, Okeda H (1954) NONYLPYRAZOLE, A New Antimicrobial Substance. J Biochem4: 183-186.
- Wang JX, Zhu ZR, Bai FY, Wang XY, Zhang XX, et al. (2015) Molecular design and the optimum synthetic route of the compounds with multi-pyrazole and its derivatives and the potential application in antibacterial agents. Polyhedron 99: 59-70.
- Reddy TS, Kulhari H, Reddy VG, Bansal V, Kamal A, et al. (2015) Design, synthesis and biological evaluation of 1,3-diphenyl-1 H -pyrazole derivatives containing benzimidazole skeleton as potential anticancer and apoptosis inducing agents. Eur J Med Chem101: 790-805.
- Alegaon SG, Alagawadi KR, Garg MK, Dushyant K (2014) 1,3,4-Trisubstituted pyrazole analogues as promising anti-inflammatory agents Bioorg. Chem54: 51-59.
- Curion DM, Chayah M, Entrena A, Lopez A, Gallo M A, et al. (2013) Synthesis and biological evaluation of 4,5-dihydro-1H-pyrazole derivatives as potential nNOS/iNOS selective inhibitors. Part 2: Influence of diverse substituents in both the phenyl moiety and the acyl group. Bioorg. Med. Chem21: 4132-4142.
- Ratkovic Z, Juranic ZD, Stanojkovic T, Manojlovic D, Vukisevic RD, et al. (2010) Synthesis, characterization, electrochemical studies and antitumor activity of some new chalcone analogues containing ferrocenyl pyrazole moiety. Bioorg Chem38: 26-32.
- Saha NC, Mandal S, Das M, Khatun N, Mitra D, et al. (2014) Synthesis, characterization, X-ray crystallography and antimicrobial activities of new Co (III) and Cu (II) complexes with a pyrazole based Schiff base ligand. Polyhedron 68: 122-130.
- Raman N, Kulandaisamy A, Jeyasubramanian K (2002) Synthesis, Structural Characterization, Redox and Antimicrobial Studies of Schiff Base Copper(II), Nickel(II), Cobalt(II), Manganese(II), Zinc(II) and Oxovanadium(II) Complexes Derived from Benzil and 2-Aminobenzyl Alcohol. Pol J Chem76: 1085.
- Tweed BG (1964) Plant extracts with metal ions as potential antimicrobial agents. Phytopathology55: 910
- Deepthi SB, Ramesh P, Trivedi R, Buddana SK, Prakasham RS (2015) Carbohydrate triazole tethered 2-pyridyl-benzimidazole ligands: Synthesis of their palladium (II) complexes and antimicrobial activities. Inorg. Chim. Acta 435: 200-205.
- Radić GP, Glodović VV, Radojević ID, Stefanović OD, Comić LR, et al. (2012) eospecific ligands and their complexes. XI: Synthesis, characterization and antimicrobial activity of palladium(II) complexes with some alkyl esters of (S,S)-ethylenediamine-N,N′-di-2-(3-methyl)-butanoic acid. Inorg Chim Acta391: 44-49.
- Deepthi SB, Trivedi R, Sujitha P, Kumar CG, Sridhar B, et al. (2012) Synthesis, characterization and cytotoxic activity of palladium (II) carbohydrate complexes. J Chem Sci 124: 1405-1413.
- Juribašić M, MolÄÂÂanov K, Kojić-Prodić B, Bellotto L, Kralj M, et al. (2011) Synthesis, characterization and cytotoxic activity of palladium (II) carbohydrate complexes. J Inorg Biochem 105: 867-879.
- Sharma K, Singh RV, Fahmi N (2011) Palladium(II) and platinum(II) derivatives of benzothiazoline ligands: Synthesis, characterization, antimicrobial and antispermatogenic activity Spectrochim. Acta Part A: Molecular and Biomolecular Spectroscopy 78: 80-87.
- Satheesh CE, Kumar PR, Sharma P, Lingaraju K, Palakshamurthy BS, Naika HR (2016) Synthesis, characterisation and antimicrobial activity of new palladium and nickel complexes containing Schiff bases Inorg Chim Acta442: 1-9.
- Efthimiadou EK, Katsarou ME, Karaliota A, Psomas G (2008) Copper(II) complexes with sparfloxacin and nitrogen-donor heterocyclic ligands: Structure-activity relationship. J Inorg Biochem102: 910-920.
- Niedenzu K, Serwatowski J, Trofimenko S (1991) Boron derivatives of 3-ferrocenylpyrazole. Inorg Chem30: 524-527.
- Obuah C, Munyaneza A, Guzei IA, Darkwa J (2014) Ferrocenylpyrazolyl palladium complexes as catalysts for the polymerisation of 1-heptene and 1-octene to highly branched polyolefins. Dalton Trans43: 8940-8950.
- Morobe IC, Obi CL, Oyedeji AO, Vasaikar SD, Mbenza LB (2013) J Microbiol Res 3: 57-65.
- Haque E, Hossen F, Islam S, Mohammod S (2006 ) J Agric Biol8: 774.
- Watanabe M, Chang WJ, Chou PT, Staykov A, Shibahara M, et al. (2015) Synthesis and electronic properties of ferrocene-containing organic dyads. Tetrahedron Lett56: 1548-1551.
- Mason WR, Gray HB (1968) Electronic structures of square-planar complexes J Am Chem Soc 90: 5721-5729.
- Less RJ, Wicks JLM, Chatterton NP, Dewey MJ, Cromhout NL, et al. (1996) Synthesis, molecular structure and palladium(II) and platinum(II) complex chemistry of 3-(ferrocen-1-yl)-1-(pyridin-2-yl)pyrazole. J Chem Soc Dalton Trans 21: 4055-4061.
- Mathiyalagan K, Gopal S, Ramasamy E, Vennila T (2012) Internat J ChemTech Res 4: 1775-1781.
- Rocha FV, Barra CV, Franchi SJS, Netto AVG, Mauro AE, et al. (2011) Study on the thermal behavior of the complexes of the type [PdX2(tdmPz)] (X = Cl−, Br−, I−, SCN−). J Therm Anal Calorim 106: 385-389.
- Netto AVG, Santana AM, Mauro AE, Frem RCG, de Almeida ET, et al. (2005) Thermal decomposition of palladium(II) pyrazolyl complexes; Part II. J Therm Anal Calorim 79: 339-342.
- Onwudiwe DC, Ekennia AC, Mogwase BMS, Olubiyi OO, Hosten E (2016) Palladium(II) and platinum(II) complexes of N-butyl-N-phenyldithiocarbamate: Synthesis, characterization, biological activities and molecular docking studies. Inorg Chim Acta450: 69-80.
- Khan MM (2010) Synth React Inorg Metal-Org Nano-Met Chem40 148-152.
- Montazerozohori M, Musavi SA, Naghiha A, Veyseh S (2014) Some new IIB group complexes of an imidazolidine ligand: Synthesis, spectral characterization, electrochemical, thermal and antimicrobial properties. J Chem Sci 126: 227-238.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences