ISSN : 2393-8862
American Journal of Pharmacology and Pharmacotherapeutics
Study of Antioxidant and Anti-Inflammatory Crude Methanol Extract and Fractions of Acacia seyal Gum
Ahmed AM Elnour1,4,Mohamed ES Mirghani1,2*, Kabbashi NA1, Md Alam Z1 and Khalid H Musa3
1 Department of Biotechnology Engineering,Bioenvironmental Engineering Research Institute (BERI), Kulliyyah (Faculty) of Engineering, International Islamic University, Kuala Lumpur, Malaysia
2 International Institute for Halal Research and Training (INHART), International Islamic University, Kuala Lumpur, Malaysia
3 Department of Food Science and Human Nutrition College of Agriculture and Veterinary Medicine Qassim University, Kingdom of Saudi Arabia
4 Department of Biochemistry and Gum Processing, Gum Arabic Research Centre, University of Kordofan, Sudan
- *Corresponding Author:
- Mohamed Elwathig Saeed Mirghani
Department of Biotechnology Engineering, Bioenvironmental Engineering Research Institute (BERI), Kulliyyah (Faculty) of Engineering, International Islamic University, Kuala Lumpur, Malaysia.
Tel: +603-6196 4442
Fax: +603-6196 5749
E-mail: elwathig@iium.edu.my
Received Date: July 05, 2018; Accepted Date: August 09, 2018; Published Date: August 16, 2018
Citation: Elnour AAM, Mirghani MES, Kabbashi NA, Md Alam Z, Musa KH (2018) Study of Antioxidant and Anti-Inflammatory Crude Methanol Extract and Fractions of Acacia seyal Gum. Am J Pharmacol Pharmacother. Vol.5 No.1:3. DOI: 10.21767/2393-8862.100012
Abstract
Acacia seyal gum (ASG) is a dried exudate from tropical plants in the family Leguminosae. It has been used in the medication of eczema, inflammation, renal failure, and hypertension in folk medicine. This study presents methanol crude extracts (MCE) and different fractions of ASG in the form of raw (ASG) and commercial type Prebio-T, (PTC) using different solvents. Evaluations were performed to observe the biological activities of the extracts and fractions with certain bioassays procedure of anti-inflammatory, total phenolic content (TPC) and radical scavenging activity (DPPH). The only MCE was assessed against inflammatory reaction using carrageenan paw oedema (in-vivo). However, the antioxidant activities of MCE and its fractions were evaluated. As a result, the highest amount of TPC was observed in the Prebio-T, with an average of 694.68 ± 3.60 mgTE/100 g DW, compared to only 155.78 ± 2.58 mg TE/100 g DW for ASG Crude Extract. The highest DPPH value was seen in methanol fraction (MF) was 235.34 ± 1.57 mg TE/100 g DW for Prebio-T, compared to ASG without any significant differences (p ≤ 0.05). Both MCE of ASG and Prebio-T at 150 mg/kg dosage developed a mean and maximum percentage inhibition of 23.63% and 23.54% respectively, during the 24h observation of the acute inflammatory test. The chemical composition of the 57 phenolic compounds in methanol crude extract (MCE), and its active fractions were detected using GC-MS/MS. The major constituents in the ASG and Prebio-T MCE were iso vitamin C (42.37%), crypton (5.86%), hydroquinone (4.86%), triacetic acid lactone (2.67%), 2,4-Di-tert-butyl phenol (2.67%), cyanidin cation (2.05%), apigenin 7-glucoside (1.9%), benzoic acid (1.83%), (+)-α-tocopherol (1.58%), methyl catechol (1.42%), and 2,6-dimethylol-pcresol (2.16%). The PBT methanolic crude extract (MCE) was found to be the most potent inhibitor of oedema formation by inducing a maximum inhibitory effect of 23.54% at the 300 mg/kg dose, during 24h post-carrageenan injection. Both PBT and ASG methanolic crude extracts showed good anti-inflammatory activities at the same dose (300 mg/kg). The results of these studies support the potential use of ASG for treating inflammation.
Keywords
Acacia seyal gum; Extraction; Antioxidant; Anti-inflammatory; GC-MS/MS
Introduction
Acacia seyal gum (ASG) from tropical plants in the Leguminosae family can be found from a particular part of Ethiopia, Eritrea, Chad, Nigeria, Burkina Faso, Senegal, Mauritania and the major gum belt in Sudan [1]. Initially, ASG was reported in various applications in food, nutrition, medicine, dyeing, and cosmetics [2]. Earlier, in the first World War, ASG solutions were injected intravenously as a blood treatment of shock and hemorrhage cases among soldiers and individual [3]. Moreover, the enriching dietary fibers lead to the extensive studies of GA as natural products where it has been mainly used as food ingredients and treatment [4]. ASG is a rich source of carbohydrates and proteins. It is rich in non-viscous soluble fibers with high dietary value, and also minerals like potassium, magnesium, and calcium [5]. According to the JEFCA (“Joint Expert Committee for Food Additives”) of FAO/WHO, it was defined like “a dried exudation obtained from the branches of A. senegal (L) Willdenow or close species from Acacia (Leguminosae family)” [6]. It includes, therefore, both A. senegal and A. seyal species. It was reported that ASG generally recognised as safe by Food Drug and Administration (FDA) [7]. Therefore, no toxicity was reported in ASG.
The solubility of ASG in solvent solutions remained challenges for scientific communities for more than decay. For example, ASG is soluble in dilute alcohol solution and precipitates when the alcohol concentration in water is of about 50%, with complete precipitation of ASG macromolecules with 60% alcohol [8]. Furthermore, with 30% alcohol, no precipitate occurs, and with 40% alcohol, a turbid/opalescent (“faint”) precipitate is obtained [9]. These findings based on ASG concentration. In this sense, alcohol precipitation has been long used to purify ASG [10]. However, it has been stated that recently, ASG during contact with alcohol-water or aqueous solution decreases the solubility of ASG and therefore, affect the antioxidant results negatively when using DPPH and ABTS radicals respectively [11]. Thus, the pure organic solvent is necessarily being the best choice of selection during antioxidant extraction form ASG.
However, the ability of alcohols (and some salts) to induce phase separation/precipitation in polysaccharides, and more generally biopolymers dispersions is not new and is called simple coacervation [12]. For this reasons, it would be useful to determine phase diagrams of AG-alcohol-water systems and to characterise in each phase the macromolecular composition. As these molecular fractions display or supposed to display different physical-chemical properties, it is likely that simple ways to obtain AG enriched in one or another of the sub-fractions could be identified. Therefore, a comprehensive study will be needed regarded antioxidant activity in ASG.
According to our knowledge, there is scant literature concerning antioxidant extraction from ASG. However, few studies have highlighted some information about ASG antioxidant properties, nephroprotectant, and other effects have also been mentioned in the recent studies [13]. This along with its role in the metabolism of lipids [14]. As a result, ASG considered being the promises many benefits in medical, food, and pharmaceutical industries. Moreover, the therapeutic use of ASG was already mentioned in Pliny, disaccharides and Theophrastus writings [15]. However, the Ebers manuscript (a medicinal papyrus written in 1550 BCE) already suggested using ASG as a contraceptive in association with dates. The famous queen Cleopatra requested the preparation of curative recipes based on ASG [16]. In the ninth century, the Arab physician Abu Zayd Hunayn Ibn Ishaq Al-Ibadi, writing in his Ten Treatises on the Eye, described ASG as an ingredient in poultices or eye compresses. It was also used to relieve topical irritation and to protect in cases of superficial excoriation, ulcers, burns, sore nipples, etc. [17]. Earlier, in the First World War, ASG solutions were injected intravenously as a blood treatment of shock and hemorrhage cases among soldiers as well as from leech bites [18]. It is, therefore, at the beginning of the 20th century; Europe consumed about 20000 tons per year of AG [19]. Thus, more research should be conducted in a biological aspect using ASG crude extract and their active fractions.
Recently, from biological studies perspective, there are several positive results in the treatment of certain degenerative diseases such as kidney failure has been presented [20]. Studies on its use for cardiovascular [21] and gastrointestinal diseases [22] have also been reported. These investigations suggest that ASG has the anti-inflammatory activity to encounter different diseases that contained within several compounds. As for their immunomodofying properties, the ASG as the raw form was found to be an anti-inflammatory agent [20]. However, studies on the anti-proliferative activity of ASG extracts and the effects of significant extract constituents on the anti-inflammatory property are yet to be conducted. The antioxidant and cytotoxic activities are yet to be comparatively evaluated, and the phytochemical composition general studies have not been conducted. Therefore, his study aimed to characterise ASG and Prebio-T extracts to compare between the natural exudates and the commercial form. The GC-MS/MS methods were employed for the quantification of the extracts main compounds and calorimetric method was used to characterize the extracts and their total phenolic content (TPC). DPPH (2, 2, -diphenyl-1-picrylhydrazyl) method was employed for the evaluation of the antioxidant properties. ASG extracts effects on anti-inflammatory activities were also reported.
Materials and Methods
Chemicals and reagents
Folin-Ciocalteu phenol reagent obtained from Merck (Darmstadt, Germany) and Sodium carbonate were from RDH (Germany). Trolox, 2, 2-diphenyl-1-picrylhydrazyl (DPPH), gallic acid, carrageenan and indomethacin were from Sigma-Aldrich (St. Louis, MO, USA). Chloroform, n-Hexan, Acetone, and Methanol were used in Fractionation process. Acacia seyal gum (ASG) as raw was obtained from Blue Nile state, Sudan seasons 2015/2016 and Prebio-T as commercial (PTC) was obtained from Perfect Life Food Containing Homogenized Ingredients Manufacturing company Dubai-U.A.E. Spectro-Star Nano spectrophotometer was used for performing the reading of sample absorbance.
Extraction and solvent-guided of methanolic crude extract
About 500 g powdered sample was extracted with methanol using ultrasonic for of 3 hours at a power of 40 kHz, and at 42.5°C. Samples were placed into 20 ml vials glass closed tightly for reducing solvent evaporation. The yield of the pure methanol crude extract from ASG was (10 g, 11.10% w/w) and from PTC gum was (12 g, 15.56% w/w) respectively. The MCE of both samples (10 g) for each was subjected to solvent-guided fractionation according to the methods of Kupchan solvent-solvent partitioning with slight modification [23]. The different solvent polarities were used, which included; Chloroform, n-Hexan, Acetone, and Methanol were used in Fractionation process. The collected solvent of active fraction was concentrated and dried under nitrogen gas flushing at room temperature.
Preparation of active fractions
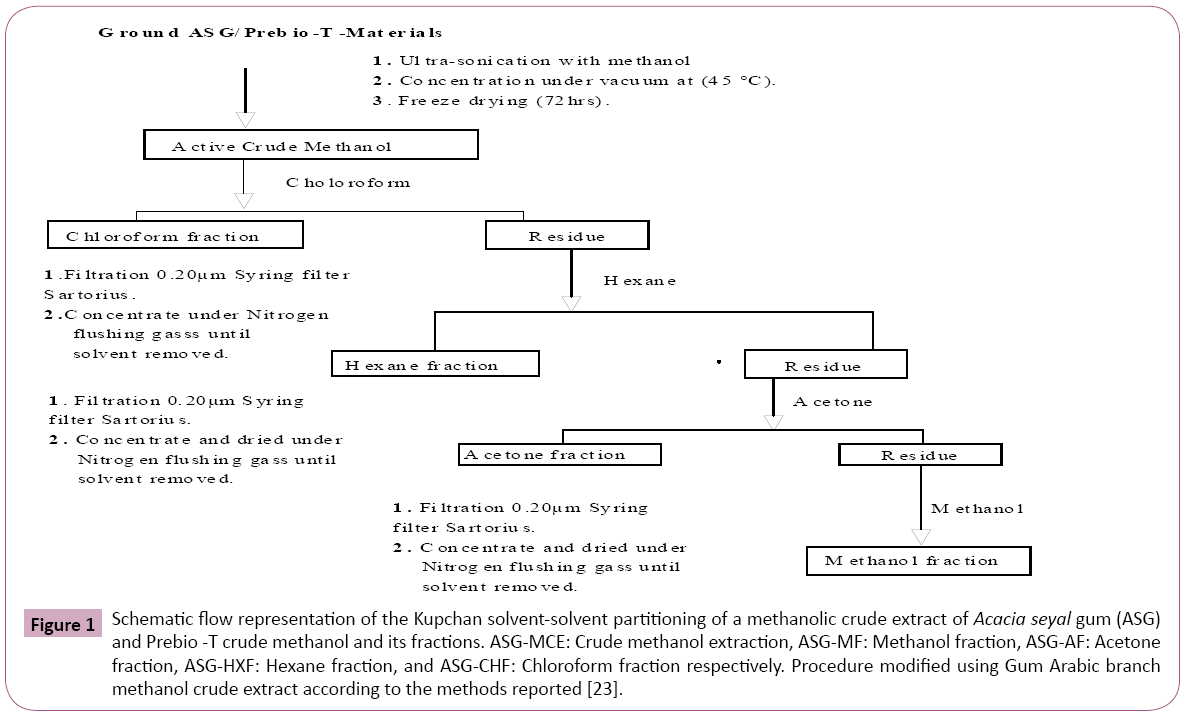
The fractionation of crude extraction will be followed by the characterisation of the fractions to select the best and active fractions. Regarding the detail steps, the following chart as described below in Figure 1.
Figure 1: Schematic flow representation of the Kupchan solvent-solvent partitioning of a methanolic crude extract of Acacia seyal gum (ASG) and Prebio -T crude methanol and its fractions. ASG-MCE: Crude methanol extraction, ASG-MF: Methanol fraction, ASG-AF: Acetone fraction, ASG-HXF: Hexane fraction, and ASG-CHF: Chloroform fraction respectively. Procedure modified using Gum Arabic branch methanol crude extract according to the methods reported [23].
Anti-oxidant activity
A. Total phenolic content (TPC): The procedure adopted follows the method described by Musa [24]. Approximately 0.5 mL diluted Folin-Ciocalteu (FC) reagent was added to 100 μL. The extraction procedure was conducted with (1.0 g) samples, and 10 mL was extracting solvent and allowed to set for 5 min before the addition of 1 mL (7.5%) of sodium carbonate (w/v). The absorbance was taken at 765 nm after 2 h using the spectrophotometer. Gallic acid was served as standard, and the results were reported as mg gallic acid equivalents mg GAE /100 g of sample dry weight (DW).
B. Anti-oxidant activity by DPPH assay: DPPH was freshly prepared by dissolving 40 mg DPPH in 1000 mL methanol to obtain the absorbance of the 1.00 ± 0.01 at 517 nm wavelength using a spectrophotometer. Sample (100 μL) was mix with 1 mL DPPH solution and kept closed in the dark for 2 h. Trolox was served as a standard, and the result was reported as mg Trolox equivalent (TE) per 100 g of dry sample (mg TE/100 g of DW).
C. Phytochemical analysis using gas chromatography-mass spectrometry (GC-MS/MS): Methanol crude extract (MCE), methanol fraction (MF), acetone fraction (AF) and active fractions were subject to GC-MS/MS to identify the phytochemical [25]. The analysis was performed by consolidating a mass-selective detector (MSD, Agilent 7000 Triple Quad) which was interfaced to a GC (Agilent Technologies 7890A), which was integrated with Agilent HP-5ms (5%-phenyl methyl polysiloxane) capillary column (with the properties of 30 m × 0.25 mm i.d. and 0.25 μm film thickness). Helium with a linear velocity of 1 ml/min was used as the process carrier gas. The injector temperature was set to 200°C with the injector at 250°C. The injected volume was administered at 1 μl of the sample. The operating parameters for MS were carried out at the ionisation potential of 70 eV, acquisition mass range of 50-600, and interface temperature of 250°C. The results for components identification were obtained by comparing their mass spectra and retention time towards the authentic compounds through a computer match-making process with NIST and WILEY library and conjointly comparing the data reported in the literature with the fragmentation pattern of the mass spectral data.
D. Preparation of animals: Adult albino rats, 120-150 g each, obtained from the Animal House in the National Research Center (Giza, Egypt) with. Acclimation was conducted for 7 days by housing the rats at 25 ± 2°C conditioned rooms featured with standard diet and tap water source. Overnight fasting was administered to the animals before each of the experiment. All experiments with animals involved were organized by conforming them to the Institutional Animal Care and Use (IACUC); International Islamic University Malaysia (IIUM), Health Guide for Care and Use of Laboratory Animals Ethics Policy Committee (AEPC), that has been approved by Senate meeting (2/2013), and along with the consent of National Research Center Ethics Committee on the use of laboratory animals, certified by Institutional Animal Care and Use (IACUC), International Islamic University Malaysia, with reference number of NHREC/12/01/2017A.
E. Carrageenan-induced rat paw oedema: Distribution was made for six treatment groups with six adult rats from per group.
• Group (I): Received saline and served as control (Distilled water in 1% Tween 80);
• Group (II): Received indomethacin drug in a dose of (20 mg/kg) 1% tween 80);
• Group (III-IV): Received crude extract in a dose of (150 and 300 mg/kg) of A. seyal gum (ASG) and Prebio-T- commercial (PTC);
• Group (V): Received indomethacin in a dose of (20 mg/kg).
Subplantar injection of 0.05 ml of 0.5% saline carrageenan suspension was used to administer the rat paw oedema into one of the hind paw plantar tissue in all groups. Vernier calipers (SMEC, Shanghai, China) was used to measure the mouse hind paws thickness before the carrageenan injection and after 1 h, 2 h, 3 h and 24 h of the injection [26] and [27], with slight modifications. The rats received vehicle or drug one hour before carrageenan injection. Carrageenan would have pronounced swelling and visible redness that was visibly occurred by four hours and lasted for more than 24 h. Water displacement plethysmometer measured the hind paw volume at the exact time before the carrageenan injection, and at the selected times after the injection [28]. The assessment of the inflammation was defined by the difference between the volume of the treated paw at zero time and the volume at the various times after the phlogistic agent administration. The outcomes were presented as the paw volume in milliliters. The percentages of inhibition and oedema were calculated from the mean effect of control and treated animals. The changes in oedema volume can be calculated for the difference between initial and subsequent readings of the paw volume for the corresponding time. Oedema control volume (Vc) and treated volume (Vt) were utilized to calculate oedema volume percentage (%) and inhibition percentage (%) by referring to the formula:
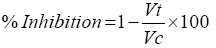 (1)
(1)
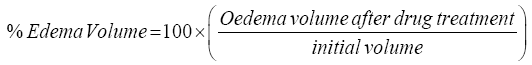 (2)
(2)
Statistical Analysis
Analysis of variance using Minitab Software version 17® was employed to determine the differences in antioxidant activities. Regarding anti-inflammatory experimental design used Dunnett multiple comparisons to analyse the final results using a one-way analysis of variance (ANOVA). An additional tool for Graph Pad Prism version 6 was also used. The results were expressed as the mean ± SEM, and the variation between means was considered to be significant at p ≤ 0.05.
Results and Discussion
The yield of crude extract and solvent-partitioned fractions
Table 1 shows the yield of the methanol crude extract (MCE) was 10 g (11.10% w/w) from Acacia seyal gum (ASG) and was 12 g (15.56% w/w) from PTC. The MCE of ASG and PTC were subjected to solvent-guided fractionation. The collected solvent fractions were methanol fraction (MF; 8.13 mg, 2.96% w/w), acetone fraction (AF; 8.74 mg, 2.34% w/w) for ASG fractions and (MF; 8.62 mg, 3.3%w/w) and (AF; 14.79 mg, 4.21% w/w) fractions for PTC.
| Plants | Extraction/Fraction | Amount (g/mg) |
Yield (% w/w) |
Antioxidant activity of methanol crude and it is active fractions | |
|---|---|---|---|---|---|
| TPC mg GAE/100 g |
DPPH mg TE/100 g |
||||
| A. seyal gum (ASG) (natural) |
ASG Crude Extract (CE) | 10.00 g | 11.19 | 155.78f ± 2.58 | 205.10d ± 1.50 |
| ASG MF | 8.13 mg | 2.96 | 285.08e ± 3.57 | 235.35a ± 1.51 | |
| ASG AF | 8.74 mg | 2.34 | 358.57d ± 1.58 | 234.85ab ± 1.57 | |
| A. seyal gum; Prebio T(commercial) | Prebio-T-Crud Extract (CE) | 10.00 g | 15.6 | 694.68a ± 3.60 | 229.01c ± 3.58 |
| Prebio-T-MF | 8.62 mg | 15.6 | 519.93c ± 1.64 | 235.34a ± 1.57 | |
| Prebio-T-AF | 14.79 mg | 4.21 | 657.81b ± 2.58 | 233.78b ± 2.57 | |
| Abbreviations: ASG-MCE: Crude Methanol Extraction, ASG-MF: Methanol Fraction, ASG-AF: Acetone Fraction, ASG-HXF: Hexane Fraction, and ASG-CHF: Chloroform Fraction, respectively. | |||||
Table 1: The antioxidant properties of active different fractions of A. seyal gum (natural exudate) (ASG) and A. seyal gum (Prebio -T) (commercial) (PTC) obtained after Kupchan-partitioning of the crude methanolic extract and its fractions*.
Total phenolic content (TPC)
Table 1 compares both methanolic crude extracts (MCE) and its active fractions of ASG and PTC. The extraction yield was almost higher with methanol fraction (MF) than with acetone (AF). Among the crude extract and solvent partitioned fractions, the highest amount of TPC was observed in the PTC, with an average value of 694.68 ± 3.60 mg GAE/100 g DW, compared to 155.78 ± 2.58 mg GAE/100 g DW for ASG methanolic Crude Extract (CE). TPC value of MF found to be 285.08 ± 3.57 mg GAE/100 g DW for ASG, whereas TPC was significantly higher (p ≤ 0.05) at 519.93 ± 1.64 mg GAE/100 g DW. Moreover, the TPC value of acetone fraction (AF) was 358.57 ± 1.58 mg GAE/100 g DW for ASG compare to the almost doubled TPC value of 657.81 ± 2.58 mg GAE/100 g DW of BBT acetone fraction. According to the results, crude extract and solvent partitioned fractions values have a descending order of MCE > AF > MF > AF for PTC and AF > MF > and MCE for ASG. Both samples revealed a significant difference (p ≤ 0.05) of antioxidant activity.
Phenolic compounds can be defined as secondary metabolites with a role of antioxidants owing to their capability of donating hydrogen, acting as metal chelators, and quenching singlet oxygen [29]. It has been confirmed that consumption of phenolicrich foods or beverages prevents diseases, such as cancer, heart disease, inflammation, arthritis, immune-related diseases, neurodegenerative diseases and diabetes [30]. This study endorsed the health benefits associated with the presence of phenolic compounds in Gum Arabic (GA).
Antioxidant activity by DPPH assay
The DPPH radical scavenging activity method has been used for the first time as an antioxidant activity test for GA fractionation (Table 1). The highest DPPH value was seen in methanol fraction (MF) at 235.34 ± 1.57 mg TE/100 g DW for PTC, in comparison with ASG at 235.35 ± 1.51 mg TE/100 g DW, without any significant differences. The DDPH antioxidant capacities of both acetone fractions (AF) were also unusually high, with AF obtained at the average of 233.78 ± 2.57 mg TE/100 g DW and 234.85 ± 1.57 mg TE/100 g DW for PTC and ASG, respectively. Within each DPPH assay, the mean values showed significant differences between the crude extract and its fractions, which also significantly affecting the antioxidant activity.
In this study, the determined antioxidant activity is of the strong type in comparison to the standard gallic acid, which also exhibits a strong correlation with the total phenolic content. The results showed the possibility of the presence of phenolic antioxidant molecules in gum Arabic and the better ability of polar solvents to extract them. Therefore, high solvent polarity increased the ability of extraction/fraction to reduce DPPH radical scavenging activity. Especially methanol and acetone fractions respectively. The results were compared to data on some rice bran protein hydrolysates [31]. Since there is no data on DPPH values of crude gum extract and gum fractionations. Thus, it was suggested that gum methanol crude extract and gum fractions could have anti-radical scavenging activity. ‘include a discussion with other studies’ (Table 1).
The chemical composition of the solvent extracts using GC-MS/MS analysis
According to our knowledge, there are no reports yet on the GC-MS/MS analysis for gum Arabic concerning extraction. The GC-MS/MS analysis of methanol crude extract (MCE) and its methanol (MF) and acetone fraction (AF) of the ASG and PTC revealed the presence of a total of 57 compounds (Table 2) that represent different classes. The major constituents in the ASG and PTC MCE were found to be isovitamin C (42.37%), crypton (5.86%), hydroquinone (4.86%), triacetic acid lactone (2.67%), 2,4-Di-tert-butyl phenol (2.67%), cyanidin cation (2.05%), apigenin 7-glucoside (1.9%), benzoic acid (1.83%), (+)-α-tocopherol (1.58%), methyl catechol (1.42%), and 2,6-dimethylol-p-cresol (2.16%). However, the same components were almost doubled in PTC -MCE (Table 2).
| No. | Compounds | Percentage of the compound in fractions Area Sum (%) | Retention time | Molecular formula |
Mw g/mol |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ASG/ MCE | ASG/ | ASG/ | Prebio-MCE | Prebio-MF | Prebio-AF | ||||||
| MF | AF | ||||||||||
| 1 | 4-Methylcatechol | 1.42 | 2.28 | 1.43 | 3.04 | 4.25 | 0.29 | 3.106 | C7H8O2 | 124 | |
| 2 | 2,5-Diamino-4.6-dihydroxypyrimidine | 1.65 | 1.8 | 0.75 | 0.98 | 1.73 | 0.59 | 3.161 | C9H13N5O2 | 223 | |
| 3 | Thiazolidin-4-0ne,5-ethyl-2-imino | 2.49 | 4.3 | 3.38 | 3.02 | 3.72 | 1.47 | 3.564 | C10H9NO3S | 223 | |
| 4 | Albuterol | 0.47 | 0.57 | 2.07 | 0.67 | 3 | 1.47 | 4.032 | C13H21NO3 | 239 | |
| 5 | 4-Methoxycinnamic acid | 1.11 | 0.57 | 0.36 | 0.35 | 0.57 | 0.22 | 4.545 | C10H10O3 | 178 | |
| 6 | Acetophenone,4'-ethyl | 0.91 | 1.46 | 1.07 | 0.54 | 0.25 | 1.14 | 4.694 | C10H12O | 148 | |
| 7 | Sinapyl alcohol | 0.98 | 0.57 | 1.12 | 1.37 | 0.8 | 1.63 | 4.765 | C23H42O4Si2 | 438 | |
| 8 | Crypton | 5.86 | 4.88 | 5.9 | 4.23 | 1.24 | 7.83 | 5.302 | C9H14O | 138 | |
| 9 | Isopinocampheol | 0.98 | 1.09 | 1.68 | 1.86 | 1.32 | 3.06 | 5.782 | C10H18O | 154 | |
| 10 | 4-Mercaptophenol | 0.76 | 0.63 | 2.02 | 3.37 | 0.54 | 1.5 | 6.292 | C6H6OS | 126 | |
| 11 | Triacetic acid lactone | 2.67 | 5.38 | 1.76 | 2.6 | 2.74 | 0.86 | 6.539 | C6H6O3 | 126 | |
| 12 | Hydroquinone | 4.86 | 4.02 | 5.23 | 5.15 | 1.33 | 5.31 | 7.003 | C6H6O2 | 110 | |
| 13 | Endo, endo-2,3-bornanediol | 0.61 | 0.93 | 0.68 | 1.88 | 2.39 | 3.84 | 7.519 | C10H18O2 | 170 | |
| 14 | Isobornyl acetate | 1.05 | 0.45 | 1.89 | 0.93 | 0.93 | 1.34 | 7.816 | C12H20O2 | 136 | |
| 15 | Apigenin 7-glucoside | 1.9 | 2.18 | 1.96 | 2.24 | 0.39 | 0.6 | 8.332 | C21H24O9 | 420 | |
| 16 | Dihydrouracil | 1.15 | 0.85 | 1 | 1.62 | 0.52 | 2.88 | 8.659 | C4H6N2O2 | 114 | |
| 17 | 1,4-Naphthoquinone, 2-acetyl-3-hydroxy-5,6,8-trimethoxy | 0.64 | 0.87 | 2.14 | 0.39 | 1.03 | 1.67 | 15.94 | C12H8O3 | 200 | |
| 18 | Fisetin | 0.74 | 0.64 | 1.9 | 0.45 | 5.81 | 2.42 | 16.087 | C15H10O6 | 286 | |
| 19 | Ferulic acid | 0.63 | 0.77 | 7.49 | 1.16 | 0.35 | 1.34 | 16.581 | C10H10O4 | 194 | |
| 20 | Resveratrol | 0.7 | 0.5 | 0.64 | 0.47 | 0.71 | 0.54 | 16.908 | C14H12O3 | 228 | |
| 21 | β-Citronellol | 0.71 | 0.78 | 1.4 | 0.5 | 0.66 | 5.84 | 16.972 | C10H20O | 156 | |
| 22 | Dihydrocarvone | 0.54 | 1.47 | 2.18 | 0.66 | 4.29 | 0.075 | 17.132 | C10H16O | 152 | |
| 23 | Patchoulol | 1.21 | 3.09 | 3.35 | 1.74 | 13.06 | 1.52 | 17.211 | C15H26O | 222 | |
| 24 | 5,7,3',4'-Tetrahydroxyflavone | 0.61 | 0.65 | 8.4 | 0.49 | 2.11 | 1.6 | 17.312 | C15H10O6 | 286 | |
| 25 | Chromone,5-hydroxy-6,7,8-trimethoxy-2,3-dimethyl- | 0.42 | 0.61 | 0.8 | 0.26 | 1.06 | 1.14 | 17.43 | C14H16O6 | 280 | |
| 26 | α-Bisabolol | 0.65 | - | 1.01 | 0.35 | 0.55 | 7.01 | 17.605 | C15H26O | 222 | |
| 27 | Isolongifolol | 0.54 | 1.44 | 1.31 | 0.27 | 0.33 | 0.68 | 17.761 | C17H28O2 | 264 | |
| 28 | Genistin | 0.67 | 0.68 | 0.37 | 0.26 | 0.49 | 1.7 | 18.338 | C12H8Cl2O3S | 302 | |
| 29 | Glycitein | 0.67 | 0.35 | 2.96 | 0.34 | 0.84 | 0.55 | 18.946 | C16H12O5 | 284 | |
| 30 | Quercetin | 0.44 | 0.4 | 0.84 | 0.33 | 0.46 | 0.49 | 19.355 | C15H10O7 | 302 | |
| 31 | Vanylglycol | 0.44 | 0.9 | 2.57 | 0.37 | 0.28 | 0.84 | 19.456 | C9H12O4 | 184 | |
| 32 | Quercetin 3-D-galactoside | 0.54 | 0.35 | 2.69 | - | 0.27 | 0.29 | 19.78 | C21H20O12 | 464 | |
| 33 | Propyl gallate | 0.58 | 0.4 | 0.39 | 0 | 0.25 | 0.46 | 20.974 | C10H12O5 | 212 | |
| 34 | 2,3,5,5,8a-Pentamethyl-6,7,8a-tetrahydo-5H-chromen-8-ol | 0.62 | 0.9 | 0 | 0 | 1.44 | 2.82 | 21.063 | C14H22O2 | 222 | |
| 35 | Glycitein | 0 | 0 | 0 | 0 | 0.33 | 0.28 | 21.389 | C16H12O5 | 284 | |
| 36 | Vanylglycol | 0 | 0 | 0 | 0 | 0.32 | 0.99 | 22.043 | C9H12O4 | 184 | |
| 37 | Quercetin 3-D-galactoside | 0 | 0 | 0 | 0 | 0.35 | 0.22 | 22.419 | C21H20O12 | 464 | |
| 38 | Vitexin | 0 | 0 | 0 | 0 | 0.25 | 0.33 | 23.424 | C21H20O10 | 432 | |
| 39 | Gengkwanin | 0 | 0 | 0 | 0 | 0.46 | 0.28 | 23.793 | C16H12O5 | 284 | |
| 40 | Flavone, 3,5,7-trimethyloxy- | 0 | 0 | 0 | 0 | 0.8 | 0.23 | 23.995 | C19H18O6 | 342 | |
| 41 | 2,3,5,5,8a-Pentamethyl-6,7,8a-tetrahydo-5H-chromen-8-ol | 0 | 0 | 0 | 0 | 0.27 | 0.35 | 24.099 | C14H22O2 | 222 | |
| 42 | Phenol,2,2'-methylenebis[6-(1,1-dimethylethyl)-4-methy- | 0 | 0 | 0 | 0 | 0.25 | 0.25 | 24.202 | C13H12O2 | 200 | |
| 43 | Lutein | 0 | 0 | 0 | 0 | 0 | 0.8 | 25 | C40H56O2 | 568 | |
| Total | 99.28 | 99.89 | 95.43 | 98.2 | 100 | 93.385 | -- | -- | -- | ||
| * Acacia seyal gum methanol (ASG); MCE: Methanol Crude Extract; MF: Methanol fraction; AF: Acetone fraction; Prebio-T(PTC): Acacia seyal gum (commercial sample). | |||||||||||
Table 2: Bioactive compounds in Acacia seyal gum (ASG) and Prebio-T (PTC) methanol crude extractions and their active fractions*.
Nine compounds, crypton (7.83%), chromone, 5-hydroxy- 6,7,8 l,-trimethoxy-2,3-dimethyl (7.01%), Phe-1,4-diol, 3,6-dimethyl (6.65%), hydroquinone (5.31%), ferulic acid (5.84%), isopinocampheol (3.06%), benzoic acid (2.02%), iso-vitamin C (1.34%), and β-carotene (1.21%), were found to be significantly high and present in both ASG-AF and PTC -MF. However, vanylglycol, quercetin 3-D-galactoside, vitexin, gengkwanin, gallic acid, retinoic acid, zearalenone, 4’,7-Dimethoxyisoflavone, and flavone, 4’-methoxy-6-acetyloxy, were calculated in methanol fraction (MF) only instead of AF and MCE for both samples. The compounds in GC-MS/MS analysis were studied based on a comparison of the mass spectra (MS) and retention time (RT) with the references present in the NIST mass spectral library.
In an earlier study, the most abundant constituents present in the volatile fractions of gum Arabic were not reported. However, in this study, most of the identified volatile compounds were reported as polyphenols, hydrocarbons, phenolic acids, fatty acids and several new constituents. Various identified compounds have already been reported as pharmacologically active. For instance, iso-vitamin C and Tocopherol, have shown antitumor activity in Hep3B hepatocellular carcinoma cells [32], and anti-inflammation [33].
Moreover, Crypton and hydroquinone are known to have potential antifungal and antibacterial activities [34]. Furthermore, long-chain unsaturated fatty acids, such as triacetic acid lactone, also show antibacterial activity and are considered to be the critical ingredients of antimicrobial, food additives and some antibacterial activities [35,36] also reported a similar investigation over the anti-inflammatory activity caused by this compound. Benzoic acid, ferulic acid and β- carotene also show anticancer and antioxidant activities [37]. Therefore, the presence of such bioactive compounds in the gum Arabic solvents extractions/ fractions were thought to play a crucial role in the everyday pharmacological activities shown by methanol, acetone crude and its fractions (Table 2).
Carrageenan-induced rat paw oedema
The results of carrageenan-induced paw oedema models (CIPE) are characterised as rising percentage (%) in paw volume at the peak of inflammation (Table 3 and Figure 2). Anti-inflammatory effect of methanol crude extract (MCE) and acetone crude extract (ACE) of the ASG and PTC, used in CIPE was dose-dependent, and indomethacin has been used as a reference drug. The maximum rise in paw volume was observed after 24 h of carrageenan injection in the vehicle-treated group. MCE and ACE at the tested doses of 150 and 300 mg/kg significantly inhibited both phases of inflammation induced by carrageenan in a dose-dependent manner.
| Groups | Percentage rise in paw edema at different time intervals | |||||
|---|---|---|---|---|---|---|
| Dose (mg/kg) | 1st hour | 2nd hours | 3rdhours | 24thhours | Mean of % inhibition | |
| Mean + SEM | Mean + SEM | Mean + SEM | Mean + SEM | |||
| Carrageenan Control | - | 68.3 ± 10.54 c | 86.6 ± 8.92 b | 88.6 ± 8.91 a | 35.3 ± 6.26 d | 69.7 |
| ASG low dose | 150 | 27.06 ± 3.93 b | 32.43 ± 6.29 a | 32.48 ± 3.92 a | 2.19 ± 1.39 c | 23.54 |
| ASG high dose | 300 | 27.43 ± 10.04 a | 22.55 ± 4.31 c | 23.32 ± 6.22 b | 2.54 ± 1.67 d | 18.96 |
| Prebio-T, low dose | 150 | 27.05 ± 3.93 b | 32.43 ± 63.29 a | 32.49 ± 3.92 a | 2.54 ± 1.67 c | 23.63 |
| Prebio-T, high dose | 300 | 27.43 ± 10.04 a | 22.55 ± 4.91 c | 23.32 ± 6.22 b | 2.54 ± 1.67 d | 18.96 |
| Indomethacin | 20 | 16.24 ± 1.27 c | 24.46 ± 1.25 b | 29.14 ± 1.54 a | 2.30 ± 1.18 d | 18.04 |
| *Abbreviations: ASG: Acacia seyal gum; Prebio-T: Acacia seyal gum (commercial sample). | ||||||
Table 3: The Effects of the Acacia seyal gum (ASG) and Prebio-T (Commercial)(PTC) Methanol Crude extract on Acute Inflammation Induced using Carrageenan*.
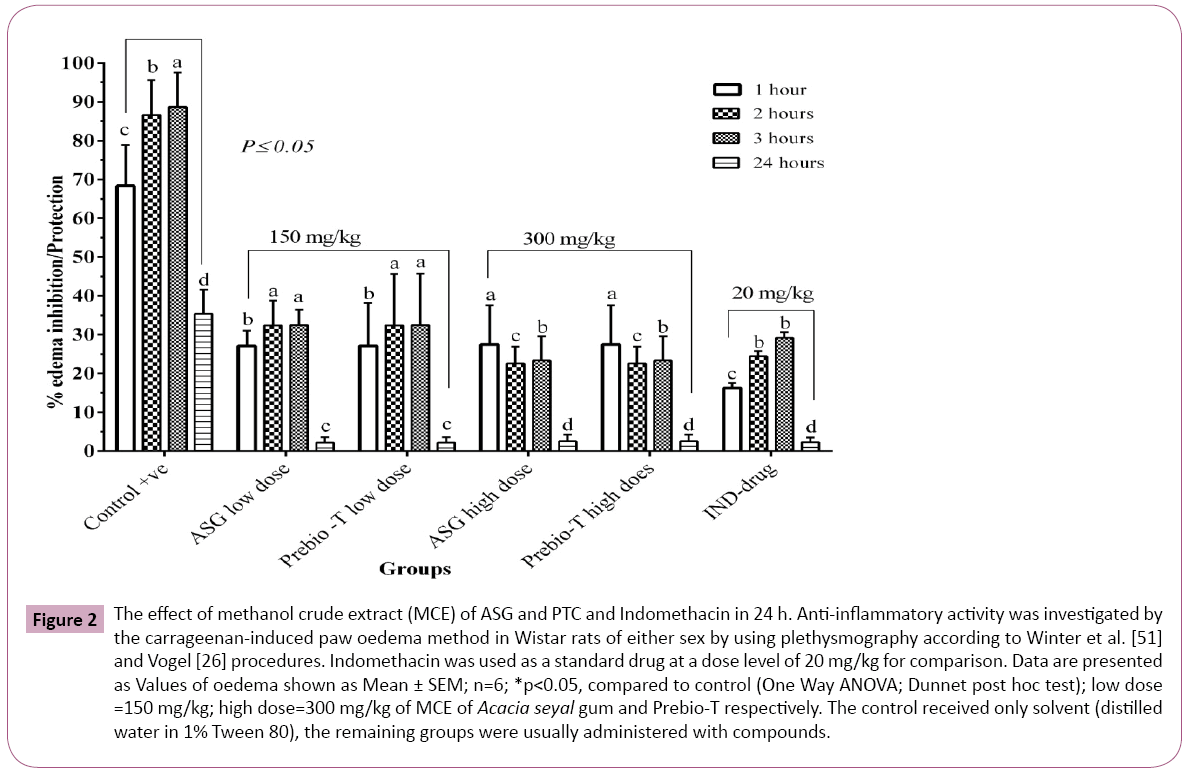
Figure 2: The effect of methanol crude extract (MCE) of ASG and PTC and Indomethacin in 24 h. Anti-inflammatory activity was investigated by the carrageenan-induced paw oedema method in Wistar rats of either sex by using plethysmography according to Winter et al. [51] and Vogel [26] procedures. Indomethacin was used as a standard drug at a dose level of 20 mg/kg for comparison. Data are presented as Values of oedema shown as Mean ± SEM; n=6; *p < 0.05, compared to control (One Way ANOVA; Dunnet post hoc test); low dose =150 mg/kg; high dose=300 mg/kg of MCE of Acacia seyal gum and Prebio-T respectively. The control received only solvent (distilled water in 1% Tween 80), the remaining groups were usually administered with compounds.
In this study, MCE and ACE of ASG and PTC were evaluated for its effects in experimental models of acute inflammation. Lambda [1] carrageenan is a natural product derived from red seaweeds belonging to a family of linear sulfated polysaccharides. Intraplantar injection of carrageenan leads to the development of inflammatory response characterized by common classical signs, including the increased volume of the injected paw, redness, as well as heat and local hypersensitivity [38]. CIPE is a standard model of acute inflammation, which has been accepted as a useful phlogistic tool for considering new antiinflammatory drugs application [39]. Previous studies stated that carrageenan-induced inflammatory response is mediated through multiple mechanisms like the release of eicosanoids, cytokines, chemokine’s, mast cell-derived products, neuropeptides, transcription factors and involvement of neutrophil migration [40].
Evaluation on methanol crude extracts antiinflammatory activity and its active fractions
The rats carrageenan-induced paw oedema (CIPO) resulted in the effect of ASG, and PTC methanolic crude extract (MCE) was observed in Table 3 and Figure 2. The investigations indicated that the anti-inflammatory activity in a dose-dependent form. The prevention of oedema formation by carrageenan was attained at 300 mg/kg dosage on both of the samples of MCE. The acute anti-inflammatory test showed that MCE of both ASG and PTC respectively, contributed to a maximum inhibition of 23.54% and a mean percentage inhibition of 23.63%, with the same dosage administration at 150 mg/kg, throughout the 24 h of examination. Furthermore, it was also notable that MCE of ASG at 300 mg/kg exhibited the same value with MCE of Prebio-T, after 24 h of examination, for the maximum percentage of inhibition at 18.96%. Between the administrations of treatment groups at 150 mg/kg, both ASG and Prebio-T MCE unfolded a similar percentage of less than 20% within the 24 h time frame (Table 3 and Figure 2). Meanwhile, the maximum percentage of inhibition for MCE was recorded at 23.54% at 150 mg/kg dosage at the end of 24 h. Nonetheless, indomethacin exhibited a higher percentage of inhibition at 18.04% at the concentration of 20 mg/kg within the same time frame. Overall, the highest inhibition rate was attained through the application of Prebio-T methanol crude extraction at 150 mg/kg with 23.63% rate of infested rat paw oedema.
In this study, oedema resulted from carrageenan was observed to be time-dependent with a progressive development towards the control rats while advancing to its maximum at 88.6 ± 8.91 mL within the first 3 h. A significant decrease was then followed at the end of 24 h with a value of 35.3 ± 6.26 mL. For a reliable comparison in the time-observation of the compounds antiinflammatory property, an investigation with 3 h, time frame for the hind paw oedema (HPO) maximum volume was presented previously [41].
Moreover, the methanolic crude extract (MCE) from both ASG and PTC samples exhibited significant activity in inhibition (p ≤ 0.05) at 150 and 300 mg/kg dosages within a period of 24 h, in carrageenan-induced inflammation models (CIIM). The activity exhibited was identical with the control rats group treated with Indomethacin. The application of 150 and 300 mg/kg MCE slightly resulted in oedema inhibition at the first hour with the lowest percentage of 27.06% at the application of 150 mg/kg, and the maximum percentage of inhibition at 27.43% at the application of 300 mg/kg MCE.
This finding is in agreement with the previous studies of CIIM that the first phase mediators inhibition of inflammation, bradykinins and prostaglandins, are discharged at the second phase of inflammation, thus causing the maximum activity of anti-inflammation in the first hour of application [42].
According to this, an argument can be built on the first phase suppression with 150 and 300 mg/kg ASG MCE application that the cause of the suppression may be the inhibition on the release of the early mediators (e.g., serotonin and histamine). The second phase may be explained with a similar interpretation over the cyclooxygenase inhibition action. The fact that the methanolic crude extract (MCE) of ASG and Prebio-T at a dose of 300 mg/kg having an equipotent inhibition for oedema inflammation, which was caused by the irritant agent for 24 h, has been described as a response to an injury from living tissues. This mechanism is well known to draw in a complex cluster of enzyme activation, fluid extravasations, mediator release, tissue breakdown and repair, as well as cell migration [43].
The compound ability to reduce local oedema on the right paw, which is induced by the irritant agent injection, can be measured by the most extensively used primary test to examine and choose the new anti-inflammatory agents [44]. For instance, the typical model for severe inflammation is CIPO in rats [44], which has been extensively used since the former time for drugs screening purpose [45]. Therefore, the assumption that explains for MCE to have longer half-life or a stable state similar to indomethacin, which not only provides 24h edema inhibition by suppressing prostaglandin synthesis but also inhibits the initiatory inflammation process where serotonin, histamine, and kinin serve as the principal mediators, can be explained by the investigation of the study performed [46]. This could present evidence that the bioactive compounds with non-selective interdependence with serotonins, histamines, prostaglandins and kinins which maintain a more prolonged duration activity were contained within the MCE of ASG and Prebio-T.
In an alluring manner, the rat paw oedema changes occurred is a biphasic process. The increased prostaglandin synthesis in the surroundings of damaged tissue, as well as histamine and serotonin, could have mediated the carrageenan model at its early phase of 1-2 h (Figure 2). The prostaglandin discharge sustained in the later phase at 3-24 h, where polymorphonuclear cells bradykinins, leukotrienes, and prostaglandins of tissue macrophages mediated the process [47].
According to our knowledge, there is no MCE study of ASG before in anti-inflammation activities. However, several researchers had been using GA as suspended materials other than antiinflammatory agents. Srimal and Dhawan [48] studied the effect of curcumin with 2% GA to reduce acute toxicity, while Marchetti and Rosnati [49], had suspended 3 mL of 5% GA for analgesic anti-inflammatory agents using raw gum Arabic. In a comparison between the results and the extract of ASG and PTC, MCE was shown to possess the highest anti-inflammatory activity towards the inflammation induced by carrageenan. Thus, with the presence of oedemogen, the significant reduction of paw oedema (p ≤ 0.05) by a highly polar methanolic extract of 300 mg/kg ASG and Prebio-T can be identified from our findings in this study. This suggests that the methanolic extract of ASG can induce the interference of histamine, bradykinin, and serotonin which leads to the preventing action of mast cell degranulation, and thus it may be critical for initial phase prevention of inflammation.
The ASG and Prebio-T content of alkaloids, flavonoids, β-carotene, as well as phenolic acids and tannins, can be identified from the earlier investigation on the MCE phytochemical constituents. Thus, the identification of flavonoids constituents in the gum Arabic may have characterised the MCE anti-inflammatory activity [50].
Finally, it is proposed that the MCE of ASG and PTC triggered the initial and later phase of inflammation PTC, with the maximum inhibition occurred during the later phase utilizing inhibition on the mast cell secretion and antihistaminic action with arachidonate metabolism interaction channeled in a distinctive means of way. Such an effect was desolated from the highly polar methanolic extract. The given results emphasise the conventional use of GA for inflammation, and the evaluation of the presence of biologically active compounds is highlighted for further investigation and clarification. However, further studies are required for the isolation and characterisation of the antiinflammatory chemical substances available from the raw and commercial forms of gum Arabic MCE (Table 3 and Figure 2).
Conclusion
This study has revealed that methanol crude extracts and its active fractions from Acacia seyal gum have unusual antioxidant activities which are enormously influenced by the solvents partition. Antioxidant extraction depends on the solubility of bioactive compounds (of gum material) in the extraction/partition solvent, hereafter a proper crude extracts namely methanol crude extract/fractions has been reported in this study. Definitive clinical studies are needed to understand the many medicinal uses of ASG fully. Future work should include the research into which bioactive compounds can be isolated and described from the different fractions of ASG for a better understanding on the underlying mechanism of their role towards cytotoxicity, antiinflammatory and antioxidant activity. Thus, there is an essential priority in the future advancement of the potential anticancer therapy through the documented potentials of cytotoxicity function by these extracts.
Original work in the field, this study conducts, for the first time, an investigation for the bioassay findings that provide some basis to justify the frequent use of Acacia seyal gum (ASG) extracts in folk medicine as treatments for cancer, and inflammatory conditions. These results provide some evidence for the medicinal value of ASG [51-62].
Acknowledgements
The authors would like to express gratitude and gratefulness to the Department of Biotechnology Engineering (BTE), Faculty of Engineering, International Islamic University Malaysia (IIUM) and the International Institute for Halal Research and Training (INHART), IIUM for allowing using their laboratories and facilities. The acknowledgement extended to Dr. Elbasheir Sallam for his continuous and unlimited financial support to the PhD candidate conducting this research.
Disclosure
The authors declare no conflict of interest.
References
- Mohamed AG (2005) Improvement of traditional Acacia senegal agroforestry: Ecophysiological characteristics as indicators for tree-crop interaction on sandy soil in western Sudan: University of Helsinki, Finland.
- Montenegro MA, Boiero ML, Valle L, Borsarelli CD (2012) Gum Arabic: More than an edible emulsifier. In: Products and applications of biopolymers: InTech.
- Bayliss W (1922a) Acacia for transfusion. JAMA 78: 1885.
- Phillips GO, Ogasawara T, Ushida K (2008) The regulatory and scientific approach to defining gum arabic (Acacia senegal and Acacia seyal) as a dietary fibre. Food Hydrocoll 22: 24-35.
- Al Assaf S, Phillips GO, Williams PA (2005) Studies on acacia exudate gums. Part I: the molecular weight of Acacia senegal gum exudate. Food Hydrocoll 19: 647-660.
- FAO (1999) The state of food insecurity in the world. Food and Nutrition, Rome, Italy.
- Middlekauff RD (1975) Food safety review-new concepts for GRAS. Food Drug Cosm 30: 288.
- Norman AG (1929) The chemical constitution of the gums: Part I. The nature of gum arabic and the biochemical classification of the gums. Biochem J 23: 524-535.
- Waters CE, Tuttle JB (1916) Some qualitative tests for gum arabic and its quantitative determination. J Ind Eng Chem 8: 413-416.
- Mukherjee S, Deb S (1962) Light-scattering studies in solutions of gum arabic. J Indian Chem Soc 39: 823.
- Mohammedelnour AA, Mirghani M, Kabbashi NA, Alam MZ, Musa KH, et al. (2017) Effect of solvent types on phenolics content and antioxidant activities of Acacia polyacantha gum. Food Res Int 24
- Bamford C, Tompa H (1950) The theory of coacervation. Transactions of the Faraday Society 46: 310-316.
- Trommer H, Neubert RH (2005) The examination of polysaccharides as potential antioxidative compounds for topical administration using a lipid model system. Int J Pharm 298: 153-163.
- Tiss A, Carrière F, Verger R (2001) Effects of gum arabic on lipase interfacial binding and activity. Anal Biochem 294: 36-43.
- Amy L (1934) Contribution à l'étude des propriétés et de la structure des solutions et des gelées de gomme: Masson, France.
- Gramatica C, Zanardelli P (2003) La gomme arabique en oenologie (1 partie). Revue des oenologues et des techniques vitivinicoles et oenologicques: Magazine trimestriel d'information professionnelle. 30: 33-35.
- Caius J, Radha K (1942) The gum arabic of the bazaars and shops of Bombay. J. Bombay Nat Hist 41: 261-271.
- Bayliss W (1922b) Acacia for transfusion. JAMA 78: 1885.
- Chevalier A (1924) Sur la production de la Gomme arabique en Afrique occidentale française. Journal d'agriculture traditionnelle et de botanique appliquée 4: 256-263.
- Ali Al-Husseni I, Beegam S, Al-Shukaili A, Nemmar A, Schierling S, et al. (2013) Effect of gum arabic on oxidative stress and inflammation in adenine-induced chronic renal failure in rats. PLOS One 8: e55242.
- Glover DA, Ushida K, Phillips AO, Riley SG (2009) Acacia (Sen) SUPERGUM™(Gum Arabic): An evaluation of potential health benefits in human subjects. Food Hydrocoll 23: 2410-2415.
- Wapnir RA, Sherry B, Codipilly CN, Goodwin LO, Vancurova I (2008) Modulation of rat intestinal nuclear factor NF-κB by gum arabic. Dig Dis Sci 53: 80-87.
- Kupchan SM, Tsou G, Sigel CW (1973). Datiscacin, a novel cytotoxic cucurbitacin 20-acetate from Datisca glomerata. J Org Chem 38: 1420-1421.
- Musa KH, Abdullah A, Jusoh K, Subramaniam V (2011) Antioxidant activity of pink-flesh guava (Psidium guajava L.): effect of extraction techniques and solvents. Food Anal Method 4: 100-107.
- Stankov V, Ilić M, Mitić V, Mihajilov-Krstev T, Simonović S (2015) Secondary metabolites of Seseli rigidum: Chemical composition plus antioxidant, antimicrobial and cholinesterase inhibition activity. J Pharm Biomed Anal 111: 78-90.
- Vogel HG, Vogel WH (2013) Drug discovery and evaluation: pharmacological assays: Springer Science & Business Media.
- Winter CA, Risley EA, Nuss GW (1962a) Carrageenin-induced oedema in hind paw of the rat as an assay for anti-inflammatory drugs. Exp Biol Med 111: 544-547.
- Chattopadhyay D, Arunachalam G, Mandal AB, Sur TK, Mandal SC, et al. (2002) Antimicrobial and anti-inflammatory activity of folklore: Mallotus peltatus leaf extract. J Ethnopharmacol 82: 229-237.
- Heleno SA, Martins A, Queiroz MJRP, Ferreira ICFR (2015) Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem 173: 501-513.
- Dubost NJ, Ou B, Beelman RB (2007) Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlation to total antioxidant capacity. Food Chem 105: 727-735.
- Phongthai S, D'Amico S, Schoenlechner R, Homthawornchoo W, Rawdkuen S (2018) Fractionation and antioxidant properties of rice bran protein hydrolysates stimulated by in vitro gastrointestinal digestion. Food Chem 240: 156-164.
- Yiang GT, Chou PL, Hung YT, Chen JN, Chang WJ, et al. (2014) Vitamin C enhances anticancer activity in methotrexate‑treated Hep3B hepatocellular carcinoma cells. Oncol Rep 32: 1057-1063.
- Teng J, Pourmand A, Mazer-Amirshahi M (2018) Vitamin C: The next step in sepsis management? J Crit Care 43: 230-234.
- Theodossiou TA, Tsiourvas D, Hothersall JS (2010) Hypericin Hydroquinone: Potential as a red‐far red photosensitizer? Photochem Photobiol 86: 18-22.
- Kraus GA, Basemann K, Guney T (2015) Selective pyrone functionalization: Reductive alkylation of triacetic acid lactone. Tetrahedron Letters 56: 3494-3496.
- Calder PC (2013) Omega‐3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol 75: 645-662.
- Bogucka-Kocka A, Zidorn C, Kasprzycka M, Szymczak G, Szewczyk K (2016) Phenolic acid content, antioxidant and cytotoxic activities of four Kalanchoë species. Saudi Journal of Biological Sciences.
- Patil S (2017) Anti-inflammatory activity of bartogenic acid containing a fraction of fruits of Barringtonia racemosa Roxb. in acute and chronic animal models of inflammation. J Tradit Complement Med 7: 86-93.
- Matsumoto K, Obara S, Kuroda Y, Kizu J (2015) Anti-inflammatory effects of linezolid on carrageenan-induced paw oedema in rats. J Infect Chemother 21: 889-891.
- Abeles AM, Pillinger MH, Abramson SB (2015) 23 - Inflammation and its mediators A2 - Hochberg, Marc C. In A. J. Silman, J. S. Smolen, M. E. Weinblatt, & M. H. Weisman (Eds.), Rheumatology (6th edn) (pp. 169-182). Philadelphia, PA, USA.
- Aragão DP, Da Silva Souza B, De Brito TV, De Araújo Bastos Santana L, De Paiva Silva RM, et al. (2017) The anti-inflammatory and antinociceptive activity of albumins from Crotalaria retusa seeds. Biomed Pharmacother 93: 536-542.
- Araújo IWF, Rodrigues JAG, Quinderé ALG, Silva JdFT, Maciel GDF (2016) Analgesic and anti-inflammatory actions on bradykinin route of a polysulfated fraction from alga Ulva lactuca. Int J Biol Macromol 92: 820-830.
- Andreasen JO, Andreasen FM, Andersson L (2013) Textbook and colour atlas of traumatic injuries to the teeth: John Wiley & Sons, NY, USA.
- Fochtman P, Raszka A, Nierzedska E (2000) The use of conventional bioassays, microbiotests, and some “rapid” methods in the selection of an optimal test battery for the assessment of pesticides toxicity. Environ Toxicol 15: 376-384.
- Rosa M (1974) Effect of non-steroidal anti-inflammatory drugs on leukocyte migration. Future Trends in Inflammation pp: 143-152.
- Dar A, Baig H, Saifullah S, Ahmad V, Yasmeen S, et al. (2007) Effect of seasonal variation on the anti-inflammatory activity of Sargassum wightii growing on the N. Arabian Sea coast of Pakistan. J Exp Mar Bio Ecol 351: 1-9.
- Nisar A, Malik AH, Zargar MA (2013) Atropa acuminata Royle Ex Lindl. Blunts production of pro-inflammatory mediators eicosanoids, leukotrienes, cytokines in vitro and in vivo models of acute inflammatory responses. J Ethnopharmacol 147: 584-594.
- Srimal R, Dhawan B (1973) Pharmacology of diferuloyl methane (curcumin), a non‐steroidal anti‐inflammatory agent. J Pharm Pharmacol 25: 447-452.
- Marchetti E, Mattalia G, Rosnati V (1968) A new class of analgetic-antiinflammatory agents. 2-Substituted 4, 5-diphenyloxazoles. J Med Chem 11: 1092-1093.
- Fongang ALM, Laure Nguemfo E, Djouatsa Nangue Y, Bogning Zangueu C, Fouokeng Y, et al. (2017) Antinociceptive and anti-inflammatory effects of the methanolic stem bark extract of Antrocaryon klaineanum Pierre (Anacardiaceae) in mice and rat. J Ethnopharmacol 203: 11-19.
- Winter CA, Risley EA, Nuss GW (1962b) Carrageenin-induced oedema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 111: 544-547.
- Bohidar H (2008) Coacervates: A novel state of soft matter: An overview. J Surf Sci Technol 24: 105-124.
- Bungenberg de Jong H (1949) Crystallisation-coacervation-flocculation. J Colloid Sci 2: 232-258.
- Gawlik-Dziki U, Dziki D, Świeca M, Nowak R (2017) Mechanism of action and interactions between xanthine oxidase inhibitors derived from natural sources of chlorogenic and ferulic acids. Food Chem 225: 138-145.
- Mukherjee S, Ghosh K (1949) Influence of concentration on the properties of Arabic acid solution. J Indian Chem Soc 26: 81-90.
- Nelson RE, Ander P (1972) Heterogeneity of gum arabic and its salts. Carbohydr Res 25: 81-98.
- Oakley HB (1935) A new type of osmometer for low pressures, with some preliminary results for gum Arabic. T Faraday Soc 31: 136-147.
- Parry E (1918) Moreover, Gums and resins: Their occurrence, properties and uses (Pitman's common commodities and industries). London: Pitman and Sons, UK.
- Spagnuolo C, Moccia S, Russo GL (2018) Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur J Med Chem 153: 105-115.
- Taft R, Malm LE (1931) Solvents for Gum Arabic. II. Trans Kans Acad Sci 34: 116-117.
- Thomas AW, Murray HA (1928) A physicochemical study of gum arabic. J Phys Chem 32: 676-697.
- Zakaria ZA, Ghani ZDFA, Nor RNSRM, Gopalan HK, Sulaiman MR, et al. (2008) Antinociceptive, anti-inflammatory, and antipyretic properties of an aqueous extract of Dicranopteris linearis leaves in experimental animal models. J Nat Med 62: 179-187.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences