ISSN : 0976-8505
Der Chemica Sinica
Study on the Preparation, Characterization of a Novel Solid Br̮̦̉̉nsted Acid Catalyst H3PW12O40/C and its Catalytic Performance in the Synthesis of Biodiesel via Esterification Reaction of Oleic Acid and Methanol
Qing Shu*, Guo-qiang Tang, Feng-sheng Liu and Hai-yan Wei
School of Metallurgy and Chemical Engineering, Jiangxi University of Science and Technology, Ganzhou, Jiangxi, China
Abstract
Waste camellia seed shell was placed in fluidized bed tubular reactor and carbonized at 700°C for the preparation of a carbon material, and which was then used as the support for the synthesis of a novel solid Brönsted acid catalyst H3PW12O40/C by impregnation of it with H3PW12O40 solution. The obtained H3PW12O40/C catalyst was characterized by scanning electron microscope, X-ray diffraction, transmission electron microscopy, Fourier transform infrared spectoscopy, thermogravimetric analysis. The catalytic activity of H3PW12O40/C was evaluated from the esterification reaction of oleic acid with methanol, which was performed in a batch reactor. Results shown that the highest conversion of oleic acid was 75.83 wt% when the molar ratio of methanol to oleic acid was 10:1, reaction temperature was 60°C and catalyst loading was 1 wt% after 5 h. The high activity can be ascribed to the waste camellia seed shell-derived carbon material is mainly consisted of hydrophobic carbon sheet, which means that the adsorption of water on its Brönsted acid sites was reduced, so the hydration between Brönsted acid sites and H2O was also reduced. And eventually, the deactivation of Brönsted acid sites was reduced.
Keywords
Biodiesel, Model acidic oil, Esterification, Solid acid, H3PW12O40/C
Introduction
Biodiesel (Fatty Acid Methyl ester, FAME) has gained widespread attention due to it has good biodegradability, non-toxicity and favorable combustion emission profile. Biodiesel can be produced by either trans-esterification of triglycerides (main component of vegetable oil and animal grease) or esterification of free fatty acid (FFA) with a short chain alcohol, mainly ethanol and methanol, using chemical and enzymatic catalysts.
According to the characteristics of biodiesel production process, it can be seen that the cost of raw material accounts for 50%~85% of the total production cost of biodiesel [1-4]. Therefore, the cost of raw materials is the most important factor in determining the price of biodiesel. At present, the major biodiesel producing countries, such as the United States, European Union and India, have chosen different types of vegetable oil as feedstock for biodiesel production according to their respective characteristics of planting of vegetable oil, including rapeseed oil, castor oil and soybean oil, palm oil [5-7]. Although China is a country with rich plant resources and broad distribution advantages, it can provide great convenience for the selection of biodiesel raw materials. However, China is a populous country, vegetable oil still needs a lot of imports and consumes as edible oil priority, which determines that China cannot use a large amount of edible vegetable oil as raw material for biodiesel production. Therefore, the industrialized development model of biodiesel in Europe and the United States is not in line with China's national conditions. At the same time, because China is a big consumer of edible oil, the annual consumption of edible oil is about 1600 million tons. Among them, about 10% of the edible oil was abandoned after use, resulting in a waste of 1 million 600 thousand tons of animal fats and vegetable oils [8]. Although there are difficulties in collecting waste animal fats and vegetable oil, and the pre-treatment of these collected waste oils is also complex. However, it can reduce the cost of raw materials due to the recovery is cheap, and the environmental pollution is also reduced after the changing of waste into treasure, so it is undoubtedly a huge cheap biodiesel sources of raw materials. Therefore, if we can reasonably use waste animal fats and vegetable oils for the production of biodiesel, it can be predicted that it will produce enormous economic and environmental benefits to our country's economic and social development. However, the composition of the waste animal fats and vegetable oil is very complex, so it put forward a severe requirement for a catalyst that can maintain high catalytic activity and stability in the reaction process when they were used as raw materials for biodiesel production.
At present, carbon based solid acid has become a hot spot in the research of solid acid catalysts at home and abroad due to it has several advantages, such as simple preparation, strong proton acidity and high catalytic activity [9-11]. Carbon based solid acid is commonly refers to a kind of material, which was prepared from the selection of a carbon material first, and then the surface of it was modified by a liquid acid (mainly concentrated sulfuric acid). However, it also found if a solid acid catalyst was prepared from the modification treatment of a carbon material by concentrated sulphuric acid alone, which will be consisted of Brönsted acid sites mainly, along with so many hydrophilic polar groups -SO3H had entered into the skeleton of carbon carrier through the bond combination action between -SO3H and carbon layer. It will further enhance the hydrophilic of the catalyst, which will lead the occurrence of hydration between Brönsted acid sites of a sulfate type carbon-based solid acid catalyst and water molecules more easily. And finally, thereby weakening and even losing of its catalytic activity. Therefore, it is difficult to take into account the advantages of high catalytic activity and stability, simultaneously.
In this study, the waste camellia oil shell was selected as raw material for the preparation of carbon material. This selection was stimulated from a previous research of our group: a carbon material that consists of a high proportion of graphitized carbon layers was obtained when petroleum asphalt (the main component of it is polycyclic aromatic hydrocarbon) was selected as raw material [12]. Due to the main component of waste camellia oil shell are cellulose and lignin, both of them also belonging to polycyclic aromatic hydrocarbon. Therefore, it can be expected to obtain a carbon carrier with a high proportion of graphitized carbon layers from the employing of waste camellia oil shell as raw material. In addition, tungstophosphoric acid (H3PW12O40) was used to replace sulfuric acid. This replacement will not change the hydrophobic surface of waste camellia seed shell-derived carbon material when compared with H2SO4, so the occurrence of hydration can be considerably reduced. And more, the dealing of the waste from the neutralization of H2SO4 can be avoided. Hence, H3PW12O40/C can be regarded as a more suitable and greener catalyst when it was compared with the sulfate type carbon-based solid acid catalyst.
Materials and Methods
Experimental
Reagent
Na3PO4·12H2O (99%, analytical pure) and (NH4)2WO4·2H2O (99%, analytical pure) were purchased from Tianjin Hengxing Chemical Reagent Co., Ltd. China (Mainland), methanol (99%, analytical pure) was purchased from Tianjin Damao Chemical Reagent Factory of China (Mainland), ethanol (99%, analytical pure) was purchased from Hengyang Kaixin chemical reagent Co. Ltd. China (Mainland), diethyl ether (99.5%, analytical pure) and oleic acid (99%, analytical pure) were purchased from Xirong chemical engineering Co. Ltd. China (Mainland), phenolphthalein (99%, analytical pure) and potassium hydroxide (96%, analytical pure) were purchased from Tianjin Guangfu Fine Chemical Research Institute Co. Ltd. China (Mainland).
Catalyst preparation
Carbon material was prepared from the carbonization treatment of waste camellia seed shell, which was carried out as follows: first, batches of 10.0 g of waste camellia seed shell were oxidized for 1.0 h at 280°C in a stream of air, and the flow rate was 300 mL·min-1; second, it was heated to 700°C with a heating rate of 2°C·min-1 under an argon atmosphere, and the flow rate was 300 mL·min-1; third, the obtaind carbon material was well grinded and sieved to separate those particles with particle size at the range of 60~80 mesh. The sorted particles were placed in the distilled water and heated to boiling for 0.5 h, which is favourable to remove ash that has adsorbed on the surface of carbon material.
The preparation of H3PW12O40 was carried out as follows: first, 3.8 g Na3PO4·12H2O and 38.4 g (NH4)2WO4·2H2O were successively dissolved into distilled water, and then heated to boiling under reflux with stirring; second, HNO3 was added drop wise into the mixed solution through separator funnel until no formation of precipitation, and heating was stopped; third, the hot mixed liquid was filtrated through Buchner funnel and transferred to a separator funnel after it has been cooled to ambient temperature, and then added some ether; and finally, it was placed in thermostatic oven with a temperature of 110°C and dried until the solvent had been evaporated, obtained H3PW12O40. The preparation principle can be expressed as reaction equation (1):
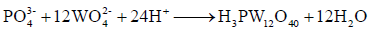 (1)
(1)
The preparation of H3PW12O40/C was performed as follows: first, 5.0 g carbon material was dispersed into 20 mL H3PW12O40 solution (with the containing of 1.5 g H3PW12O40). The solution was sonicated for 1 h, and then stirred overnight; second, the resulting suspension was dried in a vacuum drying chamber at 120°C for 5 h; and finally, it was calcined for 3 h to obtain H3PW12O40/C.
Catalyst characterization
The morphology of the carbon material, H3PW12O40 and H3PW12O40/C catalyst were observed by SEM (JSM- 7401F, JEOL). Transmission electron microscopy (TEM) was further employed to determine the strain state and crystallographic structure of H3PW12O40/C catalyst (Tecnai G2 F20, FEI). The crystalline phases of H3PW12O40/C catalyst were analysed by XRD (O8DISCOVER, Seimens) through the using of 40 mA and 40 kV Cu Kα radiation. FTIR spectra of the H3PW12O40/C catalyst were obtained by the using of a FTIR-spectrometer (Nicolet 6700 FTIR) from 4000 to 400 cm-1. Samples were pressed by a KBr disk preparation apparatus and the resolution is 4 cm-1. The thermal stability of the H3PW12O40/C catalysts was examined by TGA, which was carried out on a NETZSCH STA409 analyser. The analysis sample was heated from 25 to 800oC at a rate of 10oC·min-1 in air, and the flow rate was 90 ml·min-1.
Catalytic reaction procedure
In an industrial production, acidic oil would be used as feedstocks. In this research, oleic acid (Analytical reagent, 99%) was used as the model substitute of soapstock and reacted with methanol (Analytical reagent, 99.5%) for the investigation of the influences that were derived from the change of reaction parameters with the using of H3PW12O40/C as catalyst. The reaction was carried out in a 250 ml four-necked round-bottomed flask, equipped with a reflux condenser, a thermometer and a mechanical stirrer. The syntheses conditions with respect to catalytic activities have been optimized individually, including the molar ratio of methanol to oleic acid, reaction temperature and catalyst loading. For a typical run, the methanol and H3PW12O40/C catalyst were added into the reactor at first. And then, oleic acid was added into the reactor after the required reaction temperature was reached. The reaction was started by stirring (at 240 rpm) and stopped after 2~7 h, and the reaction mixture was poured into a separation funnel and allowed to phase separate for 12 h.
The catalytic stability test of H3PW12O40/C catalyst catalyst was carried out as follows: after the optimum experimental condition that related with H3PW12O40/C-catalysed process was determined, continuous cycle of H3PW12O40/C catalyst five times under this optimum reaction condition. In the cycling process, catalyst was separated from the reaction system by ether extraction. Reused again after drying.
Product analysis
The conversion rate of oleic acid was measured and analyzed by acid value method. The determination of acid value was carried out following the method introduced in ISO 1242:1999. Pretreatment procedure before the sample was determined as follows: 1.5 ml test sample was taken every hour, and the collected samples were placed in thermostatic oven with a temperature of 80oC for 30 minutes to evaporate residual methanol from the sample after the residual catalyst has been removed by centrifugation. Purified test sample was dissolved in 10 ml of a mixture of equal volumes of ethanol and light petroleum, neutralised with 0.1 M potassium hydroxide with the using of phenolphthale as indicator. The acid value was defined as Equation (2):
AV=(C × V × 56.1)/m (2)
Where, AV was the acid value (mg KOH/g), V was the consumed volume of KOH (mL), C was the concentration of KOH (mol/L), and the m was the mass of tested sample (g).
The conversion of oleic acid was defined as Equation (3):
X=(AV1-AV2)/AV1 (3)
Where, X was the conversion of oleic acid (wt %), AV1 was the acid value of initial oleic acid (mg KOH/g), and AV2 was the acid value of the remaining oleic acid (mg KOH/g).
Results and Discussion
Characterization of the catalyst
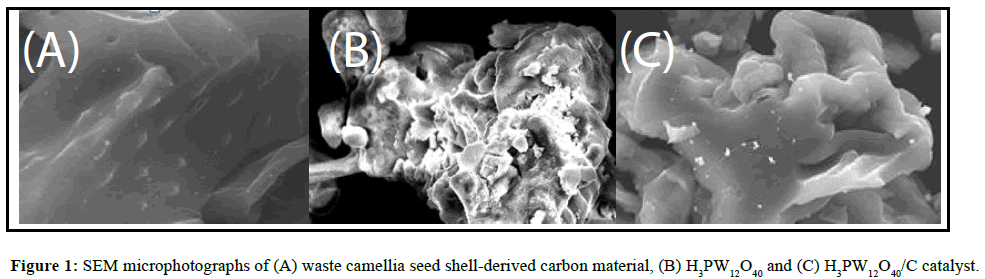
The SEM microphotographs that depicted the morphology structures of waste camellia seed shell-derived carbon material (carbon carrier), H3PW12O40 and H3PW12O40/C catalyst were shown in Figure 1.
From Figure 1A, it can be seen that the obtained carbon carrier-C exhibited a compact network structure, no obvious pores were appeared. The H3PW12O40 exhibited a disintegrated network structure with some pores (Figure 1B). After the supporting of H3PW12O40 on the carbon carrier-C by impregnation, the compact network structure of C has changed, it shown a network structure that is more like H3PW12O40 (Figure 1C).
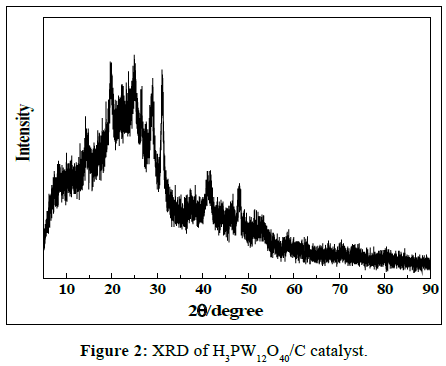
In order to attain further information of the structural property of H3PW12O40/C catalyst, it was analyzed by XRD measurement. Result was shown in Figure 2. As shown in Figure 2, the H3PW12O40/C sample has a broad strong diffraction peak in the 2θ range of 10~30° and two narrow weak peaks at 35~50°, which are attributed to the (002) and (101) faces of amorphous carbon. This indicates that the catalysts are composed of amorphous carbon with a low degree of graphitization. Some reflections were appeared at 2θ values of 16.2°, 23.7°, 26.1°, 30.2°, which are assigned to the characteristics of H3PW12O40. Hence, it can be concluded that the H3PW12O40 has been successfully supported on the surface of carbon carrier.
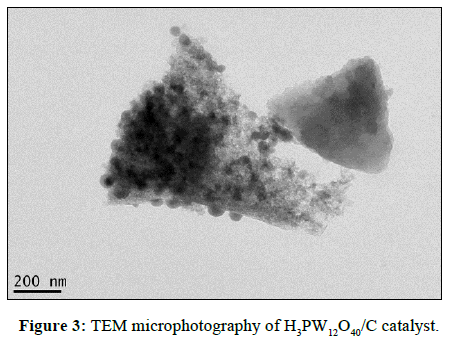
The TEM microphotography of H3PW12O40/C is illustrated in Figure 3. From this figure, a perfect layered structure could be observed obviously. The active component (dark dots) was orderly-arranged and well-distributed on the surface and the internal structure of the carbon carrier. All the active components exhibited a shape of hemispherical. The particle sizes of them are almost the same. The channel structure of carbon carrier also can be observed directly. The high dispersion of active components and organized porous structure will be convenient for the adsorption of oleic acid and methanol onto the active sites of the catalyst.
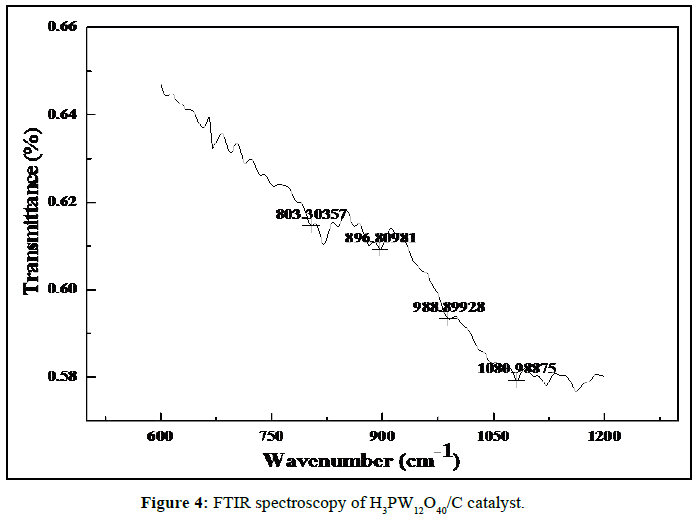
FTIR spectroscopy was employed to investigate the functional groups of the H3PW12O40/C catalyst. Result was shown in Figure 4. As is known, the characteristic absorption peaks of Keggin unit at 1080, 982, 888, 805 cm–1 belong to νas(P–O), νas(W–Od), νas(W–Ob–W), νas(W–Oc–W), respectively [13]. In Figure 4, although the characteristic absorption peaks correspond to νas(W–Od), νas(W–Ob–W) and νas(W–Oc–W) has slightly shifted towards the short wave number, the four typical adsorption peaks of Keggin unit from 700 to 1100 cm–1 still remain, which indicates that the Keggin structure of H3PW12O40 is not destroyed in the crystallization process.
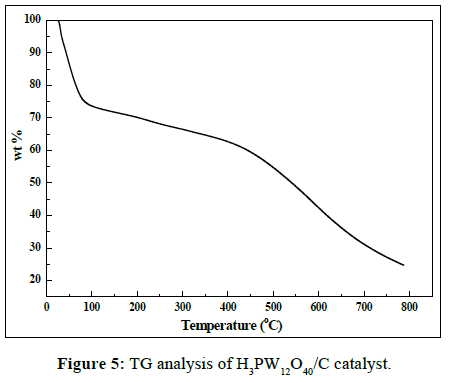
The TGA plot of the H3PW12O40/C catalyst in air was shown in Figure 5. In the temperature range of 40°C -100°C, the TGA plot of the H3PW12O40/C catalyst displayed a rapid weight loss (about 27%), the first decomposition step can be associated with the partial ligands decomposition and water loss. Then there was a slight weight loss up to 450°C, indicating the total decomposition of the remaining ligands and the oxidation of non-graphitic and graphitic carbon.
Catalytic activity
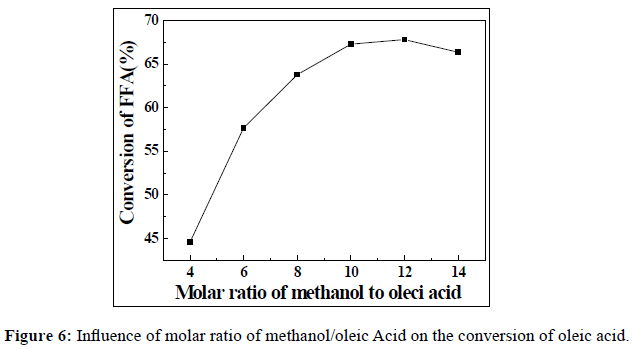
Due to the esterification reaction is reversible, so it is necessary to keep an excess of methanol in the reaction mixture, which is favourable for the proceeding of reaction towards the formation of the FAME. The effect of the molar ratio of methanol to oleic acid on the conversion of FAME was examined, and which was changed at the range of 4:1, 6:1, 8:1, 10:1, 12:1 and 14:1. The reaction time, catalyst loading and reaction temperature were fixed at 5 h, 1 wt% and 60 °C, respectively. The results were shown in Figure 6. It can be seen that the highest conversion of oleic acid was 67.8 wt% when the molar ratio of methanol to oleic acid was 12. However, with the continual increment of the molar ratio of methanol/oleic acid to 14:1, the conversion was decreased to 66.4 wt%.
It can be explained as follows: the esterification was started from the oleic acid chemisorbed on the active sites, and then protonated the carbonyl group to give an carbocation ion, which can undergo the attacking by the methanol to form esters. When the amount of methanol is high to some extent, the approach of methanol molecules to the carbocation will be enhanced. And finally, the conversion of oleic acid will increase. However, when the amount of methanol is much high, this will lead to the flooding of active sites by methanol molecule rather than oleic acid. Therefore, the increment of molar ratio will hinder the completion of oleic acid being protonated at the active sites. A molar ratio of methanol/oleic acid 12:1 is appropriate for this reaction.
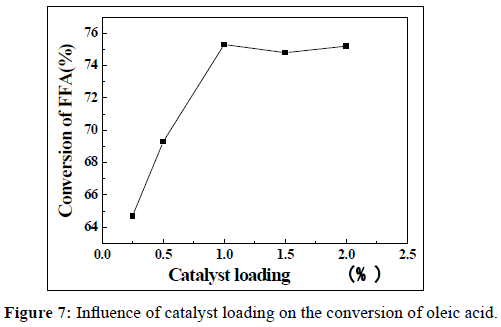
The amount of catalyst also will affect the conversion of this process. The effect of the catalyst loading on the conversion of oleic acid was studied, and which was changed at the range of 0.25, 0.5, 0.75 and 1 wt%. The reaction time, reaction temperature and the methanol/oleic acid molar ratio were fixed at 5h, 60°C and 12:1, respectively. The results were shown in Figure 7. It can be seen that the conversion of oleic acid increased with the increment of catalyst loading. The highest conversion of oleic acid was 75.3 wt% when the catalyst loading was 1 wt%. Hence, the optimum catalyst loading for this reaction was selected at 1 wt%.
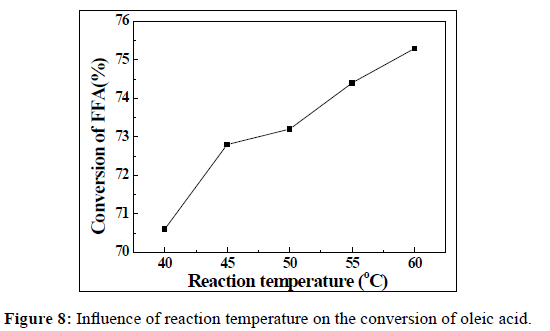
In order to study the influence of the reaction temperature on the conversion of oleic acid, experiments us were conducted at 40, 45, 50, 55 and 60°C. The reaction time, mass ratio of catalyst/oleic acid and the methanol/oleic acid molar ratio were fixed at 5h, 1 wt% and 12:1, respectively. The results were shown in Figure 8. It can be seen that the conversion of oleic acid was increased with the increment of reaction temperature. However, a high temperature increased the cost of biodiesel production. Except that a higher production cost, it also should be noted that the boiling point of methanol is 64.7°C under atmospheric pressure. Hence, the vaporization of methanol will occur when the esterification reaction was carried out at a relative reaction temperature that is much above its boiling point. This will reduce the methanol concentration in the reaction system. And finally, it will lead to a bad effect on the conversion of oleic acid. Therefore, the reaction temperature should not be much higher than the boiling point temperature of methanol when the esterification reaction was carried out under atmospheric pressure. The optimum temperature for this reaction was selected at 60°C.
The reusability of H3PW12O40/C catalyst was tested. It was carried out under the obtained-optimal reaction condition: the molar ratio of methanol/oleic acid was 12:1, reaction temperature was 60°C, catalyst loading was 1 wt%, and reaction time was 5 h with refluxing. The conversion of oleic acid was less changed (within 5%).
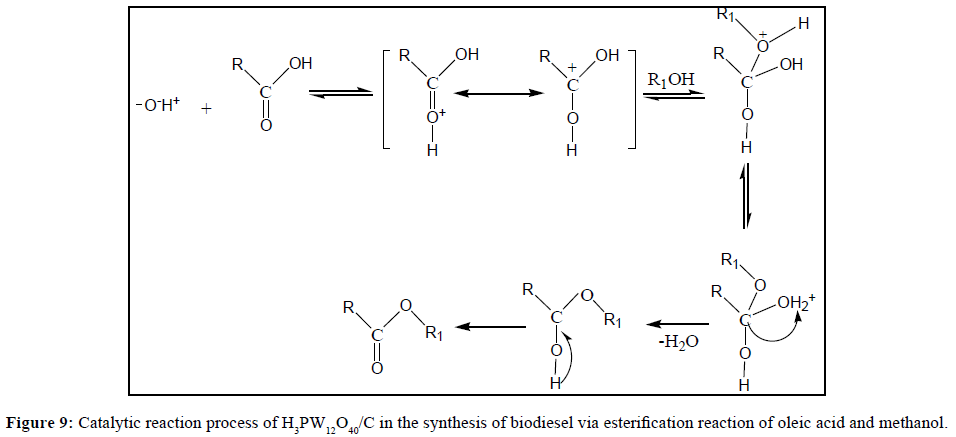
The catalytic reaction process of H3PW12O40/C in the synthesis of biodiesel via esterification reaction of oleic acid and methanol can be shown as Figure 9.
A novel solid Brönsted acid catalyst H3PW12O40/C was prepared from the using of a waste camellia seed shell-derived carbon material as the support by impregnation of it with H3PW12O40 solution. The catalytic activity of H3PW12O40/C was evaluated from the esterification reaction of model acidic oil with methanol. Results indicated that the highest conversion of model acidic oil was 75.83 wt% when the molar ratio of methanol to oleic acid was 10:1, reaction temperature was 60°C and catalyst loading of 1 wt% after 5h. It offers a way to produce biodiesel that uses a recoverable solid acid catalyst, which would be more environmentally friendly than a liquid acid catalyst.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (21206062, 21466013), Major project of Natural Science Foundation of Jiangxi Province for Youth (20143ACB21018), Program of Qingjiang Excellent Young Talents (Jiangxi University of Science and Technology), the funds from Hundred Sail Away Project in 2015 provided by Jiangxi Provincial Party Committee Organization Department and Jiangxi Association for Science and Technology.
References
- Masoumeh H, Meisam T, Mortaza A (2017) A review on the prospects of sustainable biodiesel production: a global scenario with an emphasis on waste-oil biodiesel utilization[J]. Renew Sustainable Energy Rev72: 445-464.
- Mahmudul HM, Hagos FY, Mamat R (2017) Production, characterization and performance of biodiesel as an alternative fuel in diesel engines-A review[J]. Renew Sustainable Energy Rev 72: 497-509.
- André GB, Virginia P (2016) Organic municipal solid waste (MSW) as feedstock for biodiesel production: A financial feasibility analysis [J]. Renew Energy86: 1422-1432.
- Verma P, Sharma MP (2016) Review of process parameters for biodiesel production from different feedstocks[J]. Renew Sustainable Energy Rev 62: 1063-1071.
- Jayakumar S, Yusoff MM, Rahim MH (2017) The prospect of microalgal biodiesel using agro-industrial and industrial wastes in Malaysia[J]. Renew Sustainable Energy Rev72: 33-47.
- Carneiro M, Pradelle F, Braga SL (2017) Potential of biofuels from algae: Comparison with fossil fuels, ethanol and biodiesel in Europe and Brazil through life cycle assessment (LCA)[J]. Renew Sustainable Energy Rev73: 632-653.
- Tsoutsos TD, Tournaki S, Paraíba O (2016) The used cooking oil-to-biodiesel chain in Europe assessment of best practices and environmental performance[J]. Renew Sustainable Energy Rev54: 74-83.
- Xu YJ, Li GX, Sun ZY (2016) Development of biodiesel industry in China: upon the terms of production and consumption[J]. Renew Sustainable Energy Rev54: 318-330.
- Manuel S, Lydia MP, Maribel M (2017) A sustainable method to produce biodiesel through an emulsion formation induced by a high shear mixer[J]. Fuel 189: 436-439.
- PatilPD, Reddy H, Muppaneni T (2017) Biodiesel fuel production from algal lipids using supercritical methyl acetate (glycerin-free) technology[J]. Fuel195: 201-207.
- Kuepethkaew S, Kanokphorn S, Soottawat B (2017) Optimized synthesis of biodiesel using lipase from Pacific white shrimp (Litopenaeusvannamei) hepatopancreas[J]. Renew Energy104: 139-147.
- Shu Q, Nawaz Z, Gao J (2010) Synthesis of biodiesel from waste oil feedstocks using a carbon-based solid acid catalyst: reaction and separation[J]. BioresourTechnol 101: 5374-5384
- Izumi Y, Urabe K (1981) Catalysis of heretopoly acids entrapped in activated carbon [J]. ChemLett10: 663-666.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences